Porous metal–organic frameworks for methane storage and capture: status and challenges
-
摘要: 在全球向可持续低碳经济转型的过程中,作为两大低碳能源技术,甲烷储存和甲烷捕集都面临着同样的挑战,即缺乏高效的吸附剂。MOFs(metal-organic frameworks)材料具有极高的比表面积、孔隙率和可调节的孔隙结构,在气体吸附储存领域拥有极大的潜在应用价值。本文首先介绍了MOFs的结构设计和合成方法,综述并强调了MOFs材料在CH4储存与捕集方面的研究进展及面临的问题。在CH4高压储存方面,从体积吸附量和质量吸附量两个目标出发,介绍了目前MOFs材料储存CH4的研究现状;在CH4常压捕集方面,重点强调了CH4/N2和CO2/CH4分离技术和CH4捕集技术。最后,对利用MOFs材料实现高效CH4储存和捕集存在的问题和挑战进行了分析和展望。Abstract: In the process of global transition to a sustainable low-carbon economy, the two major low-carbon energy technologies, namely, methane (CH4) storage and methane capture face the same challenge, that is, the lack of efficient adsorbents. Metal-organic framework (MOF) materials have potential value in the field of gas adsorption storage because of their high specific surface area, good porosity, and adjustable pore structure. In this study, the structural design and synthesis methods of MOFs are introduced, and the research progress and problems associated with MOF materials in methane storage and capture are reviewed. The current research status of methane storage at high pressure is introduced in terms of volumetric and gravimetric uptake. For methane capture at atmospheric pressure, emphasis is placed on CH4/N2 and CO2/CH4 separation and methane capture technologies. Finally, the problems and challenges of using MOF materials to achieve efficient methane storage and capture are analyzed and future prospects are presented.
-
Key words:
- Metal-organic frameworks /
- Methane /
- Adsorption /
- Storage /
- Capture
-
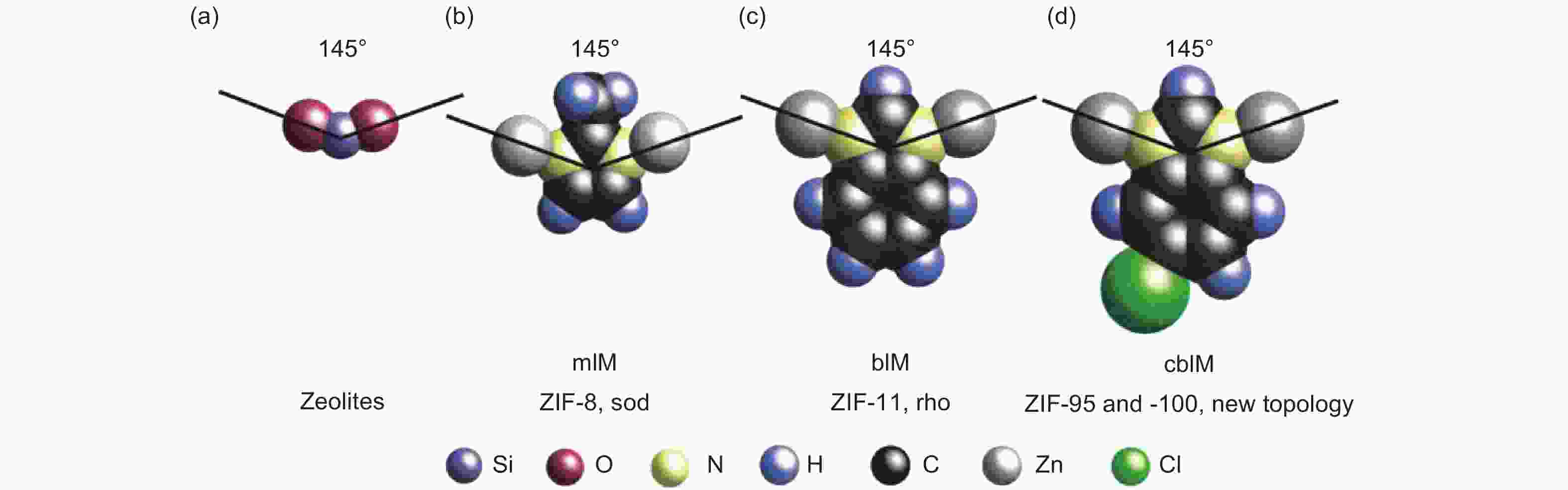
Figure 2. Bridging angles and girths in zeolites and IMs[16]. Reproduced with permission.
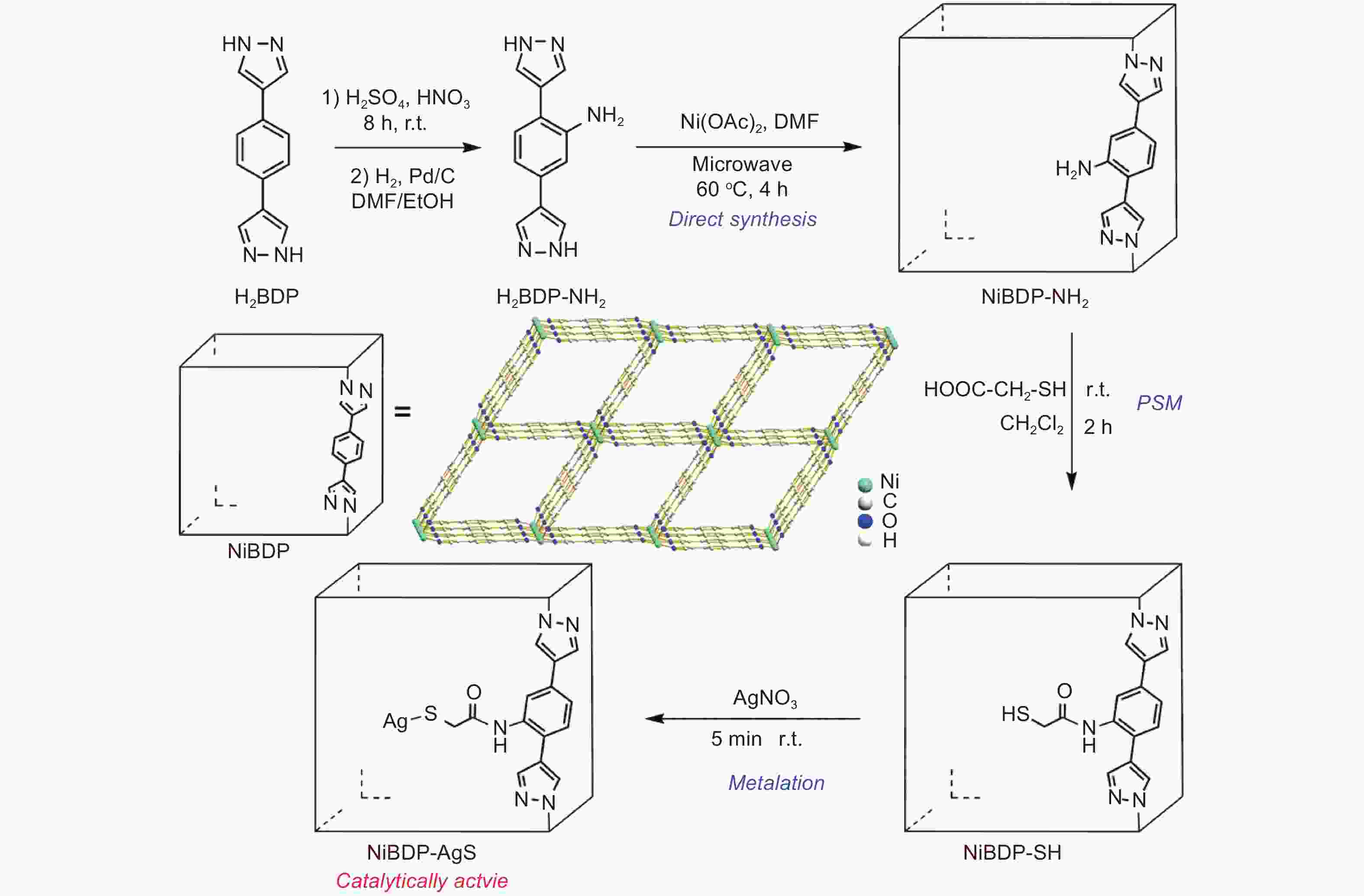
Figure 3. Synthesis flow chart of NiBDP-AgS[19]. Reproduced with permission.
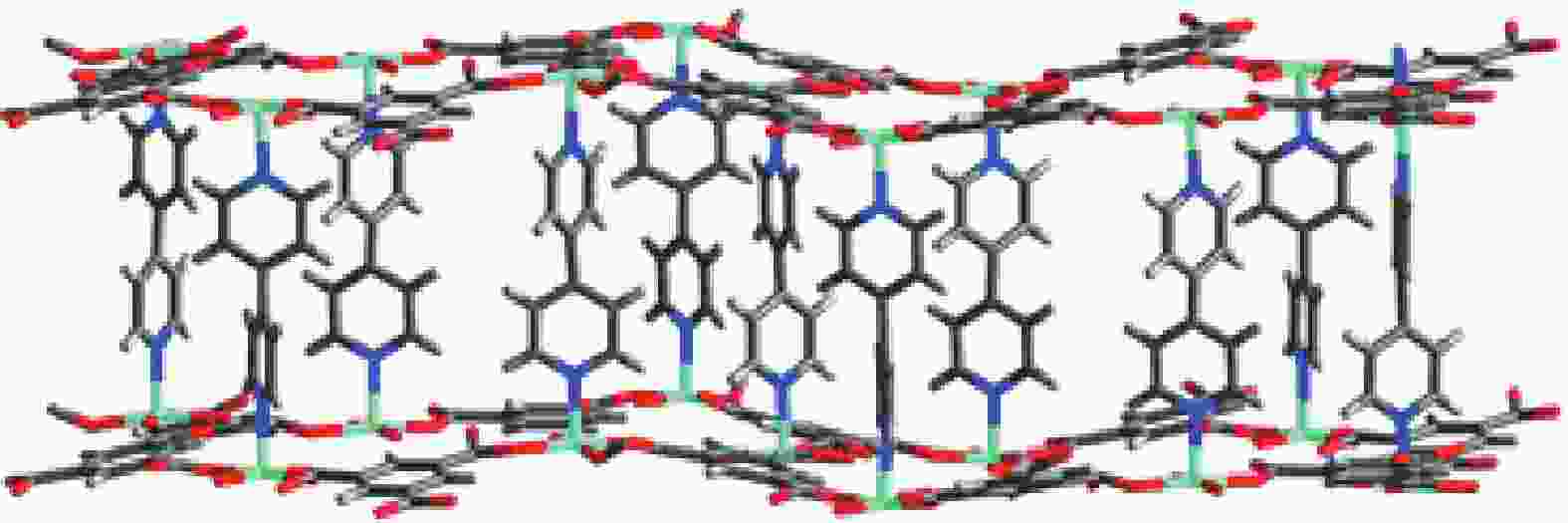
Figure 4. The three-dimensional coordination polymer, showing the pillaring of adjacent (6,3) Ni3(btc)2 sheets by 4,4’-bipy ligands[22]. Reproduced with permission.
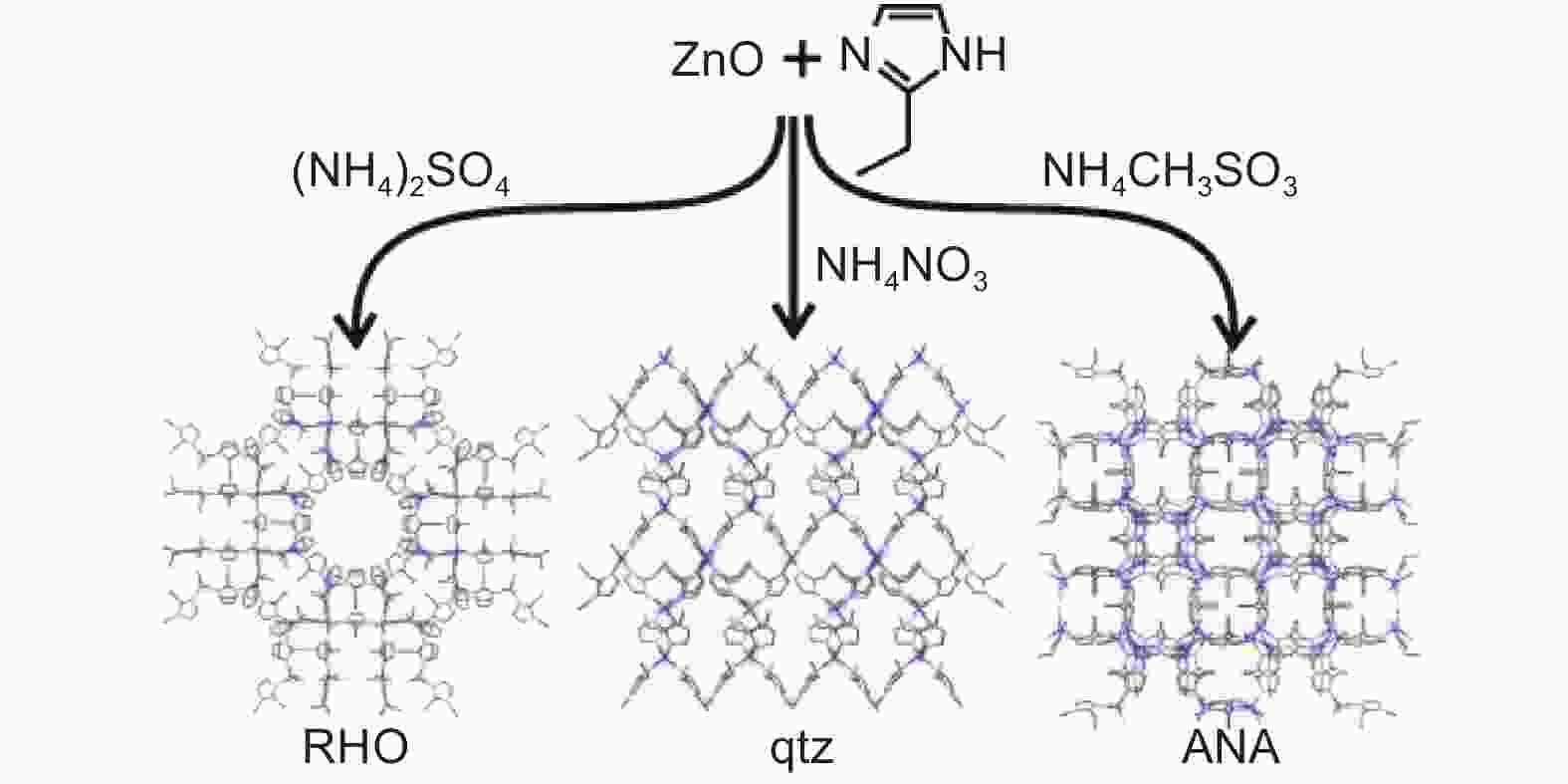
Figure 5. ZIF series produced by solid-phase reaction method[26]. Reproduced with permission.
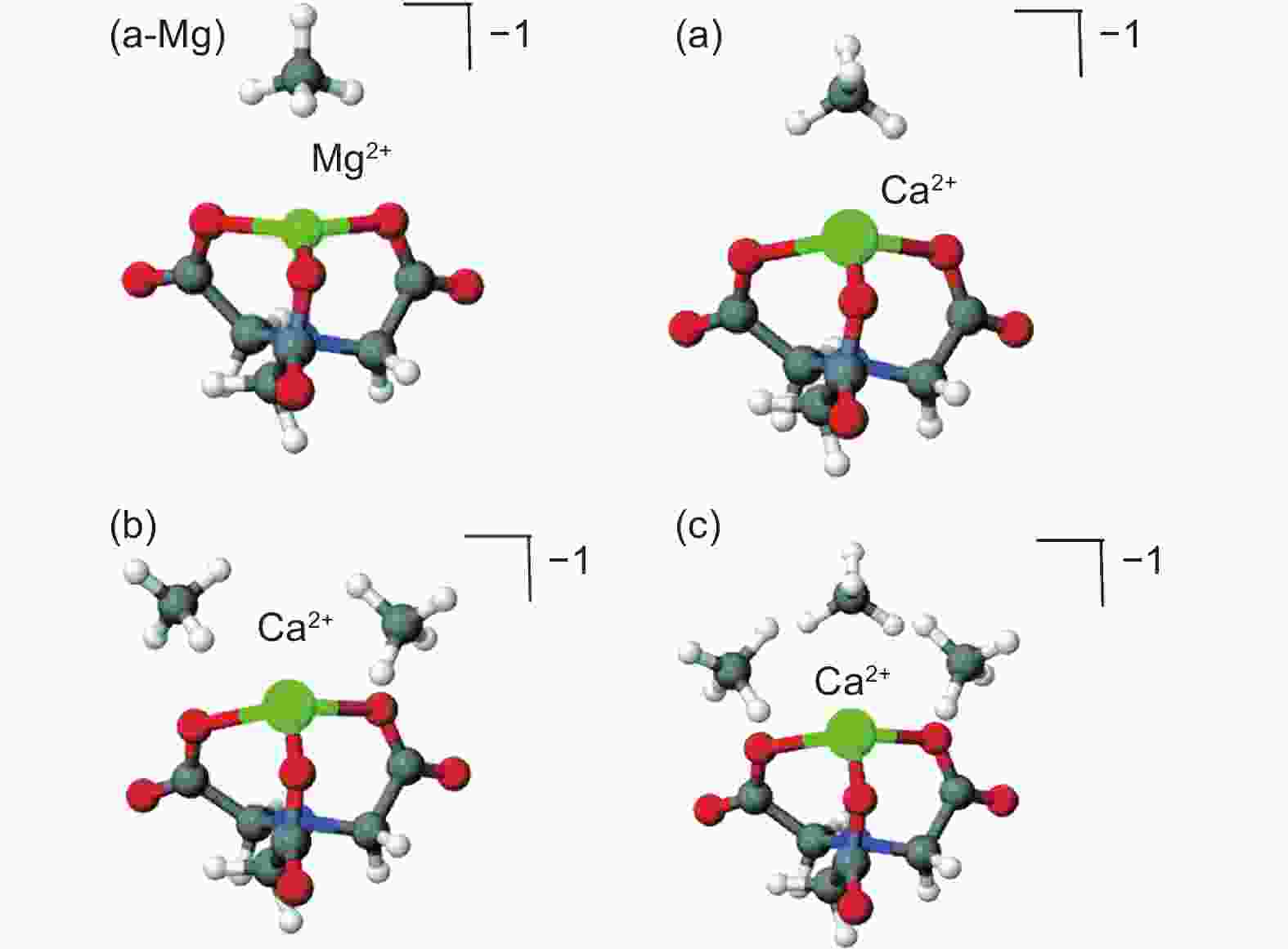
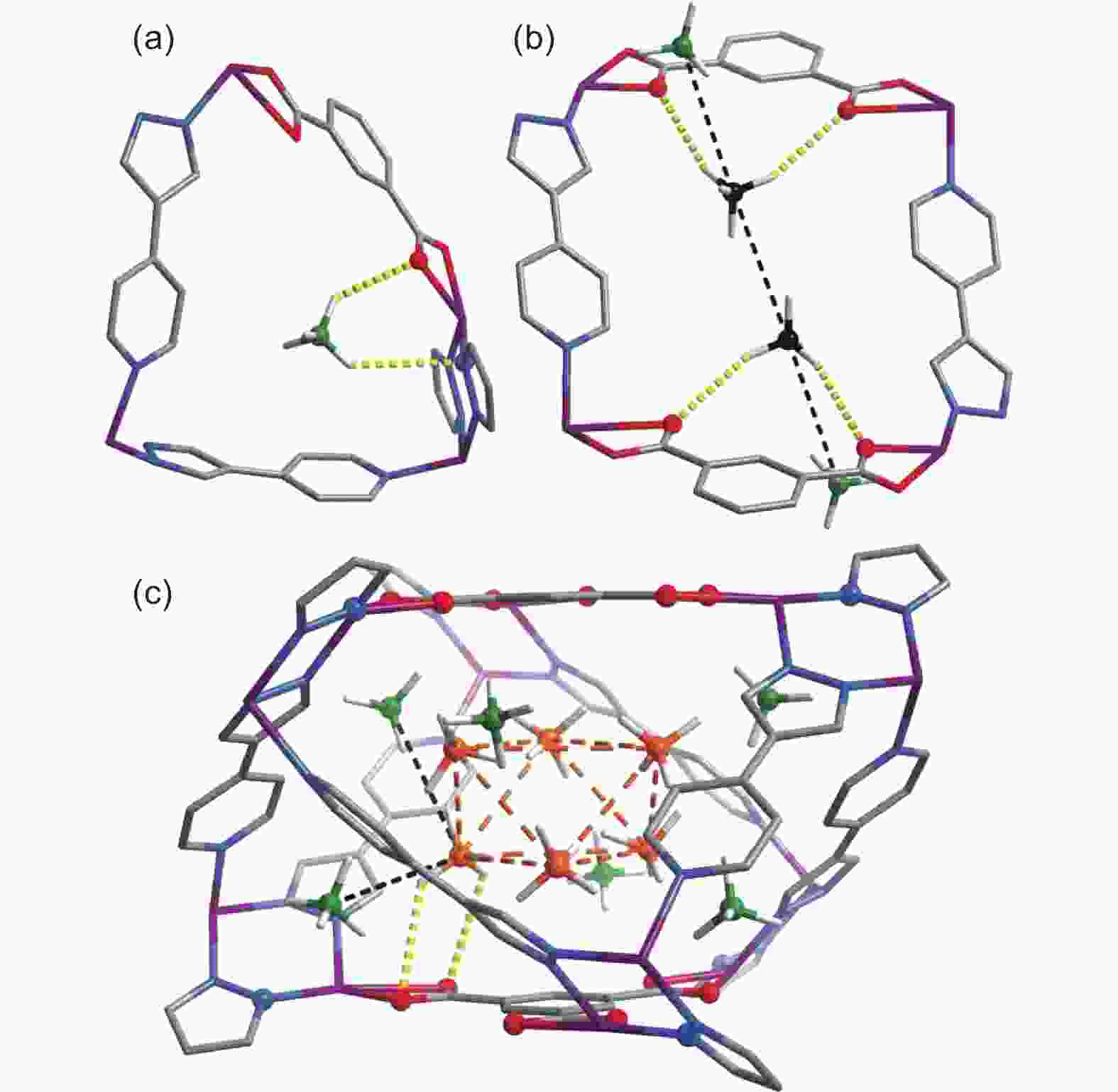
Figure 6. Clusters formed by NTA-Mg (a-Mg), NTA-Ca (Amurc) and CH4. The first adsorbed CH4 blocked the adsorption site of NTA-Mg, while the conical NTA-Ca could adsorb three CH4[73]. Reproduced with permission.
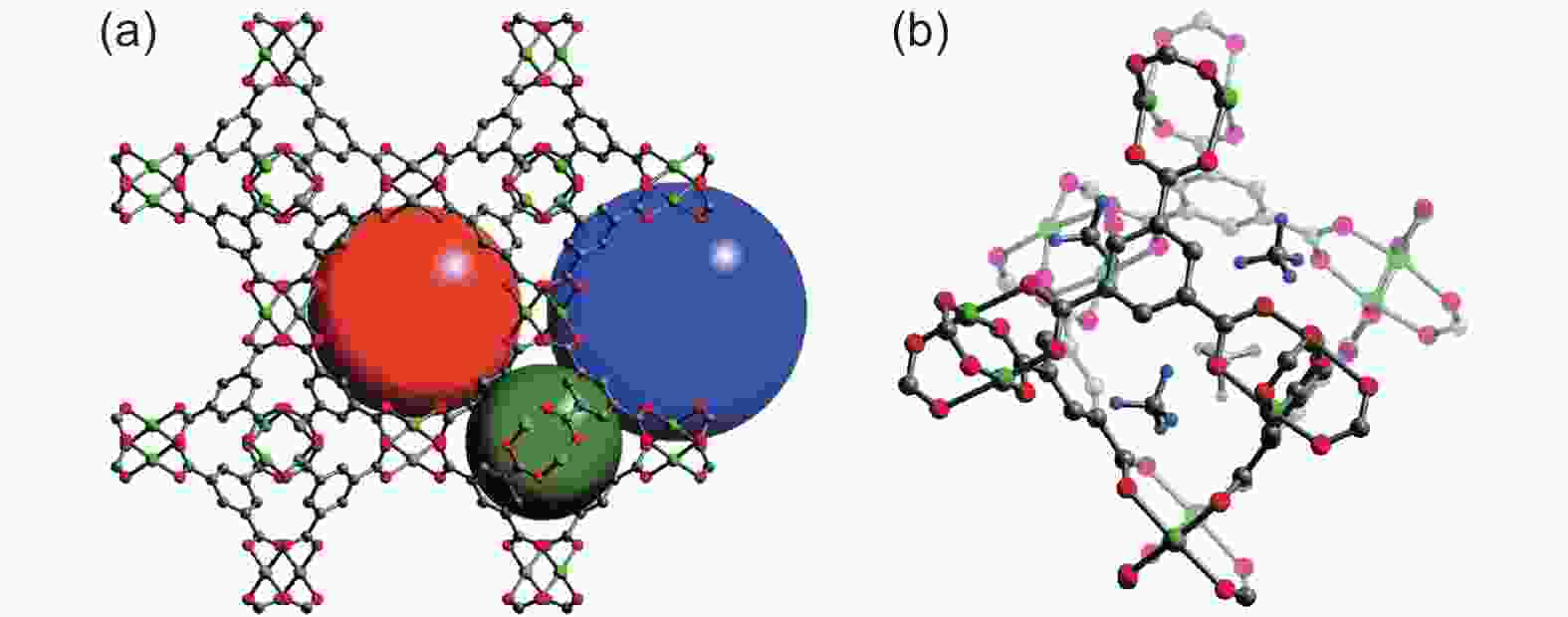
Figure 7. (a) Three types of cages in HKUST-1, and their diameters are 0.5 nm (dark green), 1.1 nm (orange) and 1.35 nm (blue); (b) The green, gray, red and light blue spheres of CD4 molecules adsorbed at the four holes of the octahedral cage represent Cu, C, O and D atoms, respectively[4]. Reproduced with permission.
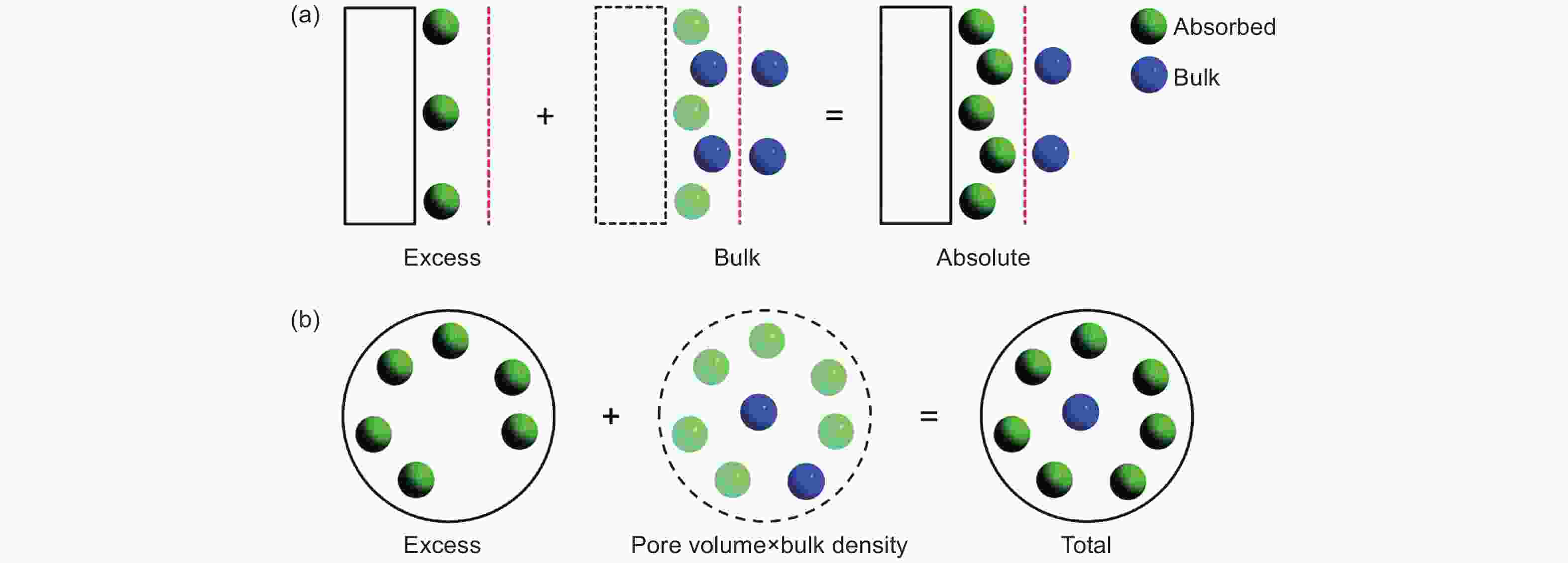
Figure 8. Schematic diagram of excess adsorption, absolute adsorption and total adsorption (the left side of the red line is the adsorption area, the right side of the red line is the unadsorbed area, the green sphere represents the adsorbed molecules, and the blue sphere represents the unadsorbed molecules)[4]. Reproduced with permission.
Figure 9. Comparison of deliverable capacity at different storage temperatures (5−65 bar)[7]. Reproduced with permission.
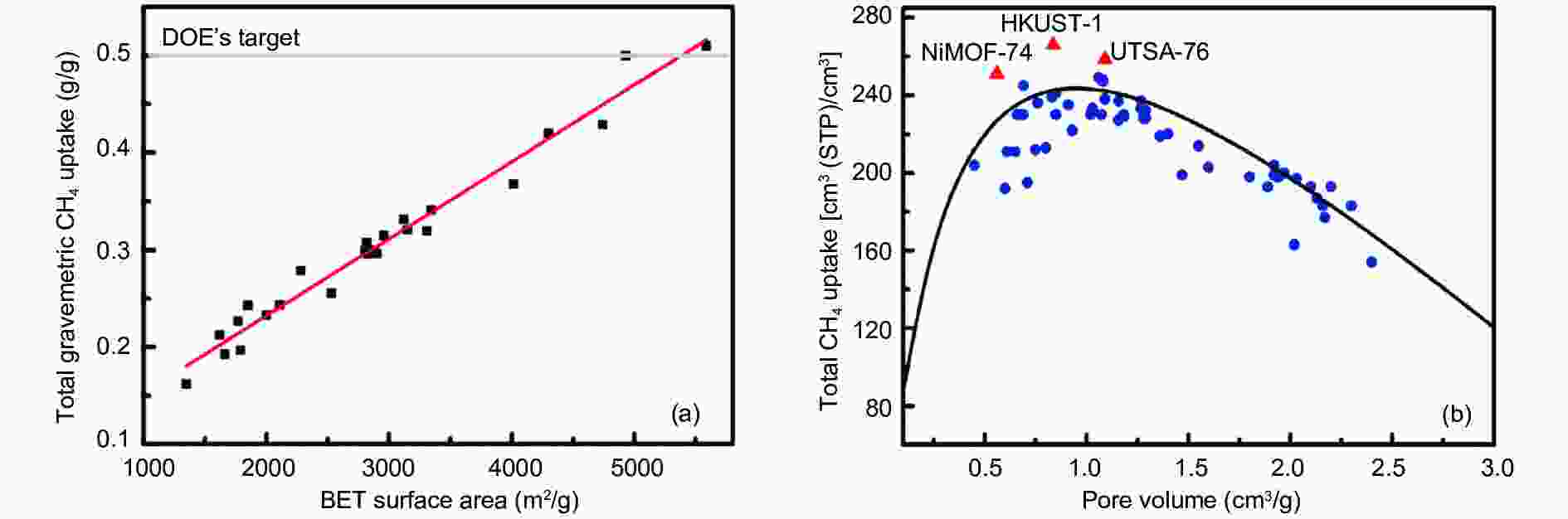
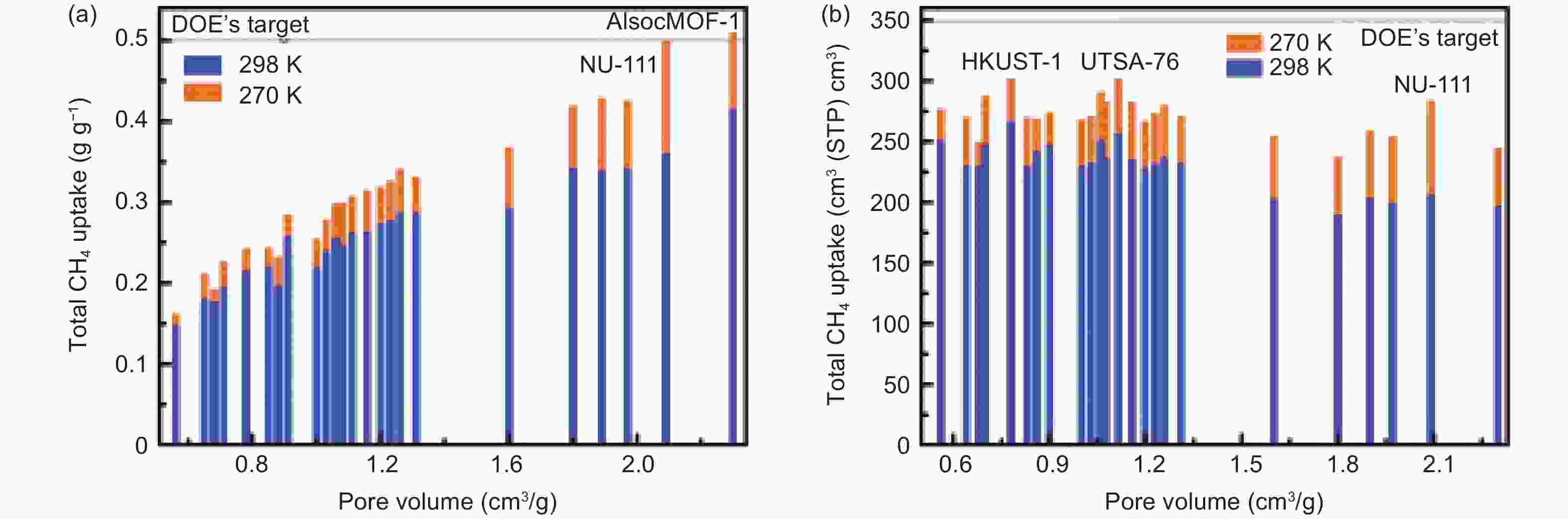
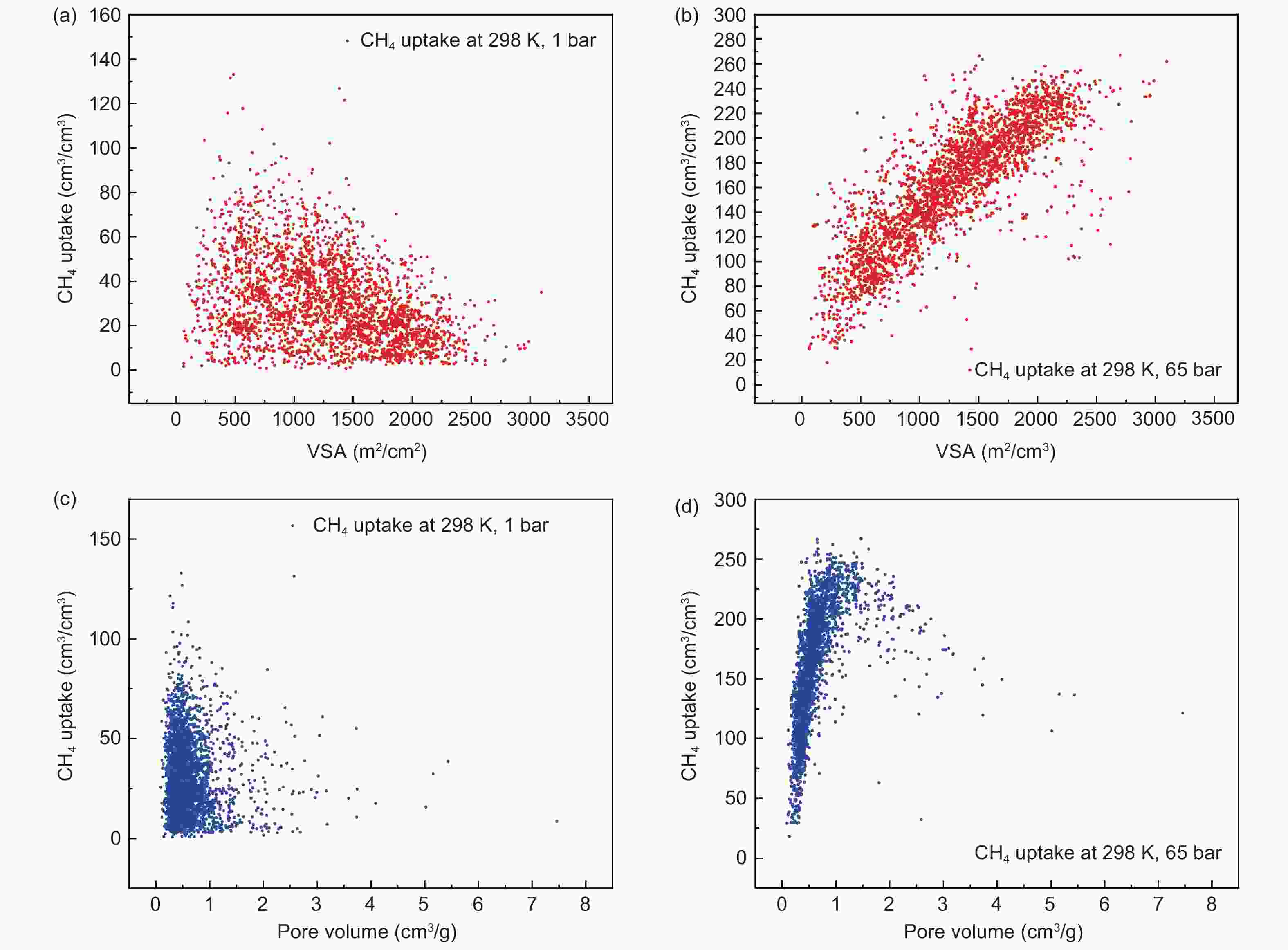
Figure 10. (a) Relationship between gravimetric uptake and BET specific surface area at 270 K; (b) Relationship between volumetric uptake and pore volume at 298 K[7]. Reproduced with permission.
Figure 11. Schematic representation of monolithic and powder MOF synthesis[92]. Reproduced with permission.
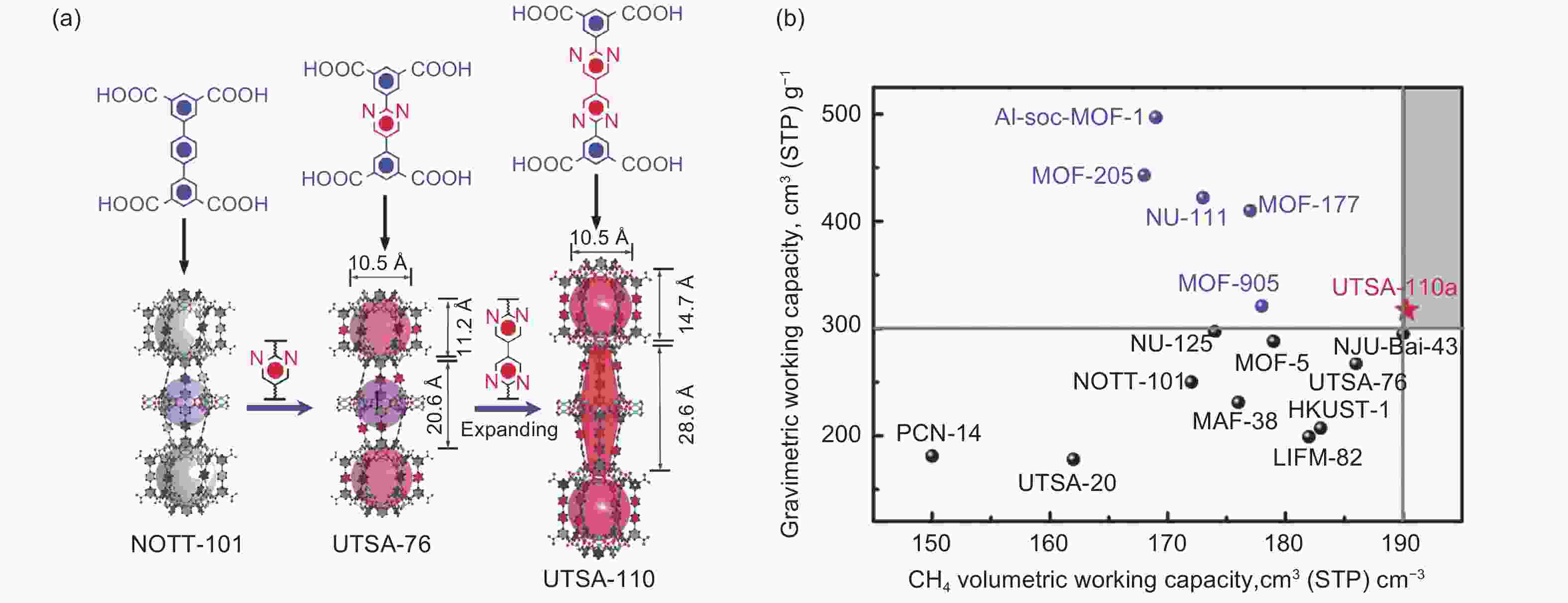
Figure 12. (a) Crystal structure of NOTT-101, UTSA-76 and UTSA-110; (b) Comparison of USTA-110 methane gravimetric/volumetric uptake with other MOFs at 298 K and 65 bar[94]. Reproduced with permission.
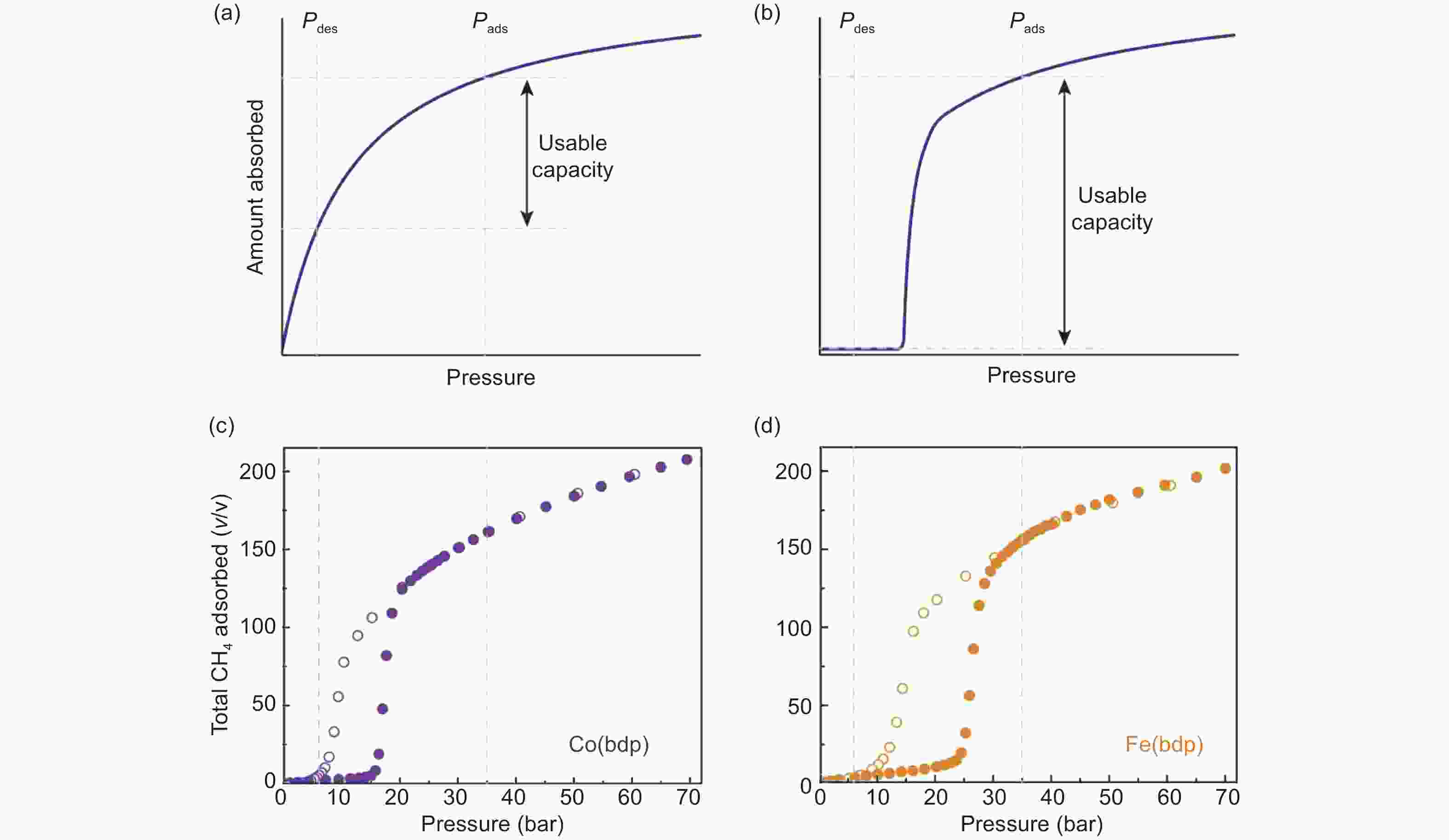
Figure 13. Comparison of deliverable capacity between (a) rigid MOFs and (b) flexible MOFs; Total CH4 uptake of (c) Co (bdp) and (d) Fe (bdp) at 25 ℃[96]. Reproduced with permission.
Figure 14. The (a) primary (green), (b) secondary (black), and (c) ternary (orange) CH4 adsorption sites in MAF-38[97]. Reproduced with permission.
Figure 15. Comparison of gravimetric/volumetric absorption of MOFs at 270 K and 298 K[7]. Reproduced with permission.
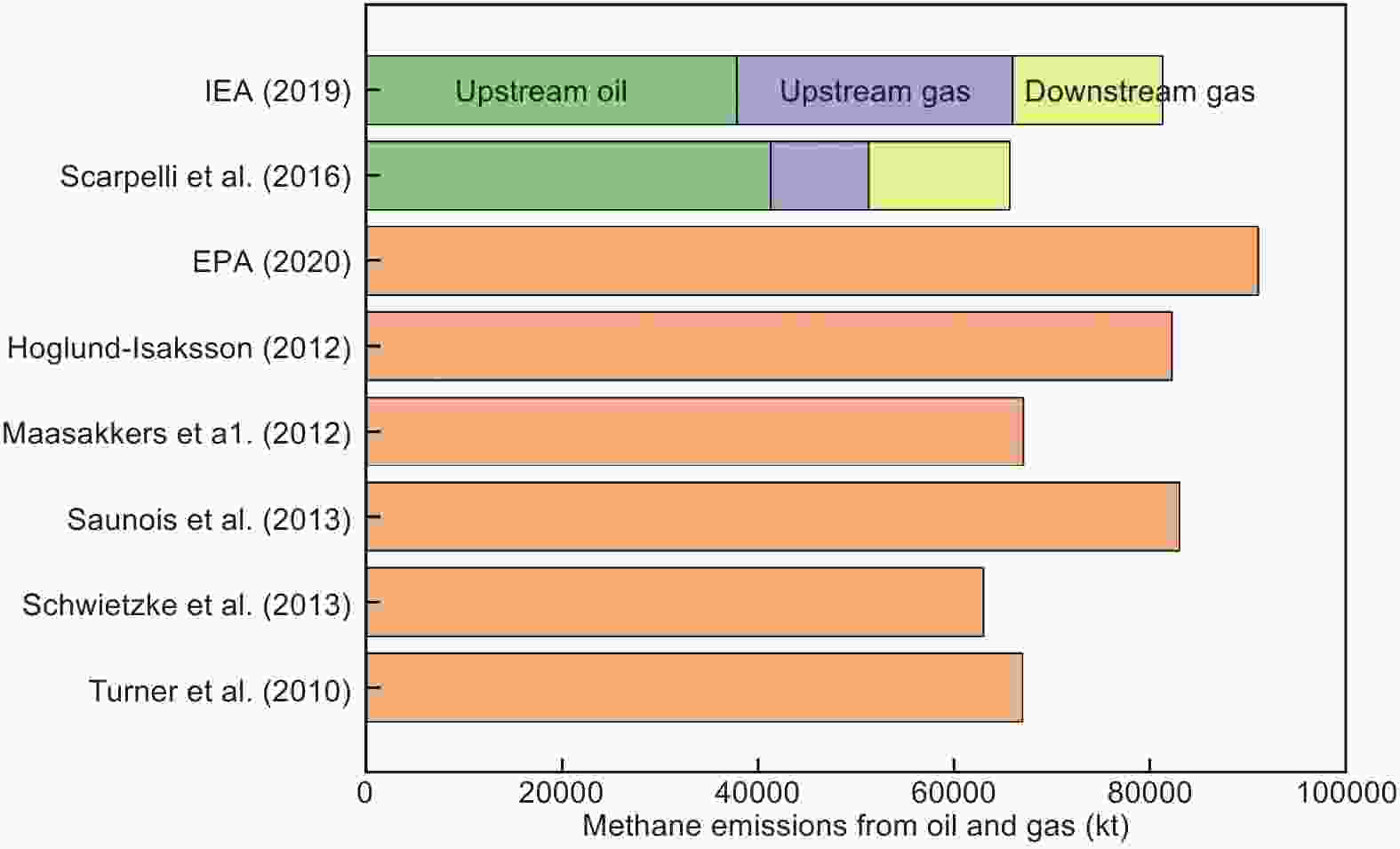
Figure 16. Methane emissions from oil and gas, comparison of IEA and other estimates[118].
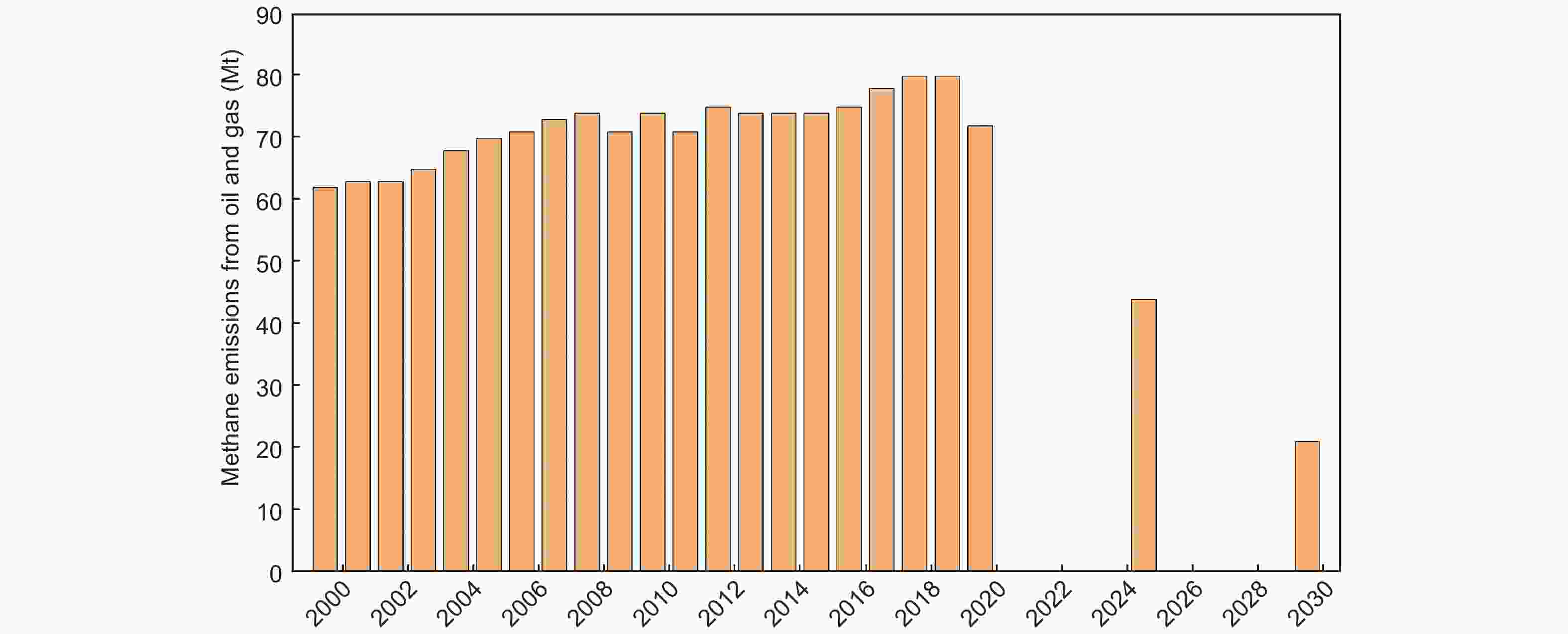
Figure 17. Oil and gas sector methane emissions 2000-2030 in the Sustainable Development Scenario[119].
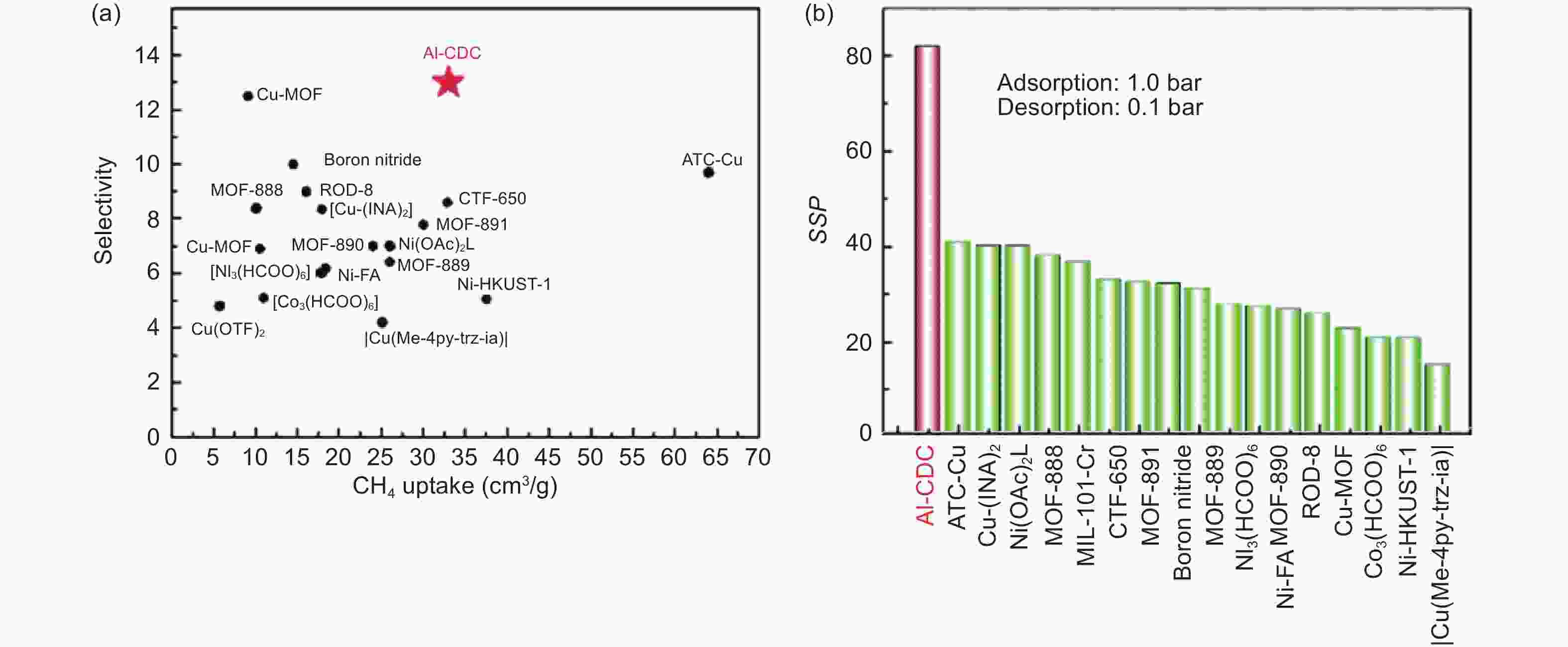
Figure 18. (a,b) Comparison of CH4/N2 separation performance of some MOFs summarized by Liu[161]. Reproduced with permission.
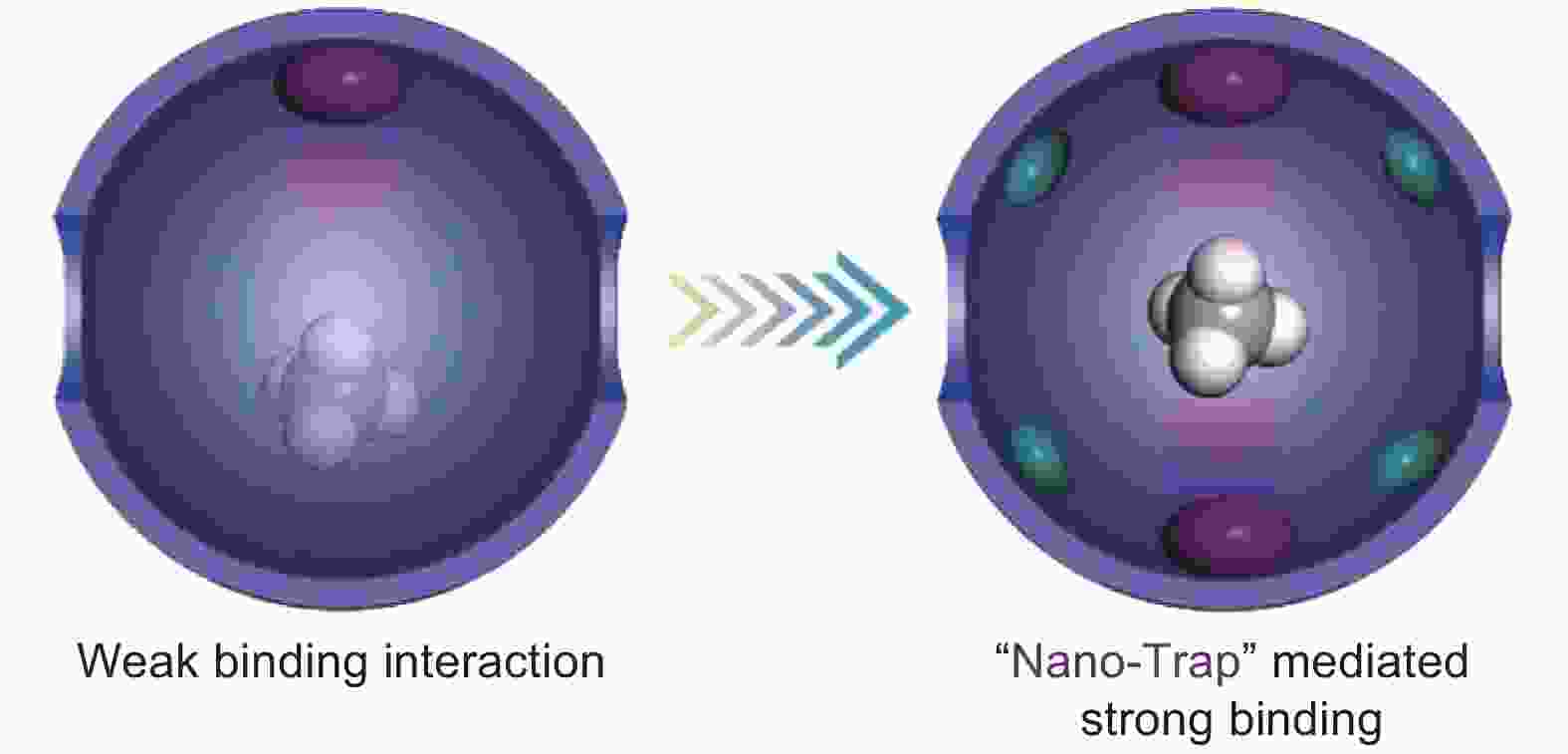
Figure 19. The comparison of traditional methane adsorbent and nano-trap. The purple and green ellipsoids represent coordinatively unsaturated metal centers and alkyl groups, respectively[159]. Reproduced with permission.
Table 1. Examples of MOF materials for methane storage.
Materials BET(m2 g−1) Vp(cm3 g−1) CH4 total uptake CH4 excess uptake(mg g−1) Delivery(cm3 cm−3) mg g−1 cm3 cm−3 T(K) P(bar) mg g−1 cm3 cm−3 T(K) P(bar) Al-soc-MOF-1[83] 5590 2.30 410 197 298 65 - - - - 201(5–80 bar) Co(bdp)[96] 2911(Langmuir) - - 203 298 65 - - - - 197 HKUST-1[100-101] 1850 0.78 216 267 298 65 178 220 298 65 190 monoHKUST-1[92] 1193 0.52 177 267 298 69 151 227 298 69 172 LIFM-82[102] 1624 0.71 210 271 298 80 - - - - 217(5–80 bar) MAF-38[97] 2022 - 247 263 298 65 - - - - 187 MFM-115a[103] 3394 1.38 278 238 298 65 - - - - 191 MIL-53(Al)[104] 1100 0.59 ≥96 155 304 35 - - - - MIL-53(Cr)[104] 1100 0.56 ≥96 165 304 35 - - - - - MIL-10[p0(Cr)[105] 1900 1.10 152 150 303 60 - - - - - MIL-101(Cr)[105-106] 4230 2.15 217.6 135 303 60 - - - - - MOF-5[107] 3800 1.55 - 132 298 35 ~160[108] - 300 60 - MOF-177[95] 4700 1.83 - 208 298 80 - - - - 185(5–80 bar) MOF-200[80] 4530 3.59 - - - - 234 - 298 80 - MOF-210[80] 6240 3.6 - - - - 264 - 298 80 157(5–80 bar) MOF-519[84] 2400 0.928 190 259 298 65 - - - - 230(5–80 bar) MOF-905[95] 3490 1.34 270 206 298 65 - - - - 203(5–80 bar) MOF-905-Naph[95] 3640 1.39 - 211 298 80 - - - - 184(5–80 bar) MOF-905-Me2[95] 3310 1.25 - 217 298 80 - - - - 188(5–80 bar) MOF-905-NO2[95] 3380 1.29 - 203 298 80 - - - - 177(5–80 bar) MOF-950[95] 3440 1.30 - 209 398 80 - - - - 174(5–80 bar) Ni-MOF-74[91, 100] 1350 0.51 148 251 298 65 125 210 298 65 129 NJU-Bai43[109] 3090 1.22 283 254 298 65 - - - - 198 NOTT-101a[81, 94] 2805 1.08 247 237 298 65 - - - - 181 NU-111[91, 98] 4930 2.09 360 205 298 65 262 150 298 65 179 NU-125[91] 3120 1.29 287 232 298 65 223 181 298 65 183 NU-135[110] 2600 1.02 219 230 298 65 - - - - 170 NU-1500-Al[99] 3560 1.46 290 200 296 65 - - - - 181(5–80 bar) NU-1501-Al[99] 7310 2.91 410 163 296 65 - - - - 174(5–80 bar) NU-1501-Fe[99] 7140 2.90 400 168 296 65 - - - - 176(5–80 bar) PCN-14[91, 112] 2000 0.85 197 230 298 65 157 183 298 65 157 PCN-66[114] 4000 1.63 - 187 398 65 177.6 110 298 35 152 PCN-68 [115] 5109 2.13 - 187 298 65 185.6 99 298 35 157 UTSA-20[91] 1620 0.66 181 230 298 65 150 191 298 65 170 UTSA-76[82, 113] 2820 1.09 263 257 298 65 - - - - 197 UTSA-110a[94] 3241 1.263 288 241 298 65 - - - - 190 ZIF-8[108] - - ~85 - 300 36 70 - 300 36 - ZJU-70 [114] 1791 0.676 - 211 298 65 - - - - - Table 2. Examples of CH4 adsorption and separation from CH4/N2 (100 kPa).
Materials CH4 total uptake (mmol g−1) CH4/N2 selectivities Temperature (K) Al-CDC[161] 1.44 13.1 298 ATC-Cu[159] 2.90 9.7 298 CAU-8-BPDC[152] 0.85 4.9 298 CAU-21-BPDC[152] 0.99 11.9 298 [Co3(C4O4)2(OH)2][153] 0.41 12.5 298 Co-MA-BPY[151] 0.92 7.2 298 Co-MOF-74[154] 1.91 3.2 298 Cu-MOF[160] 0.64 10.8 298 Mg-MOF-74[154] 1.66 1.5 298 MIL-100(Cr)[154] 0.60 3.0 298 MOF-5[155] 0.13 1.13 298 MOF-177[155] 0.56 4.0 298 Ni-MA-BPY[151] 1.01 7.4 298 [Ni3(HCOO)6][156] 0.82 6.2 298 PCN-222[157] 0.35 4.8 298 V2Cl2.8(btdd)[158] 1.00 27* 298 *represents N2/CH4 selectivity Table 3. Typical examples of CO2/CH4 separation.
Materials CO2 total uptake (mol kg−1) CO2/CH4 selectivities Pressure (kPa) Temperature(K) IITKGP-6[164] 1.67 5.1 100 295 Cu-MOF[165] - 6 100 303 MIL-53(Al)[166] 4.3(3.5bar) 4.10 100–3500 303 UTSA-16[167] 4.2 29.8 200 296 Mg-MOF-74[168] 8.56 105.1 200 296 CuBTC[169] 5.27 7.4 200 296 MIL-101[169] 2.16 9.6 200 296 SIFSIX-2-Cu[170] 1.85 5.3 100 298 SIFSIX-2-Cu-i[170] 5.41 33 100 298 SIFSIX-3-Zn[170] 2.55 231 100 298 -
[1] IEA, Methane from oil & gas-Methane Tracker 2021-Analysis-IEA, Paris. 2021.01. https://www.iea.org/fuels-and-technologies/methane-abatement. [2] Cracknell, Roger F. Gordon, Peter Gubbins, Keith E. Influence of pore geometry on the design of microporous materials for methane storage[J]. The Journal of Physical Chemistry,1993,97(2):494-499. doi: 10.1021/j100104a036 [3] V. C. Menon, S. Komarneni. Porous adsorbents for vehicular natural gas storage: A review[J]. Journal of Porous Materials,1998,5(1):43-58. [4] Jarad A. Mason, Mike Veenstra, Jeffrey R. Long. Evaluating metal–organic frameworks for natural gas storage[J]. Chemical Science,2013,5(1):32-51. [5] Mirian Elizabeth Casco, Manuel Martínez-Escandell, Enrique Gadea-Ramos, et al. High-Pressure methane storage in porous materials: are carbon materials in the pole position?[J]. Chemistry of Materials,2015,27(3):959-964. doi: 10.1021/cm5042524 [6] Wai Soong Loh, Kazi Afzalur Rahman, Anutosh Chakraborty, et al. Improved isotherm data for adsorption of methane on activated carbons[J]. Journal of Chemical & Engineering Data,2010,55(8):2840-2847. [7] Bin Li, Hui-Min Wen, Wei Zhou, et al. Porous metal-organic frameworks: promising materials for methane storage[J]. Chem,2016,1(4):557-580. [8] YG Chung, E Haldoupis, BJ Bucior, et al. Advances, updates, and analytics for the computation-ready, experimental metal–organic framework database: CoRE MOF 2019[J]. Journal of Chemical & Engineering Data,2019,64(12):5985-5998. [9] Furukawa Hiroyasu, Go Yong Bok, Ko Nakeun, et al. Isoreticular expansion of metal-organic frameworks with triangular and square building units and the lowest calculated density for porous crystals[J]. Inorganic chemistry,2011,50(18):9147-9152. [10] Stephen S. -Y. Chui, Samuel M. -F. Lo, Jonathan P. H. Charmant, et al. A chemically functionalizable nanoporous material [cu3(tma)2(h2o)3]n[J]. Science,1999,283(5405):1148-1150. [11] G. Férey, C. Mellot-Draznieks, C. Serre, et al. A chromium terephthalate-based solid with unusually large pore volumes and surface area[J]. Science,2005,309(5743):2040-2042. [12] Young Kwan Park, Sang Beom Choi, Hyunuk Kim, et al. Titelbild: crystal structure and guest uptake of a mesoporous metal–organic framework containing cages of 3.9 and 4.7 nm in diameter[J]. Angewandte Chemie,2007,119(43):8237-8237. [13] Yan Yong, Yang Sihai, Blake Alexander J, et al. A mesoporous metal-organic framework constructed from a nanosized C3-symmetric linker and [Cu24(isophthalate)24] cuboctahedra[J]. Chemical communications (Cambridge, England),2011,47(36):9995-9997. [14] Jie Peng Zhang, Yue Biao Zhang, Jian Bin Lin, et al. ChemInform abstract: metal azolate frameworks: from crystal engineering to functional materials[J]. ChemInform,2012,43(16):1001-1033. [15] Hideki Hayashi, Adrien P. Côté, Hiroyasu Furukawa, et al. Zeolite a imidazolate frameworks[J]. Nature Materials,2007,6(7):501-506. [16] Wang Bo, Côté Adrien P, Furukawa Hiroyasu, et al. Colossal cages in zeolitic imidazolate frameworks as selective carbon dioxide reservoirs[J]. Nature,2008,453(7192):207-211. [17] Banerjee Rahul, Furukawa Hiroyasu, Britt David, et al. Control of pore size and functionality in isoreticular zeolitic imidazolate frameworks and their carbon dioxide selective capture properties[J]. Journal of the American Chemical Society,2009,131(11):3875-3877. [18] R Banerjee, A Phan, B Wang, et al. High-throughput synthesis of zeolitic imidazolate frameworks and application to CO2 capture[J]. Science,2008,319(5865):939-943. doi: 10.1126/science.1152516 [19] Yang Huimin, Zhang Xu, Zhang Guiyang, et al. An alkaline-resistant Ag(i)-anchored pyrazolate-based metal-organic framework for chemical fixation of CO2[J]. Chemical communications (Cambridge, England),2018,54(35):4469-4472. [20] Putu Doddy Sutrisna, Nicholaus Prasetya, Nurul Faiqotul Himma, et al. A mini-review and recent outlooks on the synthesis and applications of zeolite imidazolate framework-8(ZIF-8) membranes on polymeric substrate[J]. Journal of Chemical Technology & Biotechnology,2020,95(11):2767-2774. [21] Yue-Biao Zhang, Hao-Long Zhou, Rui-Biao Lin, et al. Geometry analysis and systematic synthesis of highly porous isoreticular frameworks with a unique topology[J]. Nature Communications,2012,3(1):642. [22] T J Prior, D Bradshaw, S J Teat, et al. Designed layer assembly: a three-dimensional framework with 74% extra-framework volume by connection of infinite two-dimensional sheets[J]. Chemical Communications,2003,4(4):500-501. [23] Kitaura, R., Iwahori, F., Matsuda, R., et al. Rational design and crystal structure determination of a 3-D Metal-organic jungle-gym-like open framework[J]. Inorganic Chemistry,2004,43(21):6522-6524. doi: 10.1021/ic049005d [24] Yating Zhang, Peng Wang, Juan Yang, et al. Fabrication of core-shell nanohybrid derived from iron-based metal-organic framework grappled on nitrogen-doped graphene for oxygen reduction reaction[J]. Chemical Engineering Journal,2020,401:126001. doi: 10.1016/j.cej.2020.126001 [25] Zhang Yating, Wang Peng, Yang Juan, et al. Decorating ZIF-67-derived cobalt-nitrogen doped carbon nanocapsules on 3D carbon frameworks for efficient oxygen reduction and oxygen evolution[J]. Carbon,2021,177:344-356. doi: 10.1016/j.carbon.2021.02.052 [26] Patrick J. Beldon, Dr. László Fábián, Dr. Robin S. Stein, et al. Rapid room-temperature synthesis of zeolitic imidazolate frameworks by using mechanochemistry[J]. Angewandte Chemie International Edition,2010,49(50):9640-9643. [27] Crawford Deborah, Casaban José, Haydon Robert, et al. Synthesis by extrusion: continuous, large-scale preparation of MOFs using little or no solvent[J]. Chemical science,2015,6(3):1645-1649. [28] Patricia Silva, Sergio M. F. Vilela, Joao P. C. Tome, et al. ChemInform abstract: multifunctional metal-organic frameworks: from academia to industrial applications[J]. ChemInform,2015,46(46):6774-6803. [29] Albuquerque G H, Herman G S. Chemically modulated microwave-assisted synthesis of MOF-74(Ni) and preparation of MOF-matrix based membranes for removal of metal ions from aqueous media[J]. Crystal Growth & Design,2016,17(1):156-162. [30] Dreischarf Anna C, Lammert Martin, Stock Norbert, et al. Green synthesis of Zr-CAU-28: structure and properties of the first Zr-MOF based on 2, 5-furandicarboxylic acid[J]. Inorganic chemistry,2017,56(4):2270-2277. [31] Xu Lan Lan, Wang Song, Liu Yu xia, et al. Advance on research and application of MOF prepared by MW method[J]. New Chemical Materials,2019,47(04):1-5. [32] Jhung S H, Lee J H, Chang J S. Microwave synthesis of a nanoporous hybrid material, chromium trimesate[J]. Bulletin of the Korean Chemical Society,2005,26(6):880-883. doi: 10.5012/bkcs.2005.26.6.880 [33] Nesa Esmaeilian Tari, Azadeh Tadjarodi, Javad Tamnanloo, et al. Facile and fast, one pot microwave synthesis of metal organic framework copper terephthalate and study CO2 and CH4 adsorption on it[J]. Journal of Porous Materials,2015,22(5):1161-1169. [34] Hye-Young Cho, Da-Ae Yang, Jun Kim, et al. CO2 adsorption and catalytic application of Co-MOF-74 synthesized by microwave heating[J]. Catalysis Today,2012,185(1):35-40. [35] Palomino Cabello Carlos, Arean Carlos Otero, Parra José B, et al. A rapid microwave-assisted synthesis of a sodium-cadmium metal-organic framework having improved performance as a CO2 adsorbent for CCS[J]. Dalton transactions (Cambridge, England: 2003),2015,44(21):9955-9963. [36] Pradip Sarawade, Hua Tan, Vivek Polshettiwar. Shape-and morphology-controlled sustainable synthesis of Cu, Co, and in metal organic frameworks with high CO2 capture capacity[J]. ACS Sustainable Chemistry & Engineering,2012,1(1):66-74. [37] Bao Zongbi, Yu Liang, Ren Qilong, et al. Adsorption of CO2 and CH4 on a magnesium-based metal organic framework[J]. Journal of colloid and interface science,2011,353(2):549-556. [38] Chandan Dey, Tanay Kundu, Bishnu P. Biswal, et al. Crystalline metal-organic frameworks (MOFs): synthesis, structure and function[J]. Acta Crystallographica Section B,2014,70(1):3-10. [39] U. Mueller, M. Schubert, F. Teich, et al. Metal-organic frameworks—prospective industrial applications[J]. Journal of Materials Chemistry,2006,16(23):626-636. [40] Tom R. C. Van Assche, Gert Desmet, Rob Ameloot, et al. Electrochemical synthesis of thin hkust-1 layers on copper mesh[J]. Microporous and Mesoporous Materials,2012:209-213. [41] Stassen I, Styles M, Assche T V, et al. Electrochemical film deposition of the zirconium metal–organic framework UiO-66 and application in a miniaturized sorbent trap[J]. Chemistry of Materials,2015,27(5):379-391. [42] Christos Vaitsis, Georgia Sourkouni, Christos Argirusis. Metal organic frameworks (MOFs) and ultrasound: a review[J]. Ultrasonics Sonochemistry,2019:52. [43] Suslick K S, Hammerton D A, Cline R E. Sonochemical hot spot[J]. Journal of the American Chemical Society,1986,89(18):5641-5642. [44] Suslick K S. Sonochemistry[J]. Cheminform,1990,247(4949):1439-1445. [45] Qiu Ling-Guang, Li Zong-Qun, Wu Yun, et al. Facile synthesis of nanocrystals of a microporous metal-organic framework by an ultrasonic method and selective sensing of organoamines[J]. Chemical communications (Cambridge, England),2008(31):3642-3644. [46] Son Won-Jin, Kim Jun, Kim Jaheon, et al. Sonochemical synthesis of MOF-5[J]. Chemical communications (Cambridge, England),2008(47):6336-6338. [47] Zong-Qun Li, Ling-Guang Qiu, Wei Wang, et al. Fabrication of nanosheets of a fluorescent metal-organic framework [zn(bdc)(h2o)]n(bdc-1,4-benzenedicarboxylate): ultrasonic synthesis and sensing of ethylamine[J]. Inorganic Chemistry Communications,2008,11(11):1375-1377. [48] Cohen Seth M. Postsynthetic methods for the functionalization of metal-organic frameworks[J]. Chemical reviews,2012,112(2):970-1000. [49] Cai Y, Zhang Y, Huang Y, et al. Impact of alkyl-functionalized btc on properties of copper-based metal-organic frameworks[J]. Crystal Growth & Design,2012,12(7):3709-3713. [50] Yichao Lin, Chunglong Kong, Qiuju Zhang, et al. Metal-organic frameworks for carbon dioxide capture and methane storage[J]. Advanced Energy Materials,2017,7(4):1601296. [51] Christopher E. Wilmer, Michael Leaf, Chang Yeon Lee, et al. Large-scale screening of hypothetical metal-organic frameworks[J]. Nature Chemistry,2012,4(2):83-89. [52] Martin Richard L, Simon Cory M, Smit Berend, et al. In silico design of porous polymer networks: high-throughput screening for methane storage materials[J]. Journal of the American Chemical Society,2014,136(13):5006-5022. [53] Simon C M, Kim J, Gomez-Gualdron D A, et al. The materials genome in action: identifying the performance limits for methane storage[J]. Energy & Environmental Science,2015,8(4):1190-1199. [54] Simon C M, Kim J, Lin L C, et al. Optimizing nanoporous materials for gas storage[J]. Physical Chemistry Chemical Physics Pccp,2014,16(12):5499-5513. doi: 10.1039/c3cp55039g [55] YJ Colón, Fairen-Jimenez D, Wilmer C E, et al. High-throughput screening of porous crystalline materials for hydrogen storage capacity near room temperature[J]. The Journal of Physical Chemistry C,2016,118(10):5383-5389. [56] Bobbitt N S, Chen J, Snurr R Q. High-throughput screening of metal-organic frameworks for hydrogen storage at cryogenic temperature[J]. Journal of Physical Chemistry C,2016,120(48):27328-27341. [57] Gomez-Gualdron D, YJ Colón, Zhang X, et al. Evaluating topologically diverse metal–organic frameworks for cryo-adsorbed hydrogen storage[J]. Energy & Environmental Science,2016,9(10):3279-3289. doi: 10.1039.C6EE02104B [58] Han S, Huang Y, Watanabe T, et al. High-throughput screening of metal-organic frameworks for CO2 separation[J]. ACS Combinatorial Science,2012,14(4):263-267. doi: 10.1021/co3000192 [59] Yongchul G. Chung, Diego A. Gómez-Gualdrón, Peng Li, et al. In silico discovery of metal-organic frameworks for precombustion CO2 capture using a genetic algorithm[J]. Science Advances,2016,2(10):e1600909-e1600909. [60] Simon C M, Mercado R, Schnell S K, et al. What are the best materials to separate a xenon/krypton mixture?[J]. Chemistry of Materials,2015,27(12):4459-4475. [61] Curtarolo S, Hart G L W, Nardelli M B, et al. The high-throughput highway to computational materials design[J]. Nature Materials,2013,12(3):191-201. doi: 10.1038/nmat3568 [62] Canepa P, Arter C A, Conwill E M, et al. High-throughput screening of small-molecule adsorption in MOF[J]. Journal of Materials Chemistry A,2013,1:13597-13604. doi: 10.1039/c3ta12395b [63] Wollmann Philipp, Leistner Matthias, Stoeck Ulrich, et al. High-throughput screening: speeding up porous materials discovery[J]. Chemical communications (Cambridge, England),2011,47(18):5151-5153. [64] Gee J A, Zhang K, Bhattacharyya S, et al. Computational identification and experimental evaluationof metal–organic frameworks for xylene enrichment[J]. Journal of Physical Chemistry C,2016,120(22):12075-12082. [65] Demir H, Walton K S, Sholl D S. Computational screening of functionalized UiO-66 materials for selective contaminant removal from air[J]. The Journal of Physical Chemistry C,2017,121(37):20396-20406. [66] Christopher, E, WHmer, et al. Structure-property relationships of porous materials for carbon dioxide separation and capture[J]. Energy & environmental science: EES,2012,5(12):9849-9856. [67] Fernandez M, Barnard A S. Geometrical properties can predict CO2 and N2 adsorption performance of metal-organic frameworks (MOFs) at low pressure[J]. acs combinatorial science,2016,18(5):243-252. doi: 10.1021/acscombsci.5b00188 [68] Fernandez M, Woo T K, Wilmer C E, et al. Large-scale quantitative structure–property relationship (qspr) analysis of methane storage in metal–organic frameworks[J]. The Journal of Physical Chemistry C,2013,117(15):7681-7689. doi: 10.1021/jp4006422 [69] Braun E, AF Zurhelle, Thijssen W, et al. High-throughput computational screening of nanoporous adsorbents for CO2 