-
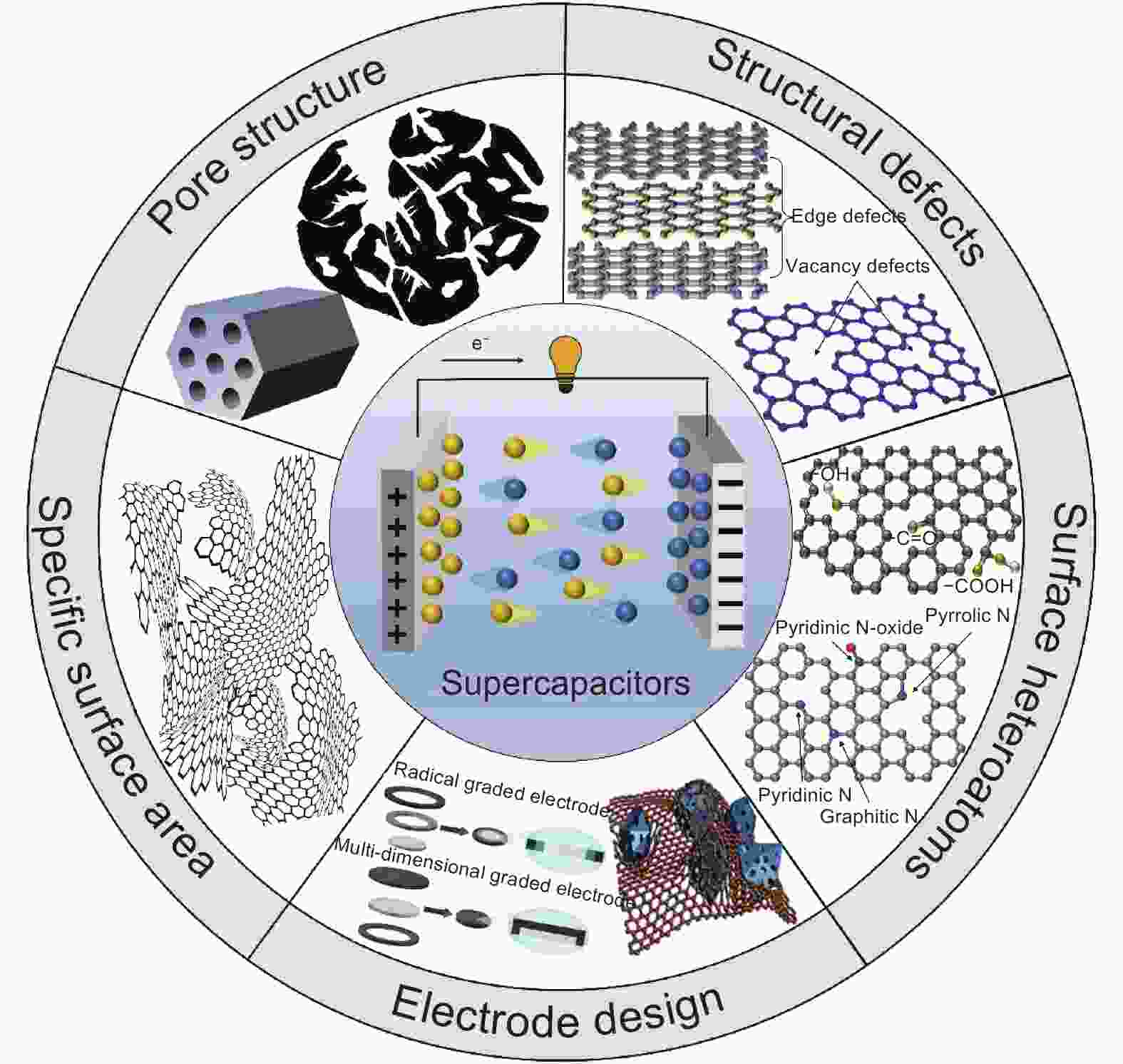
摘要: 由于具有良好的物理化学稳定性、高比表面积、可调的孔结构及优良的导电性,多孔炭广泛应用于超级电容器电极材料。它的电容性能与其比表面积、孔结构、表面杂原子、结构缺陷及电极结构密切相关。离子及表面积(有效表面)能够提供丰富的活性位点,而合适的孔结构有利于离子的传输和存储,因而共同影响着炭基电极材料的比电容和倍率性能。具有合适孔径分布、一定数量的离子传输通道及微孔/介孔比例,是提高超级电容器能量密度和功率密度的必要条件。此外,结构缺陷、表面杂原子及合理的电极结构设计对多孔炭基超级电容器的电容性能具有重要的影响。Abstract: Porous carbon-based electrode materials have been widely used in supercapacitors (SCs) because of their good physicochemical stability, high specific surface area, adjustable pore structure, and excellent electrical conductivity. The factors influencing their SC performance are analyzed, which include specific surface area, pore structure, surface heteroatoms, structural defects and electrode structure. The high surface area accessible to ions provides abundant active sites for their storage, while a suitable pore structure is important for the accommodation and diffusion of ions, thereby influencing the specific capacitance and rate performance of the electrodes. An appropriate pore size with a narrow distribution is required to increase the volumetric energy density while mesopores are favorable for ion transport, so a good balance between micro and mesopore volumes is important to improve both the energy and power densities of the SCs. Structural defects, surface heteroatoms and a rational electrode structural design all play significant roles in the capacitance performance.
-
Key words:
- Supercapacitors /
- Porous carbons /
- Specific surface area /
- Pore structure /
- Electrode structure
-
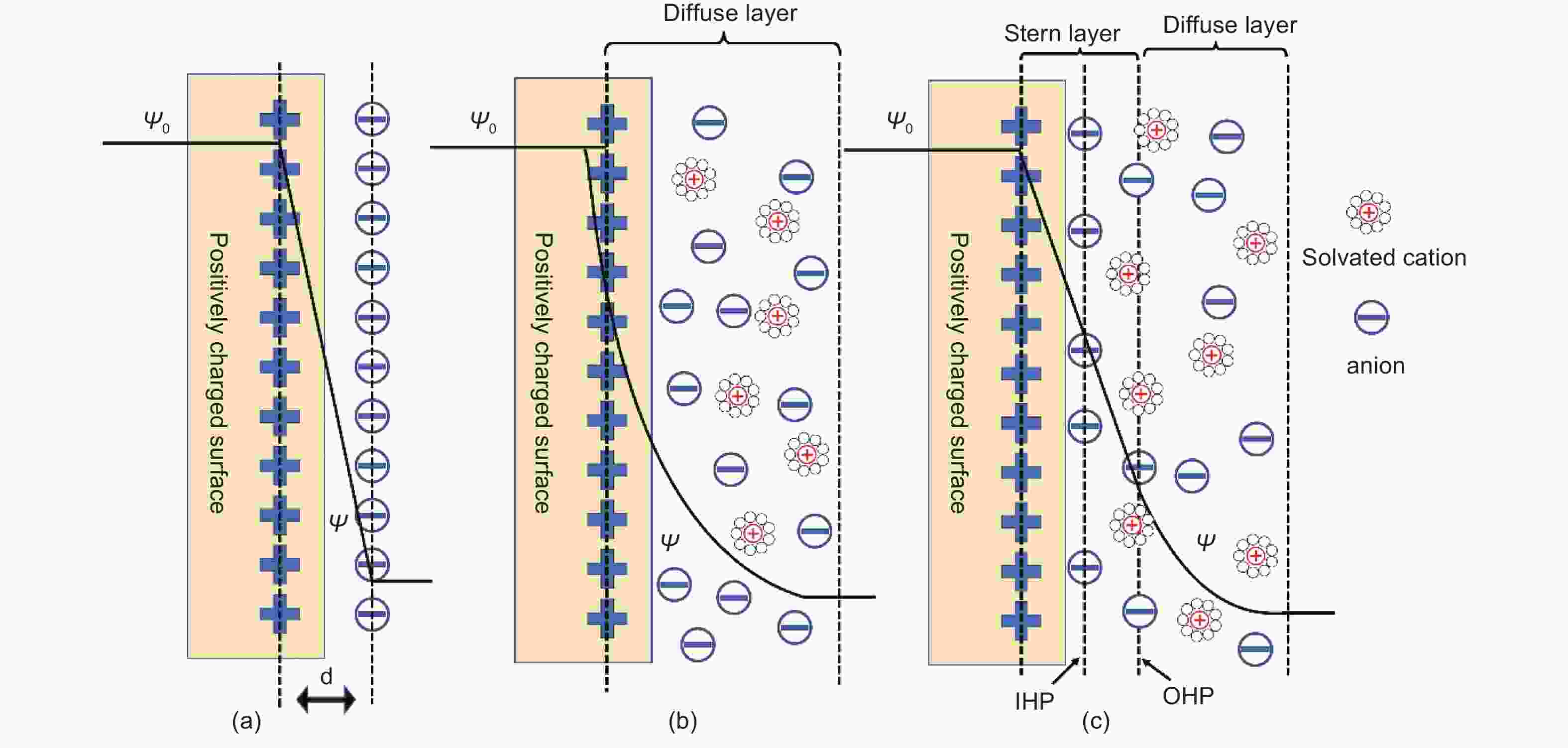
Figure 2. Different EDL models: (a) Helmholtz, (b) Gouy-Chapman and (c) Stern. d presents the electric double layer distance in Helmholtz model[23]. Ψ0 and Ψ are potentials at the electrode surface and electrode/electrolyte interface, respectively. Reprinted with permission.
Figure 3. (a) Overscreening effect at moderate voltage and (b) crowding effect at high voltage[25]. Reprinted with permission.
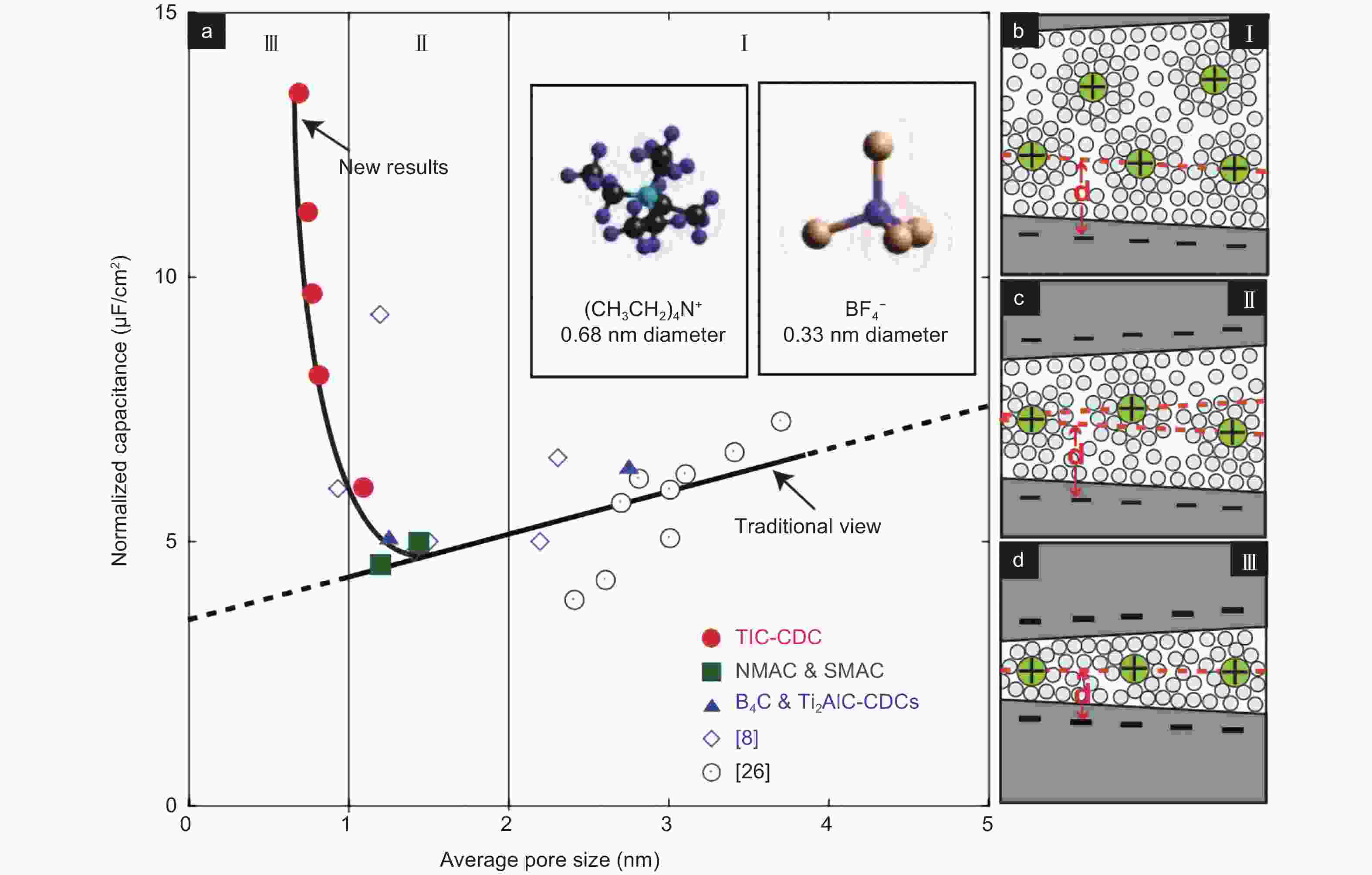
Figure 4. (a) Correlations between normalized capacitance and average pore size in an electrolyte containing 1.5 mol L–1 TEABF4 dissolved in ACN. Solvated ions residing in pores with a distance between adjacent pore walls: (b) >2 nm, (c) 1-2 nm, and (d) <1 nm[10]. Reprinted with permission.
Figure 5. Geometric confinement of ions in small pores. Ion size: TEA+ (0.67 nm) and BF4- (0.47 nm)[57]. Reprinted with permission
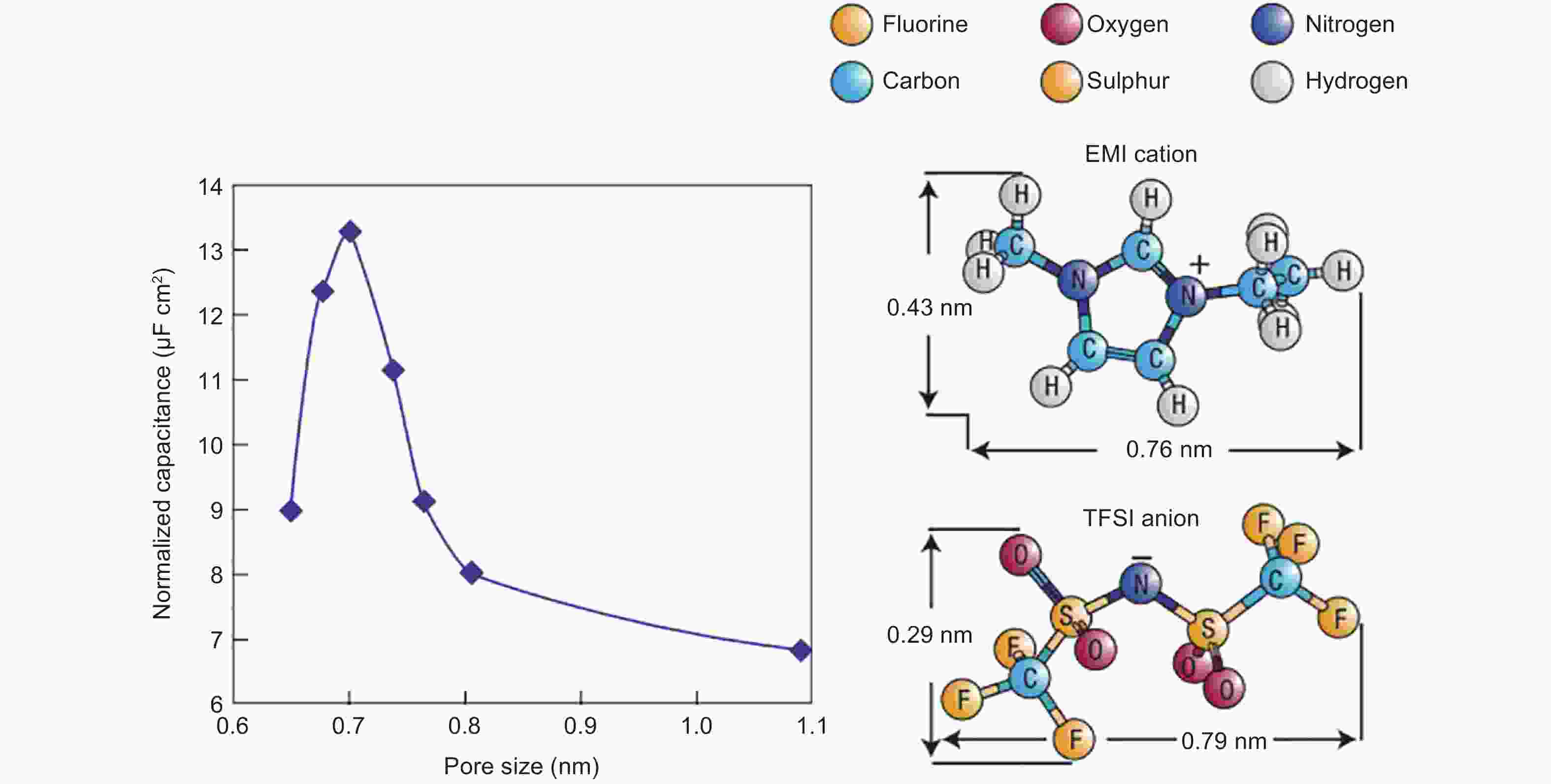
Figure 6. Plots of specific capacitance normalized by SSA as a function of average pore size obtained for various CDC electrodes in neat EMITFSI ionic liquid[11]. Reprinted with permission.
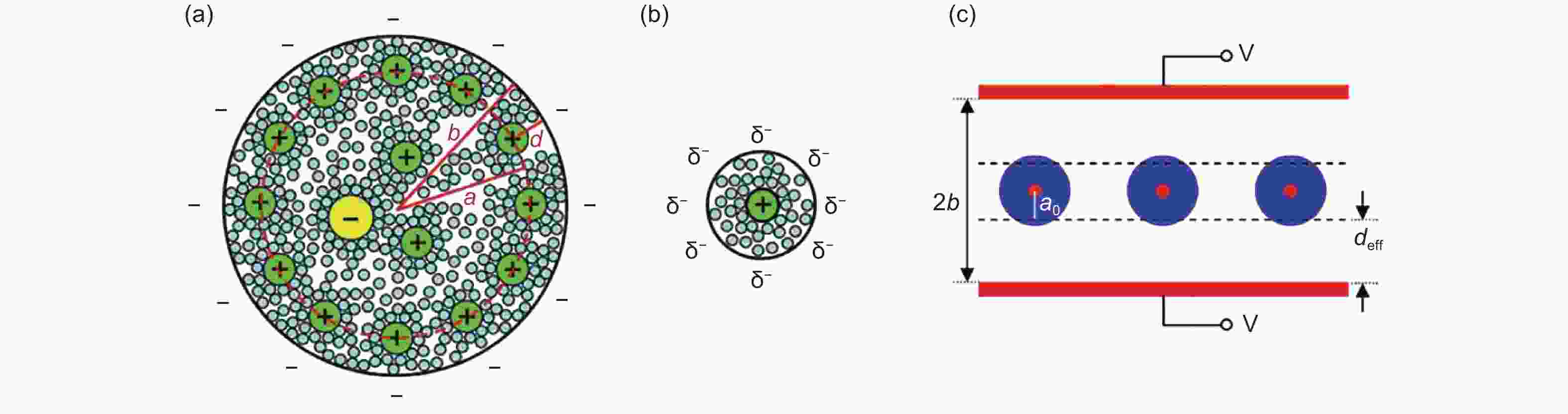
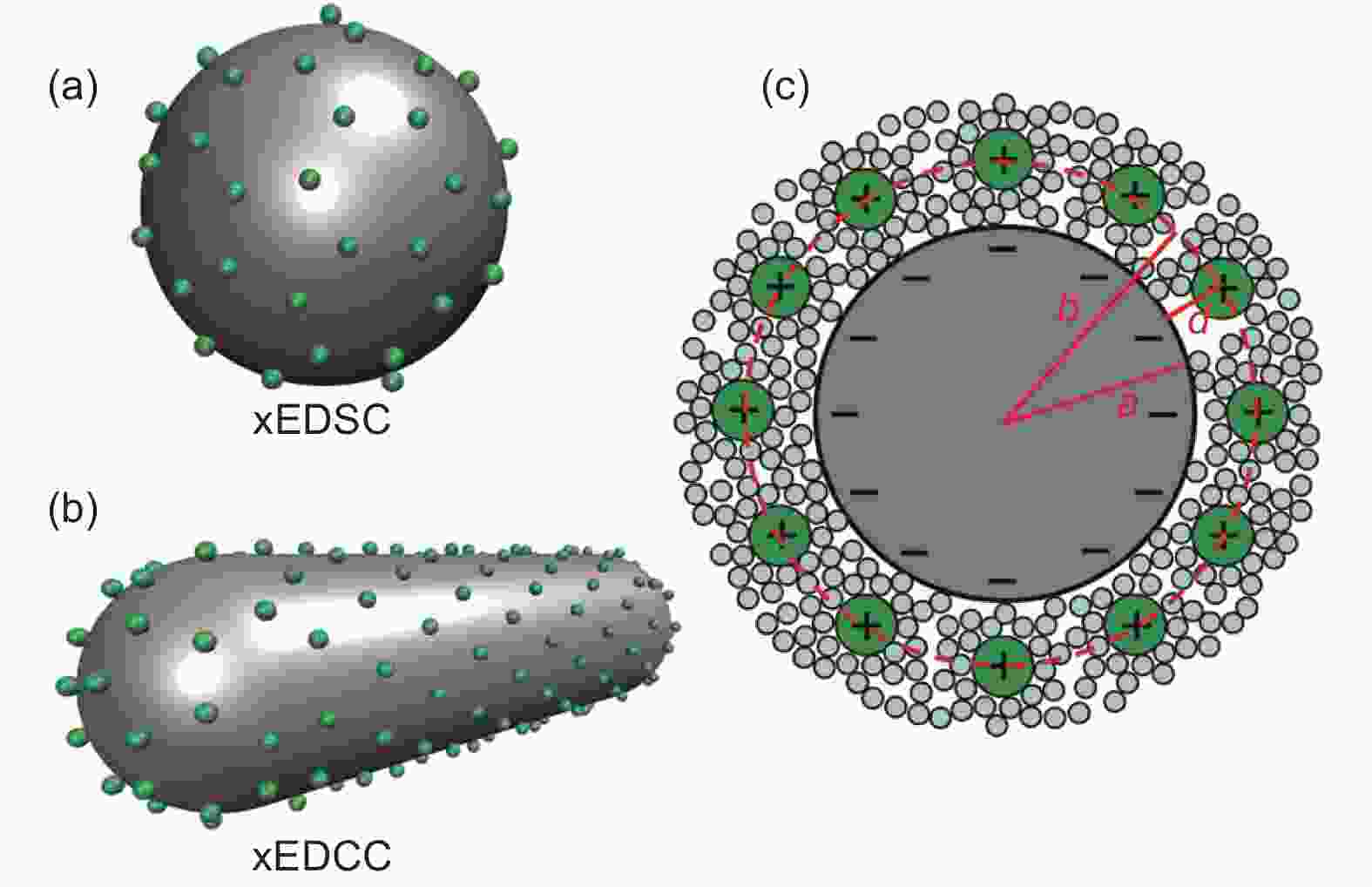
Figure 8. (a) Plots of 0D spheres and (b) 1D tubes with counter ions approaching the outer surface. (c) Schematic plot displaying the cross-section of an exohedral capacitor[81]. Reprinted with permission.
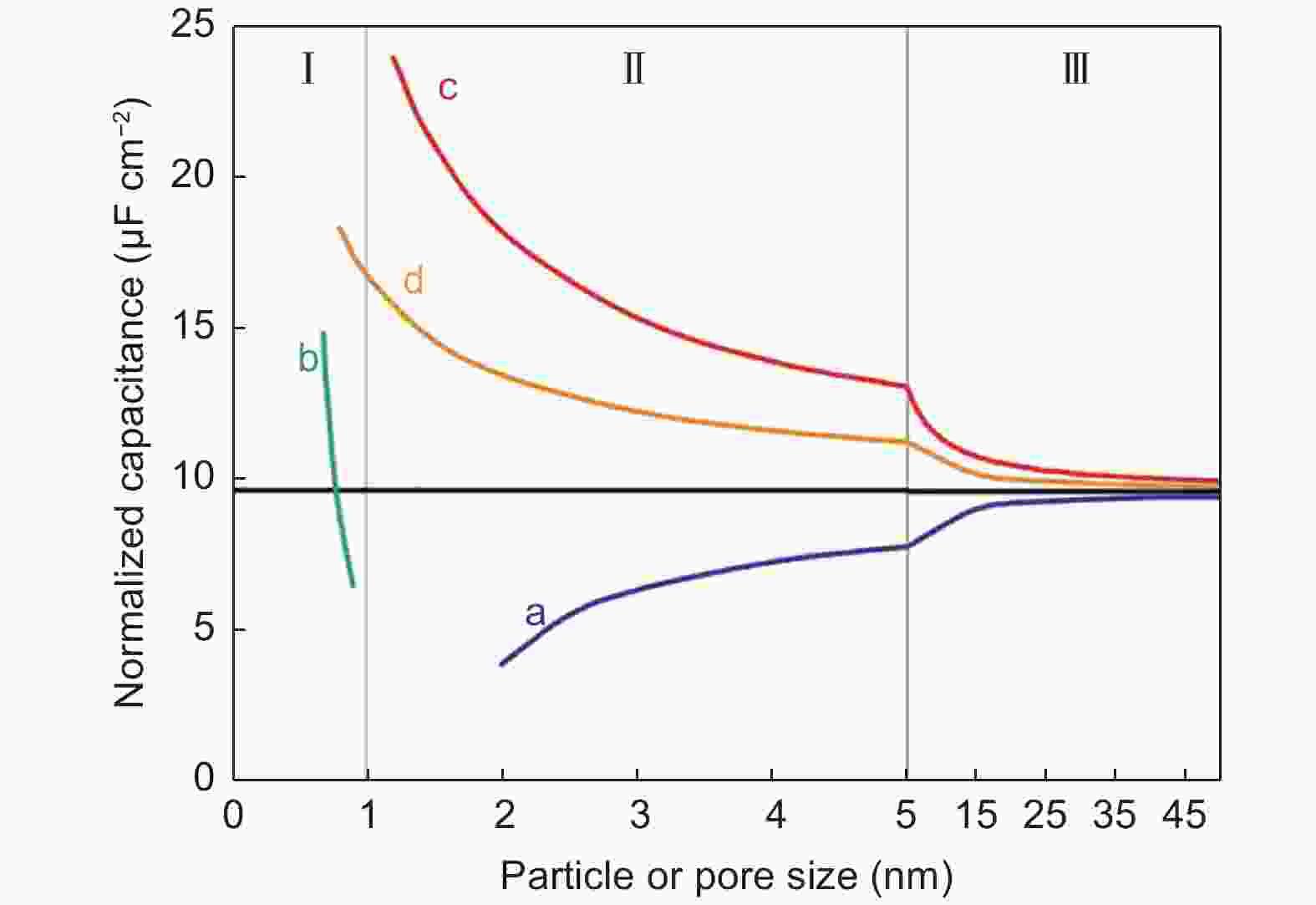
Figure 9. Correlations between normalized capacitance and particle/pore size of endohedral capacitors. Note that curves a and b represent the mesoporous and microporous carbons. For exohedral capacitors, the curves c and d represent 0D spheres and 1D tubes. The black line represents parallel-plate capacitor[81]. Reprinted with permission.
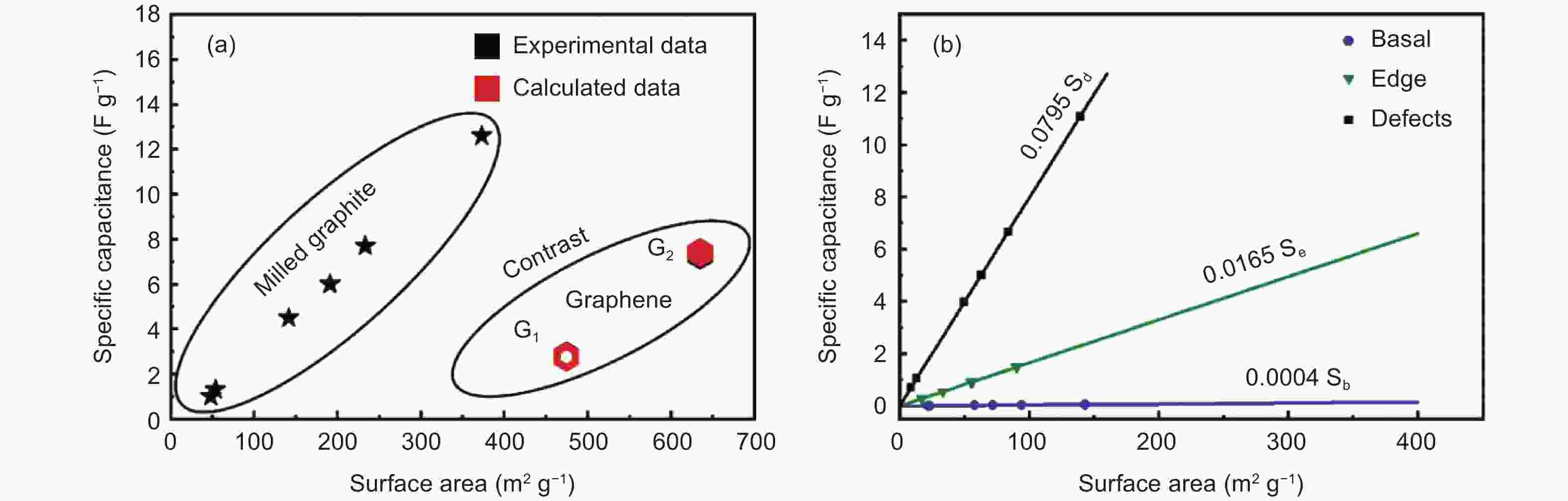
Figure 10. (a) Variation in surface area with a specific capacitance of milled-graphite and graphene. (b) Correlations between three types of surface area and specific capacitance[13]. Reprinted with permission.
Figure 11. Schematic diagrams of volume expansion in KB-AC (a) graphene-AC and (b) electrodes[147]. Reprinted with permission.
Figure 12. (a) Schematic illustration of radial and multi-dimensional graded electrodes. Rate capability (b) cycling stability at 1 A·g-1 and (c) multi-dimensional graded, radical graded, and homogeneous electrodes in 6 mol/L KOH electrolyte[150]. Reprinted with permission.
-
[1] Liu C, Li F, Ma L P, et al. Advanced materials for energy storage[J]. Advanced Materials,2010,22(8):E28-E62. doi: 10.1002/adma.200903328 [2] Muzaffar A, Ahamed M B, Deshmukh K, et al. A review on recent advances in hybrid supercapacitors: Design, fabrication and applications[J]. Renewable & Sustainable Energy Reviews,2019,101:123-145. [3] Simon P, Gogotsi Y, Dunn B. Materials science. Where do batteries end and supercapacitors begin?[J]. Science,2014,343(6176):1210-1211. doi: 10.1126/science.1249625 [4] Simon P, Gogotsi Y. Materials for electrochemical capacitors[J]. Nature Materials,2008,7(11):845-854. doi: 10.1038/nmat2297 [5] Salanne M, Rotenberg B, Naoi K, et al. Efficient storage mechanisms for building better supercapacitors[J]. Nature Energy,2016,1(6):16070. doi: 10.1038/nenergy.2016.70 [6] Zhang L L, Zhao X S. Carbon-based materials as supercapacitor electrodes[J]. Chemical Society Reviews,2009,38(9):2520-2531. doi: 10.1039/b813846j [7] González A, Goikolea E, Barrena J A, et al. Review on supercapacitors: Technologies and materials[J]. Renewable and Sustainable Energy Reviews,2016,58:1189-1206. doi: 10.1016/j.rser.2015.12.249 [8] Chen Y, Hao X, Chen G Z. Nanoporous versus nanoparticulate carbon-based materials for capacitive charge storage[J]. Energy & Environmental Materials,2020,3(3):247-264. [9] Shao H, Wu Y C, Lin Z, et al. Nanoporous carbon for electrochemical capacitive energy storage[J]. Chemical Society Reviews,2020,49(10):3005-3039. doi: 10.1039/D0CS00059K [10] Chmiola J, Yushin G, Gogotsi Y, et al. Anomalous increase in carbon capacitance at pore sizes less than 1 nanometer[J]. Science,2006,313(5794):1760-1763. doi: 10.1126/science.1132195 [11] Largeot C, Portet C, Chmiola J, et al. Relation between the ion size and pore size for an electric double-layer capacitor[J]. Journal of the American Chemical Society,2008,130(9):2730-2731. doi: 10.1021/ja7106178 [12] Jiang L L, Sheng L Z, Fan Z J. Biomass-derived carbon materials with structural diversities and their applications in energy storage[J]. Science China-Materials,2018,61(2):133-158. doi: 10.1007/s40843-017-9169-4 [13] Li Z J, Peng H N, Liu R R, et al. Quantitative assessment of basal-, edge- and defect-surfaces of carbonaceous materials and their influence on electric double-layer capacitance[J]. Journal of Power Sources,2020,457:228022. doi: 10.1016/j.jpowsour.2020.228022 [14] Lyu L, Seong K d, Ko D, et al. Recent development of biomass-derived carbons and composites as electrode materials for supercapacitors[J]. Materials Chemistry Frontiers,2019,3(12):2543-2570. doi: 10.1039/C9QM00348G [15] Wang T, Zang X, Wang X, et al. Recent advances in fluorine-doped/fluorinated carbon-based materials for supercapacitors[J]. Energy Storage Materials,2020,30:367-384. doi: 10.1016/j.ensm.2020.04.044 [16] Li D H, Chang G J, Zong L, et al. From double-helix structured seaweed to S-doped carbon aerogel with ultra-high surface area for energy storage[J]. Energy Storage Materials,2019,17:22-30. doi: 10.1016/j.ensm.2018.08.004 [17] Li J M, Jiang Q M, Wei L S, et al. Simple and scalable synthesis of hierarchical porous carbon derived from cornstalk without pith for high capacitance and energy density[J]. Journal of Materials Chemistry A,2020,8(3):1469-1479. doi: 10.1039/C9TA07864A [18] Li J X, Han K H, Wang D, et al. Fabrication of high performance structural N-doped hierarchical porous carbon for supercapacitor[J]. Carbon,2020,164:42-50. doi: 10.1016/j.carbon.2020.03.044 [19] Zhu Y Y, Chen M M, Zhang Y, et al. A biomass-derived nitrogen-doped porous carbon for high-energy supercapacitor[J]. Carbon,2018,140:404-412. doi: 10.1016/j.carbon.2018.09.009 [20] Helmholtz H v. Ueber einige gesetze der vertheilung elektrischer strome in korperlichen leitern mit anwendung auf die thierisch-elektrischen versuche[J]. Annalen der Physik,1853,165:211-233. doi: 10.1002/andp.18531650603 [21] Chapman D L. LI. A contribution to the theory of electrocapillarity[J]. The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science,1913,25(148):475-481. doi: 10.1080/14786440408634187 [22] Gouy M. Sur la constitution de la charge électrique à la surface d'un électrolyte[J]. Journal de Physique Théorique et Appliquée,1910,9(1):457-468. [23] Stern O. The theory of the electrolytic double-layer[J]. Z Elektrochem,1924,30:508-516. [24] Fedorov M V, Kornyshev A A. Ionic liquids at electrified interfaces[J]. Chemical Reviews,2014,114(5):2978-3036. doi: 10.1021/cr400374x [25] Bazant M Z, Storey B D, Kornyshev A A. Double layer in ionic liquids: Overscreening versus crowding[J]. Physical Review Letters,2011,106(4):046102. doi: 10.1103/PhysRevLett.106.046102 [26] Danish M, Ahmad T. A review on utilization of wood biomass as a sustainable precursor for activated carbon production and application[J]. Renewable and Sustainable Energy Reviews,2018,87:1-21. doi: 10.1016/j.rser.2018.02.003 [27] He Y F, Zhuang X D, Lei C J, et al. Porous carbon nanosheets: Synthetic strategies and electrochemical energy related applications[J]. Nano Today,2019,24:103-119. doi: 10.1016/j.nantod.2018.12.004 [28] Shanmuga Priya M, Divya P, et al. A review status on characterization and electrochemical behaviour of biomass derived carbon materials for energy storage supercapacitors[J]. Sustainable Chemistry and Pharmacy,2020,16:100243. doi: 10.1016/j.scp.2020.100243 [29] Tang G X, Zhand L Q, Zhu X F, et al. The preparation of activated carbon from walnut shell bio-oil distillation residues[J]. New Carbon Materials,2019,34(5):434-440. [30] Wang J S, Zhang X, Li Z, et al. Recent progress of biomass-derived carbon materials for supercapacitors[J]. Journal of Power Sources,2020,451:227794. doi: 10.1016/j.jpowsour.2020.227794 [31] Wei F, Zhang H F, He X J, et al. Synthesis of porous carbons from coal tar pitch for high-performance supercapacitors[J]. New Carbon Materials,2019,34(2):132-139. [32] Jia C, Li Y J, Yang Z, et al. Rich mesostructures derived from natural woods for solar steam generation[J]. Joule,2017,1(3):588-599. doi: 10.1016/j.joule.2017.09.011 [33] Zhu M, Li Y, Chen G, et al. Tree-inspired design for high-efficiency water extraction[J]. Advanced Materials,2017,29(44):1704107. doi: 10.1002/adma.201704107 [34] Xiao C Y, Zhang W L, Lin H B, et al. Modification of a rice husk-based activated carbon by thermal treatment and its effect on its electrochemical performance as a supercapacitor electrode[J]. New Carbon Materials,2019,34(4):341-348. doi: 10.1016/S1872-5805(19)30021-6 [35] Yang K, Peng J, Srinivasakannan C, et al. Preparation of high surface area activated carbon from coconut shells using microwave heating[J]. Bioresource Technology,2010,101(15):6163-6169. doi: 10.1016/j.biortech.2010.03.001 [36] Wang D H, Wang Y Z, Chen Y, et al. Coal tar pitch derived N-doped porous carbon nanosheets by the in-situ formed g-C3N4 as a template for supercapacitor electrodes[J]. Electrochimica Acta,2018,283:132-140. doi: 10.1016/j.electacta.2018.06.151 [37] Deng Y, Wei J, Sun Z, et al. Large-pore ordered mesoporous materials templated from non-Pluronic amphiphilic block copolymers[J]. Chemical Society Reviews,2013,42(9):4054-4070. doi: 10.1039/C2CS35426H [38] Lin Z, Liu S, Mao W, et al. Tunable self-assembly of diblock copolymers into colloidal particles with triply periodic minimal surfaces[J]. Angewandte Chemie-International Edition,2017,56(25):7135-7140. doi: 10.1002/anie.201702591 [39] Im U S, Kim J, Lee S H, et al. Preparation of activated carbon from needle coke via two-stage steam activation process[J]. Materials Letters,2019,237:22-25. doi: 10.1016/j.matlet.2018.09.171 [40] Qin L Y, Hou Z W, Lu S, et al. Porous carbon derived from pine nut shell prepared by steam activation for supercapacitor electrode material[J]. International Journal of Electrochemical Science,2019,14(9):8907-8918. [41] Lei E, Li W, Ma C H, et al. CO2-activated porous self-templated N-doped carbon aerogel derived from banana for high-performance supercapacitors[J]. Applied Surface Science,2018,457:477-486. doi: 10.1016/j.apsusc.2018.07.001 [42] Ma M J, Ying H J, Cao F F, et al. Adsorption of congo red on mesoporous activated carbon prepared by CO2 physical activation[J]. Chinese Journal of Chemical Engineering,2020,28(4):1069-1076. doi: 10.1016/j.cjche.2020.01.016 [43] Chen W M, Luo M, Yang K, et al. Microwave-assisted KOH activation from lignin into hierarchically porous carbon with super high specific surface area by utilizing the dual roles of inorganic salts: Microwave absorber and porogen[J]. Microporous and Mesoporous Materials,2020,300:110178. doi: 10.1016/j.micromeso.2020.110178 [44] Zhu Z H, Liu Y J, Ju Z J, et al. Synthesis of diverse green carbon nanomaterials through fully utilizing biomass carbon source assisted by KOH[J]. ACS Applied Materials & Interfaces,2019,11(27):24205-24211. [45] Hu L F, Zhu Q Z, Wu Q, et al. Natural biomass-derived hierarchical porous carbon synthesized by an in situ hard template coupled with NaOH activation for ultrahigh rate supercapacitors[J]. ACS Sustainable Chemistry & Engineering,2018,6(11):13949-13959. [46] Zhang Y, Song X L, Xu Y, et al. Utilization of wheat bran for producing activated carbon with high specific surface area via NaOH activation using industrial furnace[J]. Journal of Cleaner Production,2019,210:366-375. doi: 10.1016/j.jclepro.2018.11.041 [47] Chen H J, Wei H M, Fu N, et al. Nitrogen-doped porous carbon using ZnCl2 as activating agent for high-performance supercapacitor electrode materials[J]. Journal of Materials Science,2018,53(4):2669-2684. doi: 10.1007/s10853-017-1453-3 [48] Sun Q J, Jiang T Y, Zhao G Z, et al. Porous carbon material based on biomass prepared by MgO template method and ZnCl2 activation method as electrode for high performance supercapacitor[J]. International Journal of Electrochemical Science,2019,14(1):1-14. [49] Du W M, Zhang Z R, Du L G, et al. Designing synthesis of porous biomass carbon from wheat straw and the functionalizing application in flexible, all-solid-state supercapacitors[J]. Journal of Alloys and Compounds,2019,797:1031-1040. doi: 10.1016/j.jallcom.2019.05.207 [50] Endo M, Maeda T, Takeda T, et al. Capacitance and pore-size distribution in aqueous and nonaqueous electrolytes using various activated carbon electrodes[J]. Journal of the Electrochemical Society,2001,148(8):A910-A914. doi: 10.1149/1.1382589 [51] Long C L, Chen X, Jiang L L, et al. Porous layer-stacking carbon derived from in-built template in biomass for high volumetric performance supercapacitors[J]. Nano Energy,2015,12:141-151. doi: 10.1016/j.nanoen.2014.12.014 [52] J Gamby, Taberna P L, Simon P, et al. Studies and characterisations of various activated carbons used for carbon/carbon supercapacitors[J]. Journal of Power Sources,2001,101:109-116. doi: 10.1016/S0378-7753(01)00707-8 [53] Linoam E, Gregory S, Abraham S, et al. Ion sieving effects in the electrical double layer of porous carbon electrodes: Estimating effective ion size in electrolytic solutions[J]. The Journal of Physical Chemistry B,2001,105:6880-6887. doi: 10.1021/jp010086y [54] Gregory S, Abraham S, Linoam E, et al. Carbon electrodes for double-layer capacitors I. Relations between ion and pore dimensions[J]. Journal of the Electrochemical Society,2000,147(7):2486-2493. doi: 10.1149/1.1393557 [55] Raymundo-Piñero E, Kierzek K, Machnikowski J, et al. Relationship between the nanoporous texture of activated carbons and their capacitance properties in different electrolytes[J]. Carbon,2006,44(12):2498-2507. doi: 10.1016/j.carbon.2006.05.022 [56] Li Z, Gadipelli S, Li H, et al. Tuning the interlayer spacing of graphene laminate films for efficient pore utilization towards compact capacitive energy storage[J]. Nature Energy,2020,5(2):160-168. doi: 10.1038/s41560-020-0560-6 [57] Chmiola J, Largeot C, Taberna P L, et al. Desolvation of ions in subnanometer pores and its effect on capacitance and double-layer theory[J]. Angewandte Chemie-International Edition,2008,120(18):3440-3443. [58] Prehal C, Koczwara C, Jäckel N, et al. Quantification of ion confinement and desolvation in nanoporous carbon supercapacitors with modelling and in situ X-ray scattering[J]. Nature Energy,2017,2(3):16215. doi: 10.1038/nenergy.2016.215 [59] Galhena D T, Bayer B C, Hofmann S, et al. Understanding capacitance variation in sub-nanometer pores by in situ tuning of interlayer constrictions[J]. ACS Nano,2016,10(1):747-754. doi: 10.1021/acsnano.5b05819 [60] Tsai W Y, Taberna P L, Simon P. Electrochemical quartz crystal microbalance (EQCM) study of ion dynamics in nanoporous carbons[J]. Journal of the American Chemical Society,2014,136(24):8722-8728. doi: 10.1021/ja503449w [61] Jäckel N, Simon P, Gogotsi Y, et al. Increase in capacitance by subnanometer pores in carbon[J]. ACS Energy Letters,2016,1(6):1262-1265. doi: 10.1021/acsenergylett.6b00516 [62] Kalluri R K, Konatham D, Striolo A. Aqueous NaCl solutions within charged carbon-slit pores: Partition coefficients and density distributions from molecular dynamics simulations[J]. The Journal of Physical Chemistry C,2011,115(28):13786-13795. doi: 10.1021/jp203086x [63] Liu H M, Jameson C J, Murad S. Molecular dynamics simulation of ion selectivity process in nanopores[J]. Molecular Simulation,2008,34(2):169-175. doi: 10.1080/08927020801966087 [64] Shao Q, Huang L L, Zhou J, et al. Molecular simulation study of temperature effect on ionic hydration in carbon nanotubes[J]. Physical Chemistry Chemical Physics,2008,10(14):1896-1906. doi: 10.1039/b719033f [65] Kondrat S, Kornyshev A. Corrigendum: Superionic state in double-layer capacitors with nanoporous electrodes[J]. Journal of Physics: Condensed Matter,2013,25(11):119501. doi: 10.1088/0953-8984/25/11/119501 [66] Futamura R, Iiyama T, Takasaki Y, et al. Partial breaking of the Coulombic ordering of ionic liquids confined in carbon nanopores[J]. Nature Materials,2017,16(12):1225-1232. doi: 10.1038/nmat4974 [67] Chmiola J, Yushin G, Dash R, et al. Effect of pore size and surface area of carbide derived carbons on specific capacitance[J]. Journal of Power Sources,2006,158(1):765-772. doi: 10.1016/j.jpowsour.2005.09.008 [68] Kondrat S, Pérez C R, Presser V, et al. Effect of pore size and its dispersity on the energy storage in nanoporous supercapacitors[J]. Energy & Environmental Science,2012,5(4):6474-6479. [69] Wang D W, Li F, Liu M, et al. Mesopore-aspect-ratio dependence of ion transport in rodtype ordered mesoporous carbon[J]. The Journal of Physical Chemistry C,2008,112(26):9950-9955. doi: 10.1021/jp800173z [70] Wang D W, Li F, Fang H T, et al. Effect of pore packing defects in 2-D ordered mesoporous carbons on ionic transport[J]. The Journal of Physical Chemistry B,2006,110(17):8570-8575. doi: 10.1021/jp0572683 [71] Wang Q, Yan J, Fan Z J. Carbon materials for high volumetric performance supercapacitors: Design, progress, challenges and opportunities[J]. Energy & Environmental Science,2016,9(3):729-762. [72] Black J M, Andreas H A. Pore shape affects spontaneous charge redistribution in small pores[J]. The Journal of Physical Chemistry C,2010,114(27):12030-12038. doi: 10.1021/jp103766q [73] Noked M, Avraham E, Soffer A, et al. The rate-determining step of electroadsorption processes into nanoporous carbon electrodes related to water desalination[J]. Journal of Physical Chemistry C,2009,113(51):21319-21327. doi: 10.1021/jp905987j [74] Kang X, Wang C, Yin J. Hierarchically porous carbons derived from cotton stalks for high-performance supercapacitors[J]. Chemelectrochem,2017,4(10):2599-2607. doi: 10.1002/celc.201700501 [75] Shang Z, An X Y, Zhang H, et al. Houttuynia-derived nitrogen-doped hierarchically porous carbon for high-performance supercapacitor[J]. Carbon,2020,161:62-70. doi: 10.1016/j.carbon.2020.01.020 [76] Shao R, Niu J, Liang J J, et al. Mesopore- and macropore-dominant nitrogen-doped hierarchically porous carbons for high-energy and ultrafast supercapacitors in non-aqueous electrolytes[J]. ACS Applied Materials & Interfaces,2017,9(49):42797-42805. [77] Wang D W, Li F, Liu M, et al. 3D aperiodic hierarchical porous graphitic carbon material for high-rate electrochemical capacitive energy storage[J]. Angewandte Chemie-International Edition,2008,47(2):379-382. [78] Xia L C, Huang H, Fan Z, et al. Hierarchical macro-/meso-/microporous oxygen-doped carbon derived from sodium alginate: A cost-effective biomass material for binder-free supercapacitors[J]. Materials & Design,2019,182:108048. [79] Zhang Q, Han K, Li S, et al. Synthesis of garlic skin-derived 3D hierarchical porous carbon for high-performance supercapacitors[J]. Nanoscale,2018,10(5):2427-2437. doi: 10.1039/C7NR07158B [80] Zhi L, Li T, Yu H, et al. Hierarchical graphene network sandwiched by a thin carbon layer for capacitive energy storage[J]. Carbon,2017,113:100-107. doi: 10.1016/j.carbon.2016.11.036 [81] Huang J, Sumpter B G, Meunier V, et al. Curvature effects in carbon nanomaterials: Exohedral versus endohedral supercapacitors[J]. Journal of Materials Research,2011,25(8):1525-1531. [82] Huang J, Sumpter B G, Meunier V. Theoretical model for nanoporous carbon supercapacitors[J]. Angewandte Chemie-International Edtion,2008,47(3):520-524. doi: 10.1002/anie.200703864 [83] Huang J, Sumpter B G, Meunier V. A universal model for nanoporous carbon supercapacitors applicable to diverse pore regimes, carbon materials and electrolytes[J]. Chemistry-A European Journal,2008,14(22):6614-6626. doi: 10.1002/chem.200800639 [84] Feng G, Qiao R, Huang J, et al. Ion distribution in electrified micropores and its role in the anomalous enhancement of capacitance[J]. ACS Nano,2010,4(4):2382-2390. doi: 10.1021/nn100126w [85] Thommes M, Cychosz K A. Physical adsorption characterization of nanoporous materials: Progress and challenges[J]. Adsorption-Journal of the International Adsorption Society,2014,20:233-250. doi: 10.1007/s10450-014-9606-z [86] Sing K S W, Williams R T. The use of molecular probes for the characterization of nanoporous adsorbents[J]. Particle & Particle Systems Characterization,2004,21(2):71-79. [87] Silvestre-Albero J, Silvestre-Albero A, Rodríguez-Reinoso F, et al. Physical characterization of activated carbons with narrow microporosity by nitrogen (77.4 K), carbon dioxide (273 K) and argon (87.3 K) adsorption in combination with immersion calorimetry[J]. Carbon,2012,50(9):3128-3133. doi: 10.1016/j.carbon.2011.09.005 [88] García-Martínez J, Cazorla-Amorós D, Linares-Solano A. Further evidences of the usefulness of CO2 microporous solids.[J]. Studies in Surface Science and Catalysis,2000,128:485-494. doi: 10.1016/S0167-2991(00)80054-3 [89] Furmaniak S, Terzyk A P, Gauden P A, et al. The influence of carbon surface oxygen groups on Dubinin-Astakhov equation parameters calculated from CO2 adsorption isotherm[J]. Journal of Physics-Condensed Matter,2010,22(8):085003. doi: 10.1088/0953-8984/22/8/085003 [90] Zhang Z, Yang Z. Theoretical and practical discussion of measurement accuracy for physisorption with micro- and mesoporous materials[J]. Chinese Journal of Catalysis,2013,34(10):1797-1810. doi: 10.1016/S1872-2067(12)60601-9 [91] Rouquerol J, Llewellyn P, Rouquerol F. Is the BET equation applicable to microporous adsorbents?[J]. Studies in Surface Science and Catalysis,2007,160:49-56. doi: 10.1016/S0167-2991(07)80008-5 [92] Barrett E P, Joyner L G, Halenda P P. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms[J]. Journal of the American Chemical Society,1951,73(1):373-380. doi: 10.1021/ja01145a126 [93] Dubinin M M, Polyakov N S, Kataeva L I. Basic properties of equations for physical vapor adsorption in micropores of carbon adsorbents assuming a normal micropore distribution[J]. Carbon,1991,29:481-488. doi: 10.1016/0008-6223(91)90111-U [94] Dubinin M M. The potential theory of adsorption of gases and vapors for adsorbents with energetically nonuniform surfaces[J]. Chemical Reviews,1960,60:235-241. doi: 10.1021/cr60204a006 [95] Horvath G, Kawazoe, K. Method for the calculation of effective pore size distribution in molecular sieve carbon[J]. Journal of Chemical Engineering of Japan,1983,16:470-475. doi: 10.1252/jcej.16.470 [96] Saito A, Foley H C. Curvature and parametric sensitivity in models for adsorption in micropores[J]. Aiche Journal,1991,37(3):429-436. doi: 10.1002/aic.690370312 [97] Thommes M, Köhn R, Fröba M. Sorption and pore condensation behavior of nitrogen, argon, and krypton in mesoporous MCM-48 silica materials[J]. The Journal of Physical Chemistry B,2000,104(33):7932-7943. doi: 10.1021/jp994133m [98] Ravikovitch P I, Neimark A V. Density functional theory model of adsorption deformation[J]. Langmuir,2006,22(26):10864-10868. doi: 10.1021/la061092u [99] Tarazona P, Marconi U M B, Evans R. Phase equilibria of fluid interfaces and confined fluids[J]. Molecular Physics,1987,60(3):573-595. doi: 10.1080/00268978700100381 [100] Gor G Y, Thommes M, Cychosz K A, et al. Quenched solid density functional theory method for characterization of mesoporous carbons by nitrogen adsorption[J]. Carbon,2012,50(4):1583-1590. doi: 10.1016/j.carbon.2011.11.037 [101] Ravikovitch P I, Neimark A V. Density functional theory model of adsorption on amorphous and microporous silica materials[J]. Langmuir,2006,22(26):11171-11179. doi: 10.1021/la0616146 [102] Jagiello J, Olivier J P. 2D-NLDFT adsorption models for carbon slit-shaped pores with surface energetical heterogeneity and geometrical corrugation[J]. Carbon,2013,55:70-80. doi: 10.1016/j.carbon.2012.12.011 [103] Kwiatkowski M, Fierro V, Celzard A. Confrontation of various adsorption models for assessing the porous structure of activated carbons[J]. Adsorption-Journal of the International Adsorption Society,2019,25(8):1673-1682. doi: 10.1007/s10450-019-00129-y [104] Lai W D, Yang S, Jiang Y H, et al. Artefact peaks of pore size distributions caused by unclosed sorption isotherm and tensile strength effect[J]. Adsorption-Journal of the International Adsorption Society,2020,26(4):633-644. doi: 10.1007/s10450-020-00228-1 [105] Niu J, Shao R, Liang J J, et al. Biomass-derived mesopore-dominant porous carbons with large specific surface area and high defect density as high performance electrode materials for Li-ion batteries and supercapacitors[J]. Nano Energy,2017,36:322-330. doi: 10.1016/j.nanoen.2017.04.042 [106] Banks C E, Davies T J, Wildgoose G G, et al. Electrocatalysis at graphite and carbon nanotube modified electrodes: Edge-plane sites and tube ends are the reactive sites[J]. Chemical Communications,2005(7):829-841. doi: 10.1039/b413177k [107] Kim T, Lim S, Kwon K, et al. Electrochemical capacitances of well-defined carbon surfaces[J]. Langmuir,2006,22(22):9086-9088. doi: 10.1021/la061380q [108] Qu D. Studies of the activated carbons used in double-layer supercapacitors[J]. Journal of Power Sources,2002,109(2):403-411. doi: 10.1016/S0378-7753(02)00108-8 [109] Yuan W, Zhou Y, Li Y, et al. The edge- and basal-plane-specific electrochemistry of a single-layer graphene sheet[J]. Scientific Reports,2013,3:2248. doi: 10.1038/srep02248 [110] Lu Q J, Zhou S Q, Li B, et al. Mesopore-rich carbon flakes derived from lotus leaves and it's ultrahigh performance for supercapacitors[J]. Electrochimica Acta,2020,333:135481. doi: 10.1016/j.electacta.2019.135481 [111] Yan X C, Jia Y, Zhuang L Z, et al. Defective carbons derived from Macadamia nut shell biomass for efficient oxygen reduction and supercapacitors[J]. Chemelectrochem,2018,5(14):1874-1879. doi: 10.1002/celc.201800068 [112] Nath N C D, Shah S S, Qasem M A A, et al. Defective carbon nanosheets derived from syzygium cumini leaves for electrochemical energy-storage[J]. Chemistryselect,2019,4(31):9079-9083. doi: 10.1002/slct.201900891 [113] Welham N J, Berbenni V, Chapman P G. Effect of extended ball milling on graphite[J]. Journal of Alloys and Compounds,2003,349(1-2):255-263. doi: 10.1016/S0925-8388(02)00880-0 [114] Yue X, Wang H, Wang S, et al. In-plane defects produced by ball-milling of expanded graphite[J]. Journal of Alloys and Compounds,2010,505(1):286-290. doi: 10.1016/j.jallcom.2010.06.048 [115] Dong Y, Lin X, Wang D, et al. Modulating the defects of graphene blocks by ball-milling for ultrahigh gravimetric and volumetric performance and fast sodium storage[J]. Energy Storage Materials,2020,30:287-295. doi: 10.1016/j.ensm.2020.05.016 [116] Abioye A M, Ani F N. Recent development in the production of activated carbon electrodes from agricultural waste biomass for supercapacitors: A review[J]. Renewable & Sustainable Energy Reviews,2015,52:1282-1293. [117] Sun K L, Yu S S, Hu Z L, et al. Oxygen-containing hierarchically porous carbon materials derived from wild jujube pit for high-performance supercapacitor[J]. Electrochimica Acta,2017,231:417-428. doi: 10.1016/j.electacta.2017.02.078 [118] Zhao N, Deng L B, Luo D W, et al. Oxygen doped hierarchically porous carbon for electrochemical supercapacitor[J]. International Journal of Electrochemical Science,2018,13(11):10626-10634. [119] Liu M C, Kong L B, Zhang P, et al. Porous wood carbon monolith for high-performance supercapacitors[J]. Electrochimica Acta,2012,60:443-448. doi: 10.1016/j.electacta.2011.11.100 [120] Wang D W, Li F, Liu M, et al. Improved capacitance of SBA-15 templated mesoporous carbons after modification with nitric acid oxidation[J]. New Carbon Materials,2007,22(4):307-314. doi: 10.1016/S1872-5805(08)60002-5 [121] Li X R, Jiang Y H, Wang P Z, et al. Effect of the oxygen functional groups of activated carbon on its electrochemical performance for supercapacitors[J]. New Carbon Materials,2020,35(3):232-243. doi: 10.1016/S1872-5805(20)60487-5 [122] Sahoo G, Polaki S R, Ghosh S, et al. Plasma-tuneable oxygen functionalization of vertical graphenes enhance electrochemical capacitor performance[J]. Energy Storage Materials,2018,14:297-305. doi: 10.1016/j.ensm.2018.05.011 [123] Jiang L L, Sheng L Z, Long C L, et al. Functional pillared graphene frameworks for ultrahigh volumetric performance supercapacitors[J]. Advanced Energy Materials,2015,5(15):1500771. doi: 10.1002/aenm.201500771 [124] Anjos D M, McDonough J K, Perre E, et al. Pseudocapacitance and performance stability of quinone-coated carbon onions[J]. Nano Energy,2013,2(5):702-712. doi: 10.1016/j.nanoen.2013.08.003 [125] Yuan S, Huang X, Wang H, et al. Structure evolution of oxygen removal from porous carbon for optimizing supercapacitor performance[J]. Journal of Energy Chemistry,2020,51:396-404. doi: 10.1016/j.jechem.2020.04.004 [126] Shen W, Fan W. Nitrogen-containing porous carbons: Synthesis and application[J]. Journal of Materials Chemistry A,2013,1(4):999-1013. doi: 10.1039/C2TA00028H [127] Wei W, Chen Z, Zhang Y, et al. Full-faradaic-active nitrogen species doping enables high-energy-density carbon-based supercapacitor[J]. Journal of Energy Chemistry,2020,48:277-284. doi: 10.1016/j.jechem.2020.02.011 [128] Lin L, Xie H M, Lei Y, et al. Nitrogen source-mediated cocoon silk-derived N, O-doped porous carbons for high performance symmetric supercapacitor[J]. Journal of Materials Science-Materials in Electronics,2020,31(13):10825-10835. doi: 10.1007/s10854-020-03634-x [129] Guo J, Wu D L, Wang T, et al. P-doped hierarchical porous carbon aerogels derived from phenolic resins for high performance supercapacitor[J]. Applied Surface Science,2019,475:56-66. doi: 10.1016/j.apsusc.2018.12.095 [130] Jin H, Feng X, Li J, et al. Heteroatom-doped porous carbon materials with unprecedented high volumetric capacitive performance[J]. Angewandte Chemie-Internation Edition,2019,58(8):2397-2401. doi: 10.1002/anie.201813686 [131] Zhou J, Lian J, Hou L, et al. Ultrahigh volumetric capacitance and cyclic stability of fluorine and nitrogen co-doped carbon microspheres[J]. Nature Communications,2015,6:8503. doi: 10.1038/ncomms9503 [132] Zhou J, Xu L, Li L, et al. Polytetrafluoroethylene-assisted N/F co-doped hierarchically porous carbon as a high performance electrode for supercapacitors[J]. Journal of Colloid and Interface Science,2019,545:25-34. doi: 10.1016/j.jcis.2019.03.010 [133] Guo H L, Gao Q M. Boron and nitrogen co-doped porous carbon and its enhanced properties as supercapacitor[J]. Journal of Power Sources,2009,186(2):551-556. doi: 10.1016/j.jpowsour.2008.10.024 [134] Wang Y K, Zhang M K, Dai Y, et al. Nitrogen and phosphorus co-doped silkworm-cocoon-based self-activated porous carbon for high performance supercapacitors[J]. Journal of Power Sources,2019,438:227045. doi: 10.1016/j.jpowsour.2019.227045 [135] Huo S L, Zhao Y B, Zong M Z, et al. Boosting supercapacitor and capacitive deionization performance of hierarchically porous carbon by polar surface and structural engineering[J]. Journal of Materials Chemistry A,2020,8(5):2505-2517. doi: 10.1039/C9TA12170F [136] Ren G Y, Li Y N, Chen Q S, et al. Sepia-derived N, P co-doped porous carbon spheres as oxygen reduction reaction electrocatalyst and supercapacitor[J]. ACS Sustainable Chemistry & Engineering,2018,6(12):16032-16038. [137] Tang B, Zheng L P, Dai X C, et al. Nitrogen/oxygen co-doped porous carbons derived from a facilely-synthesized Schiff-base polymer for high-performance supercapacitor[J]. Journal of Energy Storage,2019,26:100961. doi: 10.1016/j.est.2019.100961 [138] Wang P Z, Luo W X, Guo N N, et al. Nitrogen and oxygen co-doped hierarchical porous carbon for high performance supercapacitor electrodes[J]. Chemical Physics Letters,2019,730:32-38. doi: 10.1016/j.cplett.2019.05.032 [139] Jia S, Wei J, Meng X T, et al. Facile and friendly preparation of N/S Co-doped graphene-like carbon nanosheets with hierarchical pore by molten salt for all-solid-state supercapacitor[J]. Electrochimica Acta,2020,331:135338. doi: 10.1016/j.electacta.2019.135338 [140] Ping Y J, Han J Z, Li J J, et al. N, S co-doped porous carbons from natural Juncus effuses for high performance supercapacitors[J]. Diamond and Related Materials,2019,100:107577. doi: 10.1016/j.diamond.2019.107577 [141] Na W, Jun J, Park J W, et al. Highly porous carbon nanofibers co-doped with fluorine and nitrogen for outstanding supercapacitor performance[J]. Journal of Materials Chemistry A,2017,5(33):17379-17387. doi: 10.1039/C7TA04406B [142] Ling Z, Wang Z Y, Zhang M D, et al. Sustainable synthesis and assembly of biomass-derived B/N co-doped carbon nanosheets with ultrahigh aspect ratio for high-performance supercapacitors[J]. Advanced Functional Materials,2016,26(1):111-119. doi: 10.1002/adfm.201504004 [143] Zhao Z C, Xie Y B. Electrochemical supercapacitor performance of boron and nitrogen co-doped porous carbon nanowires[J]. Journal of Power Sources,2018,400:264-276. doi: 10.1016/j.jpowsour.2018.08.032 [144] Wang C S, Liu T Z. Nori-based N, O, S, Cl co-doped carbon materials by chemical activation of ZnCl2 for supercapacitor[J]. Journal of Alloys and Compounds,2017,696:42-50. doi: 10.1016/j.jallcom.2016.11.206 [145] Choi J H, Kim Y, Kim B S. Multifunctional role of reduced graphene oxide binder for high performance supercapacitor with commercial-level mass loading[J]. Journal of Power Sources,2020,454:227917. doi: 10.1016/j.jpowsour.2020.227917 [146] Xu B, Wang H, Zhu Q, et al. Reduced graphene oxide as a multi-functional conductive binder for supercapacitor electrodes[J]. Energy Storage Materials,2018,12:128-136. doi: 10.1016/j.ensm.2017.12.006 [147] Yang S, Zhao F Y, Li X R, et al. Electrode structural changes and their effects on capacitance performance during preparation and charge-discharge processes[J]. Journal of Energy Storage,2019,24:100799. doi: 10.1016/j.est.2019.100799 [148] Weng Z, Su Y, Wang D W, et al. Graphene-cellulose paper flexible supercapacitors[J]. Advanced Energy Materials,2011,1(5):917-922. doi: 10.1002/aenm.201100312 [149] Liu C X, Chen J, Zhang C F, et al. Facile preparation of binder free electrode for electrochemical capacitors based on reduced graphene oxide composite film[J]. Journal of Electroanalytical Chemistry,2019,847:113133. doi: 10.1016/j.jelechem.2019.05.015 [150] Zhang X R, Yang S, Jiang Y H, et al. Multi-dimensional graded electrodes with enhanced capacitance and superior cyclic stability[J]. Journal of Power Sources,2021,481:228911. doi: 10.1016/j.jpowsour.2020.228911 -






 下载:
下载: