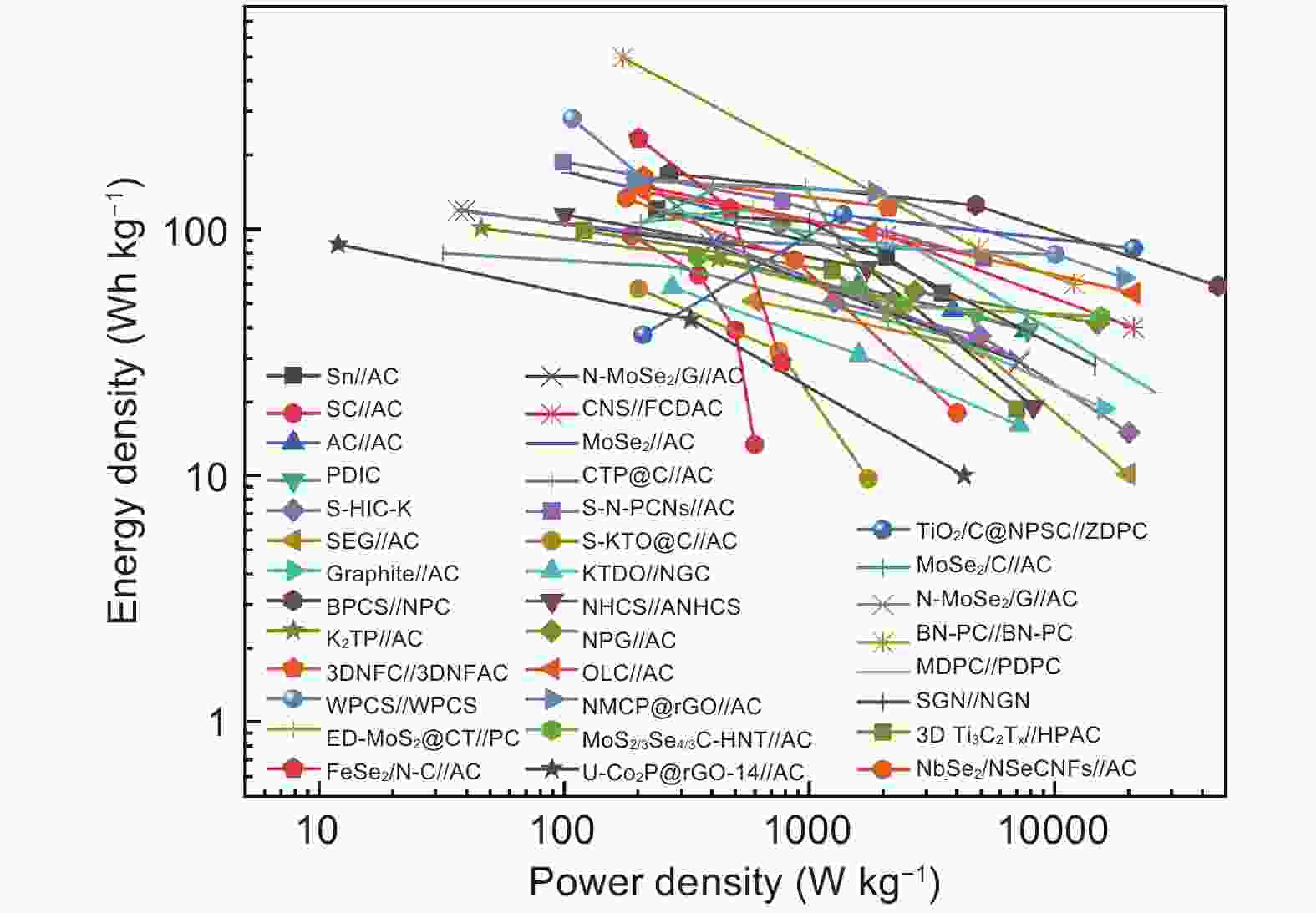
-
摘要: 钾离子电容器(PICs)是与锂离子电容器和钠离子电容器相比极具竞争力和发展前景的一种储能设备。PICs结合了电池式阳极和电容式阴极的优点,具有成本低、能量密度高、功率密度高、循环寿命长等优点。然而,在PICs中一直存在正负极比容量和动力学不匹配的问题。前期研究证明,合理选择电极材料并对其进行优化是解决这一问题的有效手段之一。本文对PICs阳极材料的研究进展进行了综述,主要包括插入型负极材料和转换型负极材料。主要讨论了炭材料(石墨、软炭、硬炭等)、KTO、MXenes、K2TP等插入型材料和金属硫化物/硒化物、金属磷化物、NASICON型磷酸盐等转化型材料。对半电池和PICs中不同电极的制备方法、结构特点和电化学性能进行总结,并进一步展望了PICs未来的发展机遇和挑战。Abstract: Potassium-ion capacitors (PICs) are promising energy storage devices, which are competitive with lithium-ion and sodium ion capacitors. PICs combine the advantages of a battery-type anode and a capacitive cathode, resulting in a low cost, high energy density, high power density and long cycle life. However, there is still a mismatch between the anode and cathode materials for achieving the optimum specific capacity and kinetics in PICs. Early studies have shown that the careful selection of electrode materials and their optimization is an effective way to solve this problem. We focus on the development of PIC anode materials including insertion-type and conversion-type materials. The insertion-type materials include carbon materials (graphite, soft carbons, hard carbons, etc.), K-based titanates, MXenes, and dipotassium terephthalate. The conversion-type materials include metal sulfides/selenides, metal phosphides and sodium super ionic conductor-type phosphates (NASICON). Their preparation, structural characteristics, electrochemical performance as anode materials in half-cell and PIC devices are summarized and discussed. The future prospects and challenges of PICs are also considered.
-
Key words:
- Potassium-ion capacitors /
- Carbon materials /
- Anode /
- Energy storage devices
-
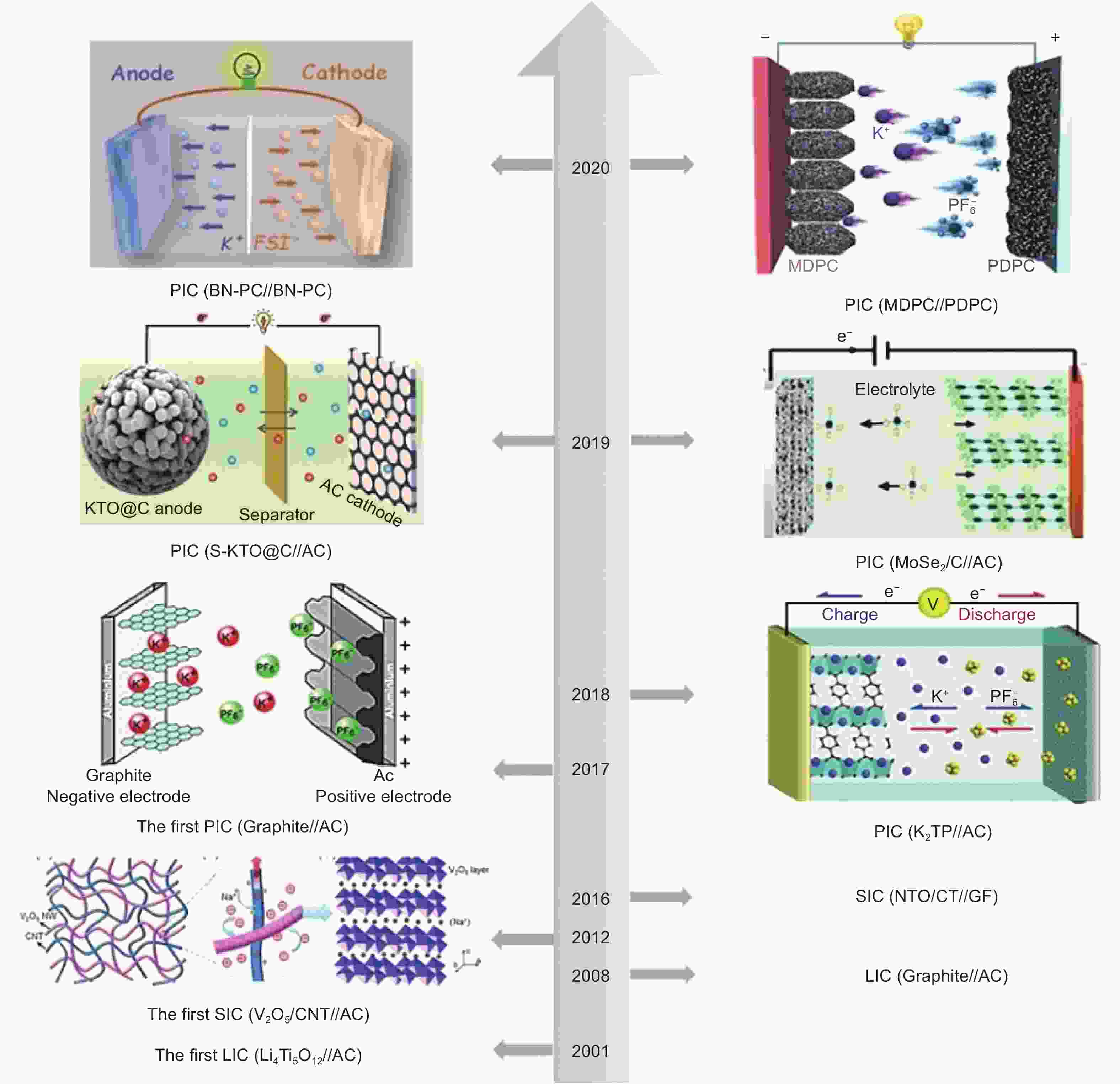
Figure 1. Development of metal-ion capacitors. The hybrid capacitors included in the picture: LIC: Li4Ti5O12//AC[7], graphite//AC[8], SIC: V2O5/CNT//AC[9] (Reprinted with permission by John Wiley and Sons), NTO/CT//GF[10], PIC: graphite//AC[11] (Reprinted with permission by Elsevier), K2TP//AC[12], MoSe2/C//AC[13], S-KTO@C//AC[14] , MDPC//PDPC[15], and BN-PC//BN-PC[16] (Reprinted with permission by John Wiley and Sons).
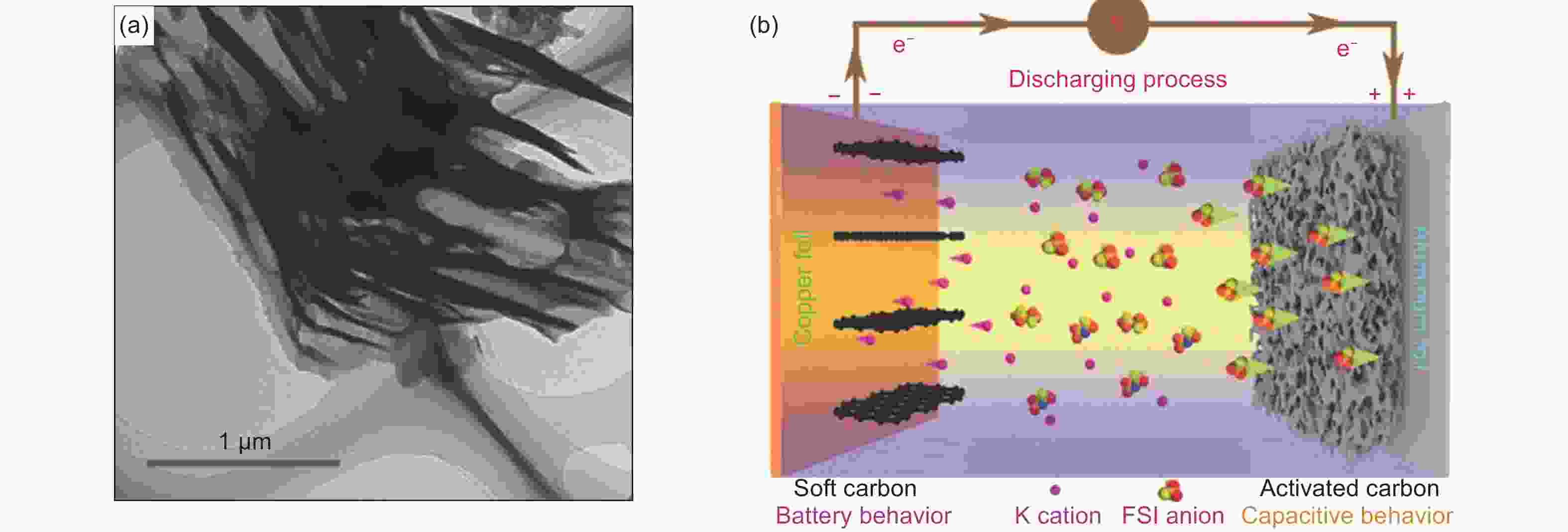
Figure 2. (a) TEM image of SC and (b) schematic illustration of discharging mechanism of SC//AC PICs[38] (Reprinted with permission by John Wiley and Sons).
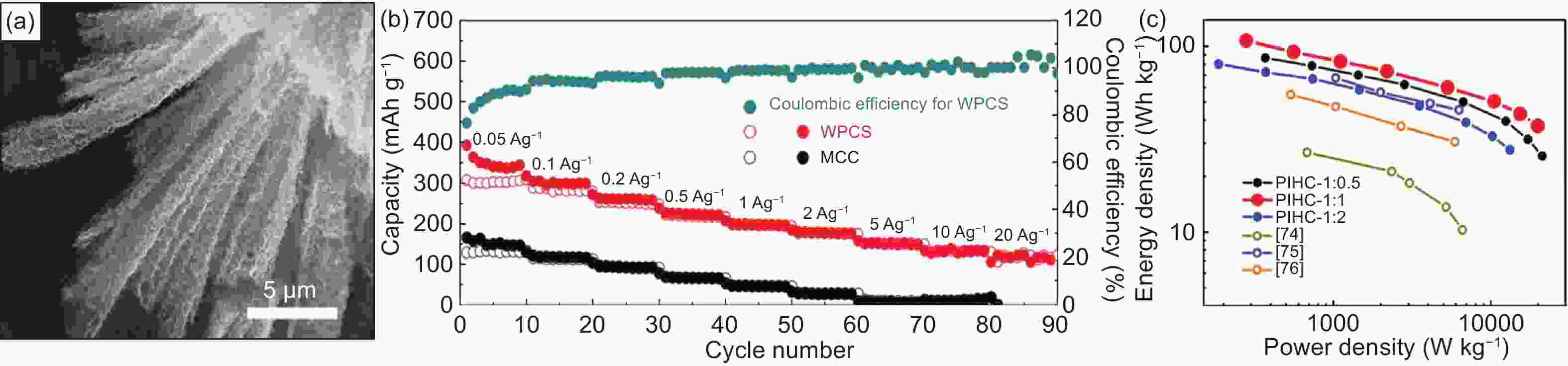
Figure 3. (a) SEM image of WPCS, (b) active mass normalized Ragone plots based on total mass of both the cathode and anode compared with previously reported WPCS-based PICs, (c) rate capability profiles for WPCS electrode[46] (Reprinted with permission by American Chemical Society).
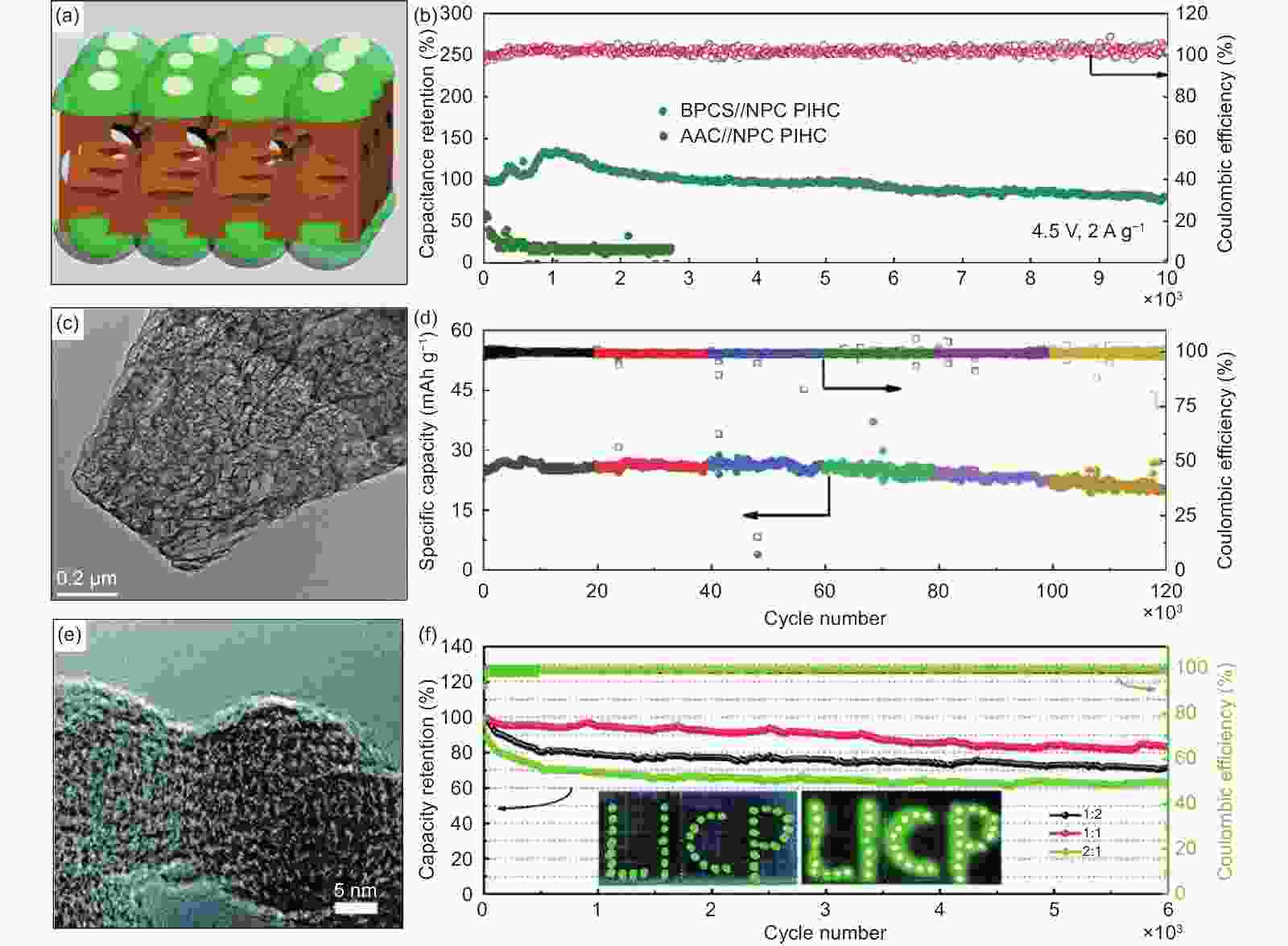
Figure 4. (a) The schematic image of the BPCS structure made by the “tree crown-stem” model, (b) cycling performance of the PIC tested at 2 A g−1[47] (Reprinted with permission by American Chemical Society), (c) TEM image of MDPC, d) long‐term cycling performance at 2 A g−1[15] (Reprinted with permission by John Wiley and Sons), (e) SEM image of OLC, (f) cycling performance of the assembled OLC//AC PICs with different mass ratios (1∶2, 1∶1 and 2∶1) at the current density of 2 A g−1[74] (Reprinted with permission by Royal Society of Chemistry).
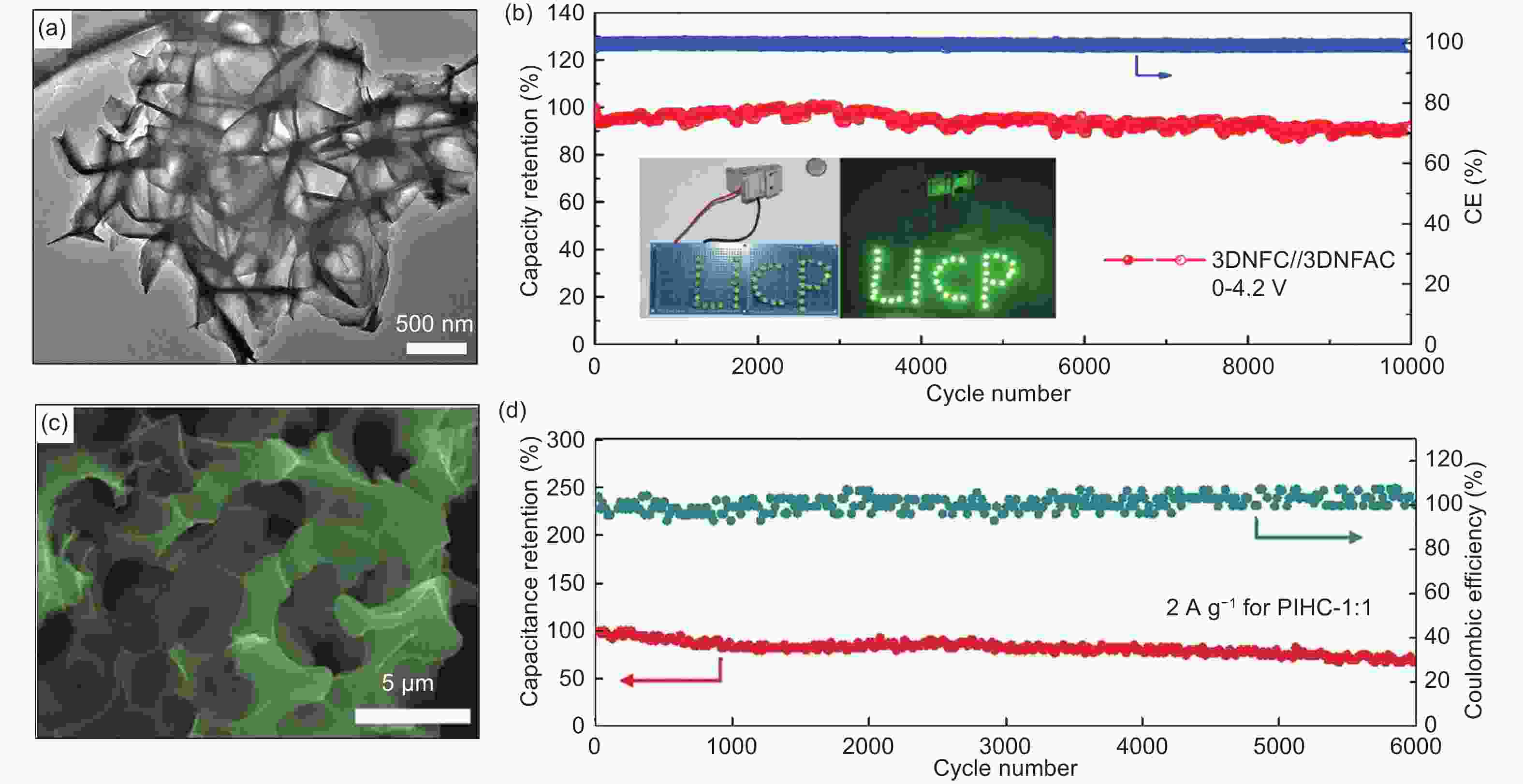
Figure 5. (a) SEM image of 3DNFC, (b) cycling stability of the optimal 3DNFC//3DNFAC device tested at 2 A g−1 for 10000 cycles within the voltage window of 0−4.2 V[80] (Reprinted with permission by Elsevier), (c) SEM images of BN-PC, and (d) cycling performance of PIC-1∶1 tested at a current density of 2 A g−1[16] (Reprinted with permission by John Wiley and Sons).
Figure 6. (a) Schematic illustration for preparation of S-N-PCNs, (b) rate capabilities of the S-N-PCNs, N-PCNs and PCNs electrodes, (c) long-term cycle performance of the AC//S-N-PCNs at a current density of 1 A g−1[83] (Reprinted with permission by John Wiley and Sons).
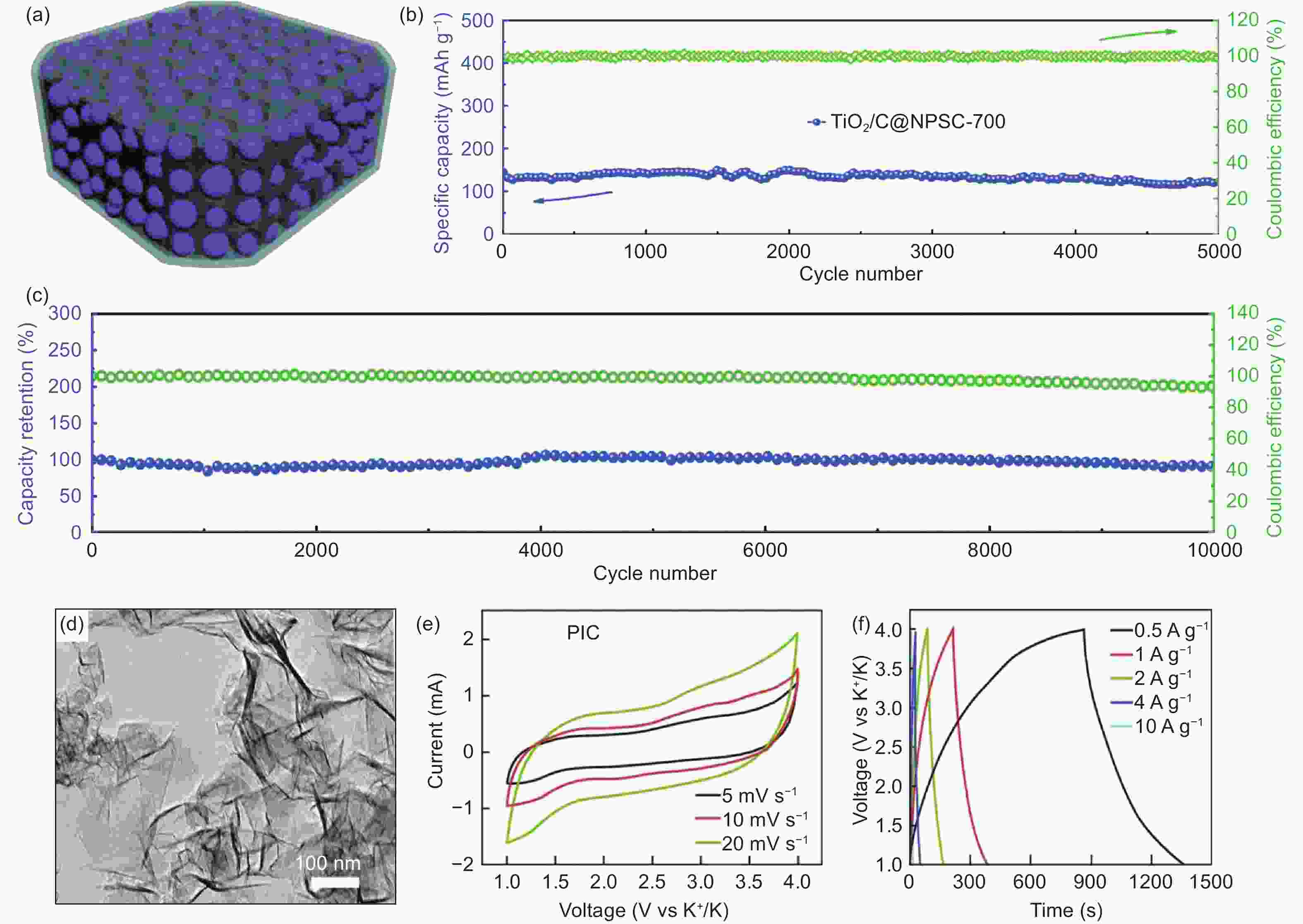
Figure 7. (a) Schematic illustration showing the structural characteristics of the TiO2/C@NPSC material, (b) the cycling stability and CE of TiO2/C@NPSC at 1 A g−1, (c) the long cycle stability and CE of the as-assembled TiO2/C@NPSC//ZDPC PIC[84] (Reprinted with permission by Royal Society of Chemistry). (d) TEM images of NPG nanosheets, (e) CV curves at scan rates of 5, 10, and 20 mV s−1 and (f) galvanostatic charge–discharge curves at current densities of 0.5−10 A g−1[88] (Reprinted with permission by Springer Nature).
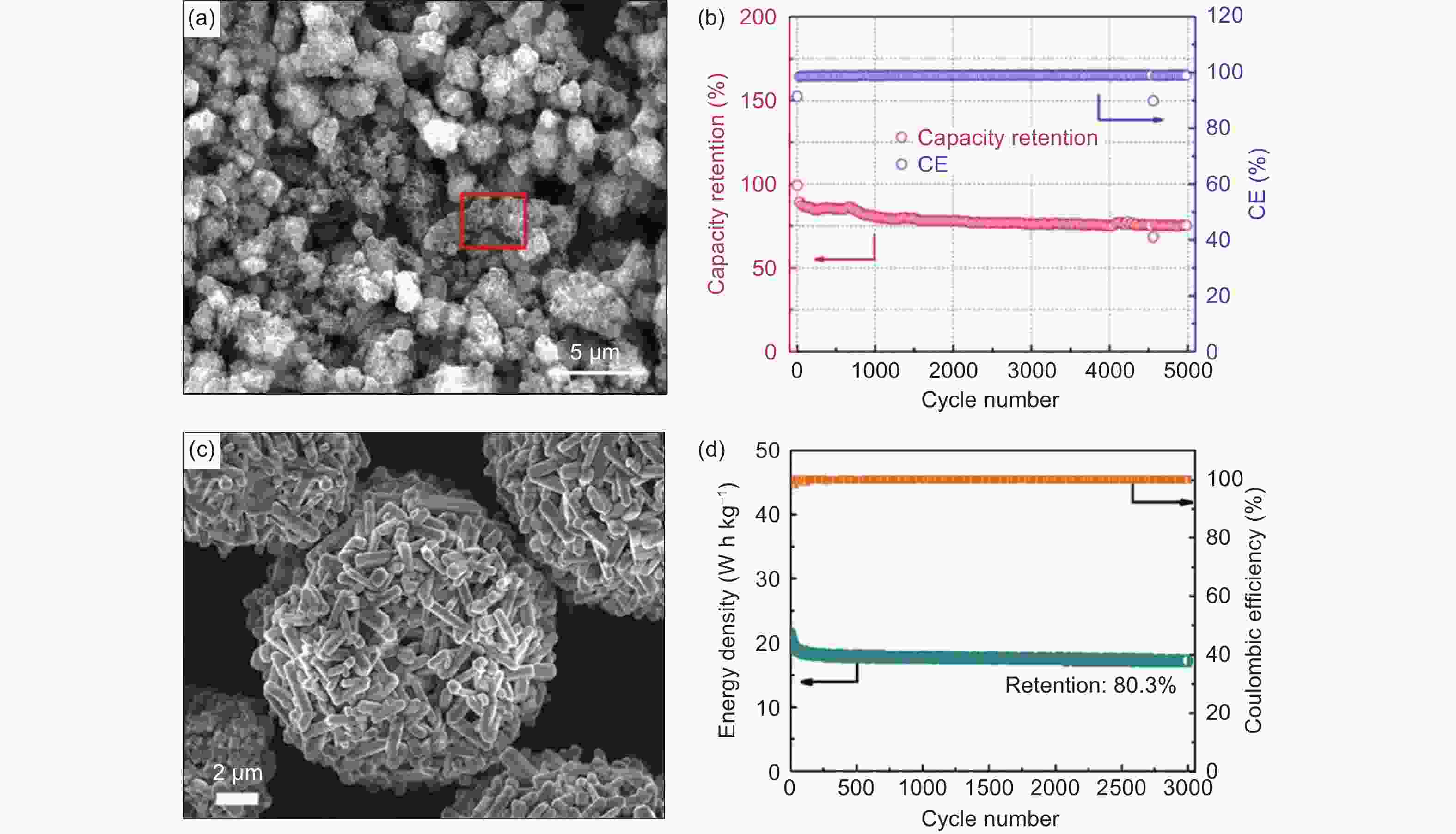
Figure 8. (a) SEM images of K2Ti6O13 (KTO), (b) long-term cycling stability at the current density of 1 A g−1[93] (Reprinted with permission by American Chemical Society), (c) SEM images of S-KTO@C and (d) long-term cycling stability of the PIC device at 5 A g−1 for 3000 cycles[14] (Reprinted with permission by John Wiley and Sons).
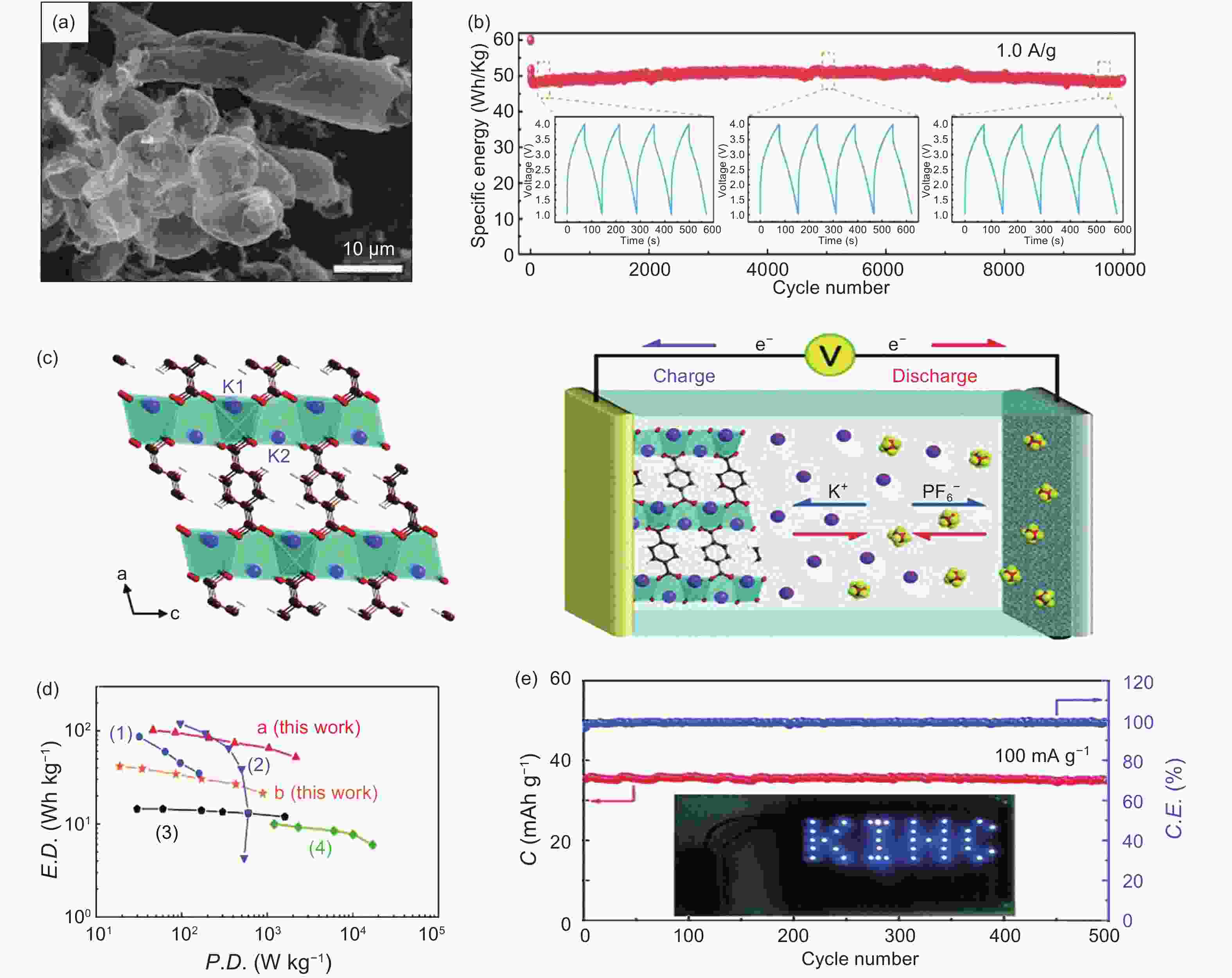
Figure 9. (a) Morphology and structure characters of the 3D-Ti3C2 , (b) electrochemical performance of PICs (3D-Ti3C2//HPAC)[97] (Reprinted with permission by John Wiley and Sons), (c) schematic illustration of the PIC with the configuration K2TP//DME-based electrolyte//AC, (d) ragone plots of the PIC of K2TP//AC, and other reported PICs and (e) cycling performance of the PIC at 100 mA g−1 for 500 cycles[12] (Reprinted with permission by Royal Society of Chemistry).
Figure 10. (a) SEM images of N-MoSe2/G, (b) long-term cyclic performance at 1 A g−1[13] (Reprinted with permission by John Wiley and Sons), (c) SEM images of MoSe2/C-700, (d) cycling performance of MoSe2/C-700, (e) cycling performance of the PIC at 1.0 A g−1[105] (Reprinted with permission by Royal Society of Chemistry), (f) SEM images of FeSe2 and (g) the cycle stability of the PIC at 500 mA g−1[106] (Reprinted with permission by John Wiley and Sons).
Figure 11. (a) SEM images of ED-MoS2@CT, (b) cycling performance for ED-MoS2@CT//PC PIC with an optimal mass ratio tested at 10 A g−1 between 0 and 4.0V[35] (Reprinted with permission by John Wiley and Sons, (c) low magnification TEM image of MoS2/3Se4/3/CHNTs, (d) cycling performance of MoS2/3Se4/3/C-HNTs and pure MoS2/C-HNTs at 0.2 A g−1, (e) cycling stability of PIC based on the MoS2/3Se4/3/C-HNT anode and AC cathode at 2.0 A g−1[115] (Reprinted with permission by Royal Society of Chemistry), (f) SEM image of NbSe2/NSeCNFs composite and (g) the cycling stability of the NbSe2/NSeCNFs//AC PICs at 2 A g−1[120] (Reprinted with permission by John Wiley and Sons).
Figure 12. (a) Low-magnification TEM images of the U-Co2P@rGO-14 nanocomposite, (b) long-cycle performance of the AC//U-Co2P@rGO-14 PIC device[124] (Reprinted with permission by Royal Society of Chemistry, (c) SEM images of CTP@C and (d) long-cycle performance and the charge–discharge curves (inset) of PIC[127] (Reprinted with permission by John Wiley and Sons).
Table 1. A comparison of the physical properties of Li, Na and K.
Li Na K Atomic number 3 11 19 Melting point (°C) 180 97.72 63.25 Density (g/cm3) 0.534 0.968 0.86 Atomic radius (pm) 123 154 203 Voltage vs S.H.E (V) −3.04 −2.71 −2.93 Price (USD/Ton) 13900 152 790 Element content in the crust (%) 0.0017 2.36 2.09 -
[1] Miller J R, Simon P. Materials science - electrochemical capacitors for energy management[J]. Science,2008,321(5889):651-652. doi: 10.1126/science.1158736 [2] Yu X W, Manthiram A. Electrochemical energy storage with mediator-ion solid electrolytes[J]. Joule,2017,1(3):453-462. doi: 10.1016/j.joule.2017.10.011 [3] Lee H Y, Goodenough J B. Supercapacitor behavior with kcl electrolyte[J]. Journal of Solid State Chemistry,1999,144(1):220-223. doi: 10.1006/jssc.1998.8128 [4] Gogotsi Y, Simon P. True performance metrics in electrochemical energy storage[J]. Science,2011,334(6058):917-918. doi: 10.1126/science.1213003 [5] Yoon S, Lee J W, Hyeon T, et al. Electric double-layer capacitor performance of a new mesoporous carbon[J]. Journal of the Electrochemical Society,2000,147(7):2507-2512. doi: 10.1149/1.1393561 [6] Forse A C, Merlet C, Griffin J M, et al. New perspectives on the charging mechanisms of supercapacitors[J]. Journal of the American Chemical Society,2016,138(18):5731-5744. doi: 10.1021/jacs.6b02115 [7] Amatucci G G, Badway F, Du Pasquier A, et al. An asymmetric hybrid nonaqueous energy storage cell[J]. Journal of the Electrochemical Society,2001,148(8):A930-A939. doi: 10.1149/1.1383553 [8] Khomenko V, Raymundo-Pinero E, Beguin F. High-energy density graphite/AC capacitor in organic electrolyte[J]. Journal of Power Sources,2008,177(2):643-651. doi: 10.1016/j.jpowsour.2007.11.101 [9] Chen Z, Augustyn V, Jia X L, et al. High-performance sodium-ion pseudocapacitors based on hierarchically porous nanowire composites[J]. Acs Nano,2012,6(5):4319-4327. doi: 10.1021/nn300920e [10] Dong S Y, Shen L F, Li H S, et al. Flexible sodium-ion pseudocapacitors based on 3D Na2Ti3O7 nanosheet arrays/carbon textiles anodes[J]. Advanced Functional Materials,2016,26(21):3703-3710. doi: 10.1002/adfm.201600264 [11] Le Comte A, Reynier Y, Vincens C, et al. First prototypes of hybrid potassium-ion capacitor (KIC): An innovative, cost-effective energy storage technology for transportation applications[J]. Journal of Power Sources,2017,363:34-43. doi: 10.1016/j.jpowsour.2017.07.005 [12] Luo Y W, Liu L J, Lei K X, et al. A nonaqueous potassium-ion hybrid capacitor enabled by two-dimensional diffusion pathways of dipotassium terephthalate[J]. Chemical Science,2019,10(7):2048-2052. doi: 10.1039/C8SC04489A [13] Yi Y Y, Sun Z T, Li C, et al. Designing 3D biomorphic nitrogen-doped MoSe2/graphene composites toward high-performance potassium-ion capacitors[J]. Advanced Functional Materials,2020,30(4):1903878. doi: 10.1002/adfm.201903878 [14] Zhao S Q, Dong L B, Sun B, et al. K2Ti2O5@C microspheres with enhanced K+ intercalation pseudocapacitance ensuring fast potassium storage and long-term cycling stability[J]. Small,2020,16(4):1906131. doi: 10.1002/smll.201906131 [15] Shao M J, Li C X, Li T, et al. Pushing the energy output and cycling lifespan of potassium-ion capacitor to high level through metal-organic framework derived porous carbon microsheets anode[J]. Advanced Functional Materials,2020,30(51):2006561. doi: 10.1002/adfm.202006561 [16] Feng W T, Feng N Y, Liu W, et al. Liquid-state templates for constructing B, N, co-doping porous carbons with a boosting of potassium-ion storage performance[J]. Advanced Energy Materials,2020:2003215. [17] Cericola D, Kotz R. Hybridization of rechargeable batteries and electrochemical capacitors: Principles and limits[J]. Electrochimica Acta,2012,72:1-17. doi: 10.1016/j.electacta.2012.03.151 [18] Zuo W H, Li R Z, Zhou C, et al. Battery-supercapacitor hybrid devices: Recent progress and future prospects[J]. Advanced Science,2017,4(7):1600539. doi: 10.1002/advs.201600539 [19] Li B, Dai F, Xiao Q F, et al. Nitrogen-doped activated carbon for a high energy hybrid supercapacitor[J]. Energy & Environmental Science,2016,9(1):102-106. [20] Dubal D P, Ayyad O, Ruiz V, et al. Hybrid energy storage: The merging of battery and supercapacitor chemistries[J]. Chemical Society Reviews,2015,44(7):1777-1790. doi: 10.1039/C4CS00266K [21] Aida T, Yamada K, Morita M. An advanced hybrid electrochemical capacitor that uses a wide potential range at the positive electrode[J]. Electrochemical and Solid State Letters,2006,9(12):A534-A536. doi: 10.1149/1.2349495 [22] Choi H S, Park C R. Theoretical guidelines to designing high performance energy storage device based on hybridization of lithium-ion battery and supercapacitor[J]. Journal of Power Sources,2014,259:1-14. doi: 10.1016/j.jpowsour.2014.02.001 [23] Naoi K, Ishimoto S, Miyamoto J, et al. Second generation 'nanohybrid supercapacitor': Evolution of capacitive energy storage devices[J]. Energy & Environmental Science,2012,5(11):9363-9373. [24] Camara M B, Gualous H, Gustin F, et al. Design and new control of DC/DC converters to share energy between supercapacitors and batteries in hybrid vehicles[J]. Ieee Transactions on Vehicular Technology,2008,57(5):2721-2735. doi: 10.1109/TVT.2008.915491 [25] Huang Y, Zhu M S, Huang Y, et al. Multifunctional energy storage and conversion devices[J]. Advanced Materials,2016,28(38):8344-8364. doi: 10.1002/adma.201601928 [26] Jezowski P, Crosnier O, Deunf E, et al. Safe and recyclable lithium-ion capacitors using sacrificial organic lithium salt[J]. Nature Materials,2018,17(2):167-173. doi: 10.1038/nmat5029 [27] Wu X, Chen Y L, Xing Z, et al. Advanced carbon-based anodes for potassium-ion batteries[J]. Advanced Energy Materials,2019,9(21):1900343. doi: 10.1002/aenm.201900343 [28] Cao B, Zhang Q, Liu H, et al. Graphitic carbon nanocage as a stable and high power anode for potassium-ion batteries[J]. Advanced Energy Materials,2018,8(25):1801149. doi: 10.1002/aenm.201801149 [29] Hou H S, Banks C E, Jing M J, et al. Carbon quantum dots and their derivative 3D porous carbon frameworks for sodium-ion batteries with ultralong cycle life[J]. Advanced Materials,2015,27(47):7861-7866. doi: 10.1002/adma.201503816 [30] Pramudita J C, Sehrawat D, Goonetilleke D, et al. An initial review of the status of electrode materials for potassium-ion batteries[J]. Advanced Energy Materials,2017,7(24):1602911. doi: 10.1002/aenm.201602911 [31] Dong S Y, Shen L F, Li H S, et al. Pseudocapacitive behaviours of Na2Ti3O7@CNT coaxial nanocables for high-performance sodium-ion capacitors[J]. Journal of Materials Chemistry A,2015,3(42):21277-21283. doi: 10.1039/C5TA05714K [32] Li H S, Zhu Y, Dong S Y, et al. Self-assembled Nb2O5 nanosheets for high energy-high power sodium ion capacitors[J]. Chemistry of Materials,2016,28(16):5753-5760. doi: 10.1021/acs.chemmater.6b01988 [33] Yang B J, Chen J T, Lei S L, et al. Spontaneous growth of 3D framework carbon from sodium citrate for high energy- and power-density and long-life sodium-ion hybrid capacitors[J]. Advanced Energy Materials,2018,8(10):1702409. doi: 10.1002/aenm.201702409 [34] Roh H K, Kim M S, Chung K Y, et al. A chemically bonded NaTi2(PO4)3/rGO microsphere composite as a high-rate insertion anode for sodium-ion capacitors[J]. Journal of Materials Chemistry A,2017,5(33):17506-17516. doi: 10.1039/C7TA05252A [35] Cui Y P, Liu W, Feng W T, et al. Controlled design of well-dispersed ultrathin MoS2 nanosheets inside hollow carbon skeleton: Toward fast potassium storage by constructing spacious "houses" for K ions[J]. Advanced Functional Materials,2020,30(10):1908755. doi: 10.1002/adfm.201908755 [36] Jian Z L, Luo W, Ji X L. Carbon electrodes for K-ion batteries[J]. Journal of the American Chemical Society,2015,137(36):11566-11569. doi: 10.1021/jacs.5b06809 [37] Tian B B, Zheng J, Zhao C X, et al. Carbonyl-based polyimide and polyquinoneimide for potassium-ion batteries[J]. Journal of Materials Chemistry A,2019,7(16):9997-10003. doi: 10.1039/C9TA00647H [38] Fan L, Lin K R, Wang J, et al. A nonaqueous potassium-based battery-supercapacitor hybrid device[J]. Advanced Materials,2018,30(20):1800804. doi: 10.1002/adma.201800804 [39] Qiu D P, Guan J Y, Li M, et al. Kinetics enhanced nitrogen-doped hierarchical porous hollow carbon spheres boosting advanced potassium-ion hybrid capacitors[J]. Advanced Functional Materials,2019,29(32):1903496. doi: 10.1002/adfm.201903496 [40] Yang J L, Ju Z C, Jiang Y, et al. Enhanced capacity and rate capability of nitrogen/oxygen dual-doped hard carbon in capacitive potassium-ion storage[J]. Advanced Materials,2018,30(4):1700104. doi: 10.1002/adma.201700104 [41] Xu Z Q, Wu M Q, Chen Z, et al. Direct structure-performance comparison of all-carbon potassium and sodium ion capacitors[J]. Advanced Science,2019,6(12):1802272. doi: 10.1002/advs.201802272 [42] Hwang J Y, Kim J, Yu T Y, et al. Development of P3-K0.69CrO2 as an ultra-high-performance cathode material for K-ion batteries[J]. Energy & Environmental Science,2018,11(10):2821-2827. [43] Liu M Q, Chang L M, Le Z Y, et al. Emerging potassium-ion hybrid capacitors[J]. Chemsuschem,2020,13(22):5837-5862. doi: 10.1002/cssc.202000578 [44] Charlier J C, Eklund P C, Zhu J, et al. Electron and phonon properties of graphene: Their relationship with carbon nanotubes[J]. Carbon Nanotubes,2008,111:673-709. [45] Chen Z, Li W L, Yang J, et al. Excellent electrochemical performance of potassium ion capacitor achieved by a high nitrogen doped activated carbon[J]. Journal of the Electrochemical Society,2020,167(5):050506. doi: 10.1149/1945-7111/ab6a84 [46] Cui Y P, Liu W, Wang X, et al. Bioinspired mineralization under freezing conditions: An approach to fabricate porous carbons with complicated architecture and superior K+ storage performance[J]. Acs Nano,2019,13(10):11582-11592. doi: 10.1021/acsnano.9b05284 [47] Feng W T, Cui Y P, Liu W, et al. Rigid-flexible coupling carbon skeleton and potassium-carbonate-dominated solid electrolyte interface achieving superior potassium-ion storage[J]. Acs Nano,2020,14(4):4938-4949. doi: 10.1021/acsnano.0c01073 [48] Nishi Y. The development of lithium ion secondary batteries[J]. Chemical Record,2001,1(5):406-413. doi: 10.1002/tcr.1024 [49] Aravindan V, Gnanaraj J, Lee Y S, et al. LiMnPO4 - a next generation cathode material for lithium-ion batteries[J]. Journal of Materials Chemistry A,2013,1(11):3518-3539. doi: 10.1039/c2ta01393b [50] Aravindan V, Lee Y S, Madhavi S. Research progress on negative electrodes for practical Li-ion batteries: Beyond carbonaceous anodes[J]. Advanced Energy Materials,2015,5(13):1402225. doi: 10.1002/aenm.201402225 [51] Underhill C, Krapchev T, Dresselhaus M S. Synthesis and characterization of high stage alkali-metal donor compounds[J]. Synthetic Metals,1980,2(1-2):47-55. doi: 10.1016/0379-6779(80)90031-4 [52] Dresselhaus M S, Dresselhaus G. Intercalation compounds of graphite[J]. Advances in Physics,1981,30(2):139-326. doi: 10.1080/00018738100101367 [53] Yao F, Pham D T, Lee Y H. Carbon-based materials for lithium-ion batteries, electrochemical capacitors, and their hybrid devices[J]. Chemsuschem,2015,8(14):2284-2311. doi: 10.1002/cssc.201403490 [54] An Y L, Fei H F, Zeng G F, et al. Commercial expanded graphite as a low cost, long-cycling life anode for potassium-ion batteries with conventional carbonate electrolyte[J]. Journal of Power Sources,2018,378:66-72. doi: 10.1016/j.jpowsour.2017.12.033 [55] Winter M, Besenhard J O, Spahr M E, et al. Insertion electrode materials for rechargeable lithium batteries[J]. Advanced Materials,1998,10(10):725-763. doi: 10.1002/(SICI)1521-4095(199807)10:10<725::AID-ADMA725>3.0.CO;2-Z [56] Zhang J, Shi Z Q, Wang C Y. Effect of pre-lithiation degrees of mesocarbon microbeads anode on the electrochemical performance of lithium-ion capacitors[J]. Electrochimica Acta,2014,125:22-28. doi: 10.1016/j.electacta.2014.01.040 [57] Griffith K J, Wiaderek K M, Cibin G, et al. Niobium tungsten oxides for high-rate lithium-ion energy storage[J]. Nature,2018,559(7715):556-563. doi: 10.1038/s41586-018-0347-0 [58] Liu X, Elia G A, Qin B S, et al. High-power Na-ion and K-ion hybrid capacitors exploiting cointercalation in graphite negative electrodes[J]. Acs Energy Letters,2019,4(11):2675-2682. doi: 10.1021/acsenergylett.9b01675 [59] Fan L, Liu Q, Chen S H, et al. Soft carbon as anode for high-performance sodium-based dual ion full battery[J]. Advanced Energy Materials,2017,7(14):1602778. doi: 10.1002/aenm.201602778 [60] Cui Y P, Wang H L, Mao N, et al. Tuning the morphology and structure of nanocarbons with activating agents for ultrafast ionic liquid-based supercapacitors[J]. Journal of Power Sources,2017,9(361):182-194. [61] Wang G, Xiong X H, Xie D, et al. Chemically activated hollow carbon nanospheres as a high-performance anode material for potassium ion batteries[J]. Journal of Materials Chemistry A,2018,6(47):24317-24323. doi: 10.1039/C8TA09751H [62] Zhao D Y, Zhao R Z, Dong S H, et al. Alkali-induced 3D crinkled porous Ti3C2 mxene architectures coupled with NiCoP bimetallic phosphide nanoparticles as anodes for high-performance sodium-ion batteries[J]. Energy & Environmental Science,2019,12(8):2422-2432. [63] Ming J, Cao Z, Wahyudi W, et al. New insights on graphite anode stability in rechargeable batteries: Li ion coordination structures prevail over solid electrolyte interphases[J]. Acs Energy Letters,2018,3(2):335-340. doi: 10.1021/acsenergylett.7b01177 [64] Heiskanen S K, Kim J, Lucht B L. Generation and evolution of the solid electrolyte interphase of lithium-ion batteries[J]. Joule,2019,3(10):2322-2333. doi: 10.1016/j.joule.2019.08.018 [65] Wang H, Yu D D, Wang X, et al. Electrolyte chemistry enables simultaneous stabilization of potassium metal and alloying anode for potassium-ion batteries[J]. Angewandte Chemie-International Edition,2019,58(46):16451-16455. doi: 10.1002/anie.201908607 [66] Han X Q, Xu G J, Zhang Z H, et al. An in situ interface reinforcement strategy achieving long cycle performance of dual-ion batteries[J]. Advanced Energy Materials,2019,9(16):1804022. doi: 10.1002/aenm.201804022 [67] Zhao Y, Zhu J J, Ong S J H, et al. High-rate and ultralong cycle-life potassium ion batteries enabled by in situ engineering of yolk-shell FeS2@C structure on graphene matrix[J]. Advanced Energy Materials,2018,8(36):1802565. doi: 10.1002/aenm.201802565 [68] Adams R A, Varma A, Pol V G. Mechanistic elucidation of thermal runaway in potassium-ion batteries[J]. Journal of Power Sources,2018,375:131-137. doi: 10.1016/j.jpowsour.2017.11.065 [69] Lei Y, Han D, Dong J H, et al. Unveiling the influence of electrode/electrolyte interface on the capacity fading for typical graphite-based potassium-ion batteries[J]. Energy Storage Materials,2020,1(24):319-328. [70] Benitez L, Seminario J M. Electron transport and electrolyte reduction in the solid-electrolyte interphase of rechargeable lithium ion batteries with silicon anodes[J]. Journal of Physical Chemistry C,2016,120(32):17978-17988. doi: 10.1021/acs.jpcc.6b06446 [71] Huang W, Wang J Y, Braun M R, et al. Dynamic structure and chemistry of the silicon solid-electrolyte interphase visualized by cryogenic electron microscopy[J]. Matter,2019,1(5):1232-1245. doi: 10.1016/j.matt.2019.09.020 [72] Wang R T, Jin D D, Zhang Y B, et al. Engineering metal organic framework derived 3D nanostructures for high performance hybrid supercapacitors[J]. Journal of Materials Chemistry A,2017,5(1):292-302. doi: 10.1039/C6TA09143A [73] Iijima S. Direct observation of the tetrahedral bonding in graphitized carbon-black by high-resolution electron-microscopy[J]. Journal of Crystal Growth,1980,50(3):675-683. doi: 10.1016/0022-0248(80)90013-5 [74] Chen J T, Yang B J, Li H X, et al. Candle soot: Onion-like carbon, an advanced anode material for a potassium-ion hybrid capacitor[J]. Journal of Materials Chemistry A,2019,7(15):9247-9252. doi: 10.1039/C9TA01653H [75] Paraknowitsch J P, Thomas A. Functional carbon materials from ionic liquid precursors[J]. Macromolecular Chemistry and Physics,2012,213(10-11):1132-1145. doi: 10.1002/macp.201100573 [76] Chen W M, Wan M, Liu Q, et al. Heteroatom-doped carbon materials: Synthesis, mechanism, and application for sodium-ion batteries[J]. Small Methods,2019,3(4):1800323. doi: 10.1002/smtd.201800323 [77] Ying T, Feiwen Z, Lei J, et al. Heteroatom-doped carbon materials for capacitive deionization[J]. Functional Materials,2017,48(8):08001-08006. [78] Sennu P, Aravindan V, Lee Y S. Marine algae inspired pre-treated SnO2 nanorods bundle as negative electrode for Li-ion capacitor and battery: An approach beyond intercalation[J]. Chemical Engineering Journal,2017,324:26-34. doi: 10.1016/j.cej.2017.05.003 [79] Sun Y, Wang H, Wei W, et al. Sulfur-rich graphene nanoboxes with ultra-high potassiation capacity at fast charge: Storage mechanisms and device performance[J]. Acs Nano,2020,15(1):1652-1665. [80] Yang B J, Chen J T, Liu L Y, et al. 3D nitrogen-doped framework carbon for high-performance potassium ion hybrid capacitor[J]. Energy Storage Materials,2019,23:522-529. doi: 10.1016/j.ensm.2019.04.008 [81] Liu M R, Hong Q L, Li Q H, et al. Cobalt boron imidazolate framework derived cobalt nanoparticles encapsulated in B/N codoped nanocarbon as efficient bifunctional electrocatalysts for overall water splitting[J]. Advanced Functional Materials,2018,28(26):1801136. doi: 10.1002/adfm.201801136 [82] Lee W H, Yang H N, Park K W, et al. Synergistic effect of boron/nitrogen co-doping into graphene and intercalation of carbon black for Pt-BCN-Gr/CB hybrid catalyst on cell performance of polymer electrolyte membrane fuel cell[J]. Energy,2016,96:314-324. doi: 10.1016/j.energy.2015.12.088 [83] Hu X, Liu Y J, Chen J X, et al. Fast redox kinetics in Bi-heteroatom doped 3D porous carbon nanosheets for high-performance hybrid potassium-ion battery capacitors[J]. Advanced Energy Materials,2019,9(42):1901533. doi: 10.1002/aenm.201901533 [84] Li H X, Chen J T, Zhang L, et al. A metal-organic framework-derived pseudocapacitive titanium oxide/carbon core/shell heterostructure for high performance potassium ion hybrid capacitors[J]. Journal of Materials Chemistry A,2020,8(32):16302-16311. doi: 10.1039/D0TA04912C [85] Qie L, Chen W M, Xu H H, et al. Synthesis of functionalized 3D hierarchical porous carbon for high-performance supercapacitors[J]. Energy & Environmental Science,2013,6(8):2497-2504. [86] Zheng F C, Yang Y, Chen Q W. High lithium anodic performance of highly nitrogen-doped porous carbon prepared from a metal-organic framework[J]. Nature Communications,2014,5:1-10. [87] Zhang C, Wang X, Liang Q F, et al. Amorphous phosphorus/nitrogen-doped graphene paper for ultrastable sodium-ion batteries[J]. Nano Letters,2016,16(3):2054-2060. doi: 10.1021/acs.nanolett.6b00057 [88] Luan Y T, Hu R, Fang Y Z, et al. Nitrogen and phosphorus dual-doped multilayer graphene as universal anode for full carbon-based lithium and potassium ion capacitors[J]. Nano-Micro Letters,2019,11(1):30. doi: 10.1007/s40820-019-0260-6 [89] Shen L F, Uchaker E, Zhang X G, et al. Hydrogenated Li4Ti5O12 nanowire arrays for high rate lithium ion batteries[J]. Advanced Materials,2012,24(48):6502-6506. doi: 10.1002/adma.201203151 [90] Xiong H, Slater M D, Balasubramanian M, et al. Amorphous TiO2 nanotube anode for rechargeable sodium ion batteries[J]. Journal of Physical Chemistry Letters,2011,2(20):2560-2565. doi: 10.1021/jz2012066 [91] Zhao L, Hu Y S, Li H, et al. Porous Li4Ti5O12 coated with N-doped carbon from ionic liquids for li-ion batteries[J]. Advanced Materials,2011,23(11):1385-1388. doi: 10.1002/adma.201003294 [92] Han J, Xu M W, Niu Y B, et al. Exploration of K2Ti8O17 as an anode material for potassium-ion batteries[J]. Chemical Communications,2016,52(75):11274-11276. doi: 10.1039/C6CC05102B [93] Dong S, Li Z, Xing Z, et al. Novel potassium-ion hybrid capacitor based on an anode of K2Ti6O13 microscaffolds[J]. ACS Appl Mater Interfaces,2018,10(18):15542-15547. doi: 10.1021/acsami.7b15314 [94] Fang Y Z, Zhang Y, Zhu K, et al. Lithiophilic three-dimensional porous Ti3C2Tx-rGO membrane as a stable scaffold for safe alkali metal (Li or Na) anodes[J]. Acs Nano,2019,13(12):14319-14328. doi: 10.1021/acsnano.9b07729 [95] Fang Y Z, Lian R Q, Li H P, et al. Induction of planar sodium growth on mxene (Ti3C2Tx)-modified carbon cloth hosts for flexible sodium metal anodes[J]. Acs Nano,2020,14(7):8744-8753. doi: 10.1021/acsnano.0c03259 [96] Mounet N, Gibertini M, Schwaller P, et al. Two-dimensional materials from high-throughput computational exfoliation of experimentally known compounds[J]. Nature Nanotechnology,2018,13(3):246-252. doi: 10.1038/s41565-017-0035-5 [97] Fang Y Z, Hu R, Zhu K, et al. Aggregation-resistant 3D Ti3C2Tx Mxene with enhanced kinetics for potassium ion hybrid capacitors[J]. Advanced Functional Materials,2020,30(50):2005663. doi: 10.1002/adfm.202005663 [98] Sultana I, Ramireddy T, Rahman M M, et al. Tin-based composite anodes for potassium-ion batteries[J]. Chemical Communications,2016,52(59):9279-9282. doi: 10.1039/C6CC03649J [99] Chen Y N, Luo W, Carter M, et al. Organic electrode for non-aqueous potassium-ion batteries[J]. Nano Energy,2015,18:205-211. doi: 10.1016/j.nanoen.2015.10.015 [100] Lei K X, Li F J, Mu C N, et al. High K-storage performance based on the synergy of dipotassium terephthalate and ether-based electrolytes[J]. Energy & Environmental Science,2017,10(2):552-557. [101] Sannyal A, Zhang Z Q, Gao X F, et al. Two-dimensional sheet of germanium selenide as an anode material for sodium and potassium ion batteries: First-principles simulation study[J]. Computational Materials Science,2018,154:204-211. doi: 10.1016/j.commatsci.2018.08.002 [102] Zhai Z B, Huang K J, Wu X. Superior mixed co-Cd selenide nanorods for high performance alkaline battery-supercapacitor hybrid energy storage[J]. Nano Energy,2018,47:89-95. doi: 10.1016/j.nanoen.2018.02.059 [103] Niu F E, Yang J, Wang N N, et al. MoSe2-covered N,P-doped carbon nanosheets as a long-life and high-rate anode material for sodium-ion batteries[J]. Advanced Functional Materials,2017,27(23):1700522. doi: 10.1002/adfm.201700522 [104] Huang H W, Cui J, Liu G X, et al. Carbon-coated MoSe2/Mxene hybrid nanosheets for superior potassium storage[J]. Acs Nano,2019,13(3):3448-3456. doi: 10.1021/acsnano.8b09548 [105] Shen Q, Jiang P J, He H C, et al. Encapsulation of MoSe2 in carbon fibers as anodes for potassium ion batteries and nonaqueous battery-supercapacitor hybrid devices[J]. Nanoscale,2019,11(28):13511-13520. doi: 10.1039/C9NR03480C [106] Ge J M, Wang B, Wang J, et al. Nature of FeSe2/N-C anode for high performance potassium ion hybrid capacitor[J]. Advanced Energy Materials,2020,10(4):1903277. doi: 10.1002/aenm.201903277 [107] Wang R T, Wang S J, Zhang Y B, et al. Sodium storage in a promising MoS2-carbon anode: Elucidating structural and interfacial transitions in the intercalation process and conversion reactions[J]. Nanoscale,2018,10(23):11165-11175. doi: 10.1039/C8NR02620C [108] Wang R T, Wang S J, Peng X, et al. Elucidating the intercalation pseudocapacitance mechanism of MoS2-carbon monolayer interoverlapped superstructure: Toward high-performance sodium-ion-based hybrid supercapacitor[J]. Acs Applied Materials & Interfaces,2017,9(38):32745-32755. [109] Pumera M, Sofer Z, Ambrosi A. Layered transition metal dichalcogenides for electrochemical energy generation and storage[J]. Journal of Materials Chemistry A,2014,2(24):8981-8987. doi: 10.1039/C4TA00652F [110] Chhowalla M, Shin H S, Eda G, et al. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets[J]. Nature Chemistry,2013,5(4):263-275. doi: 10.1038/nchem.1589 [111] Xing L D, Yu Q Y, Jiang B, et al. Carbon-encapsulated ultrathin MoS2 nanosheets epitaxially grown on porous metallic TiNb2O6 microspheres with unsaturated oxygen atoms for superior potassium storage[J]. Journal of Materials Chemistry A,2019,7(10):5760-5768. doi: 10.1039/C8TA12497C [112] Chen B A, Lu H H, Zhou J W, et al. Porous MoS2/carbon spheres anchored on 3D interconnected multiwall carbon nanotube networks forultrafast na storage[J]. Advanced Energy Materials,2018,8(15):1702909. doi: 10.1002/aenm.201702909 [113] Jia G C, Chao D L, Tiep N H, et al. Intercalation Na-ion storage in two-dimensional MoS2-xSex and capacity enhancement by selenium substitution[J]. Energy Storage Materials,2018,14:136-142. doi: 10.1016/j.ensm.2018.02.019 [114] Wang S S, Liu B C, Zhi G L, et al. Relaxing volume stress and promoting active sites in vertically grown 2D layered mesoporous MoS2(1-x)Se2x/rGO composites with enhanced capability and stability for lithium ion batteries[J]. Electrochimica Acta,2018,268:424-434. doi: 10.1016/j.electacta.2018.02.102 [115] Gao J Y, Wang G R, Liu Y, et al. Ternary molybdenum sulfoselenide based hybrid nanotubes boost potassium-ion diffusion kinetics for high energy/power hybrid capacitors[J]. Journal of Materials Chemistry A,2020,8(28):13946-13954. doi: 10.1039/D0TA01786H [116] Gao H, Zhou T F, Zheng Y, et al. Cos quantum dot nanoclusters for high-energy potassium-ion batteries[J]. Advanced Functional Materials,2017,27(43):1702634. doi: 10.1002/adfm.201702634 [117] Ma G Y, Li C J, Liu F, et al. Metal-organic framework-derived Co0.85Se nanoparticles in N-doped carbon as a high-rate and long-lifespan anode material for potassium ion batteries[J]. Materials Today Energy,2018,10:241-248. doi: 10.1016/j.mtener.2018.09.013 [118] Dong C F, Liang J W, He Y Y, et al. NiS1.03 hollow spheres and cages as superhigh rate capacity and stable anode materials for half/full sodium-ion batteries[J]. Acs Nano,2018,12(8):8277-8287. doi: 10.1021/acsnano.8b03541 [119] Zhu S H, Li Q D, Wei Q L, et al. NiSe2 nanooctahedra as an anode material for high-rate and long-life sodium-ion battery[J]. Acs Applied Materials & Interfaces,2017,9(1):311-316. [120] Chen M X, Wang L, Sheng X H, et al. An ultrastable nonaqueous potassium-ion hybrid capacitor[J]. Advanced Functional Materials,2020,30(40):2004247. doi: 10.1002/adfm.202004247 [121] Li N, Song H W, Cui H, et al. Self-assembled growth of Sn@CNTs on vertically aligned graphene for binder-free high Li-storage and excellent stability[J]. Journal of Materials Chemistry A,2014,2(8):2526-2537. doi: 10.1039/c3ta14217e [122] Xie X Q, Kretschmer K, Zhang J Q, et al. Sn@CNT nanopillars grown perpendicularly on carbon paper: A novel free-standing anode for sodium ion batteries[J]. Nano Energy,2015,13:208-217. doi: 10.1016/j.nanoen.2015.02.022 [123] Zhang Y, Zhou Q, Zhu J X, et al. Nanostructured metal chalcogenides for energy storage and electrocatalysis[J]. Advanced Functional Materials,2017,27(35):1702317. doi: 10.1002/adfm.201702317 [124] Wang Y X, Zhang Z Y, Wang G X, et al. Ultrafine Co2P nanorods wrapped by graphene enable a long cycle life performance for a hybrid potassium-ion capacitor[J]. Nanoscale Horizons,2019,4(6):1394-1401. doi: 10.1039/C9NH00211A [125] Chen S Q, Wu C, Shen L F, et al. Challenges and perspectives for nasicon-type electrode materials for advanced sodium-ion batteries[J]. Advanced Materials,2017,29(48):1700431. doi: 10.1002/adma.201700431 [126] Han J, Niu Y B, Bao S J, et al. Nanocubic KTi2(PO4)3 electrodes for potassium-ion batteries[J]. Chemical Communications,2016,52(78):11661-11664. doi: 10.1039/C6CC06177J [127] Zhang Z, Li M, Gao Y, et al. Fast potassium storage in hierarchical Ca0.5Ti2(PO4)3@C microspheres enabling high-performance potassium-ion capacitors[J]. Advanced Functional Materials,2018,28(36):1802684. doi: 10.1002/adfm.201802684 -





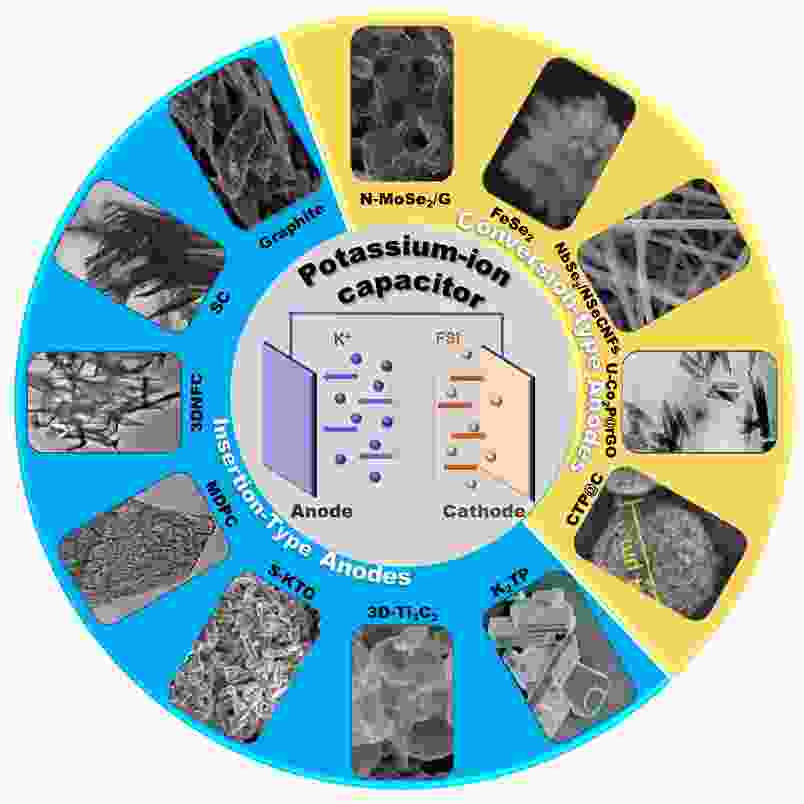
 下载:
下载: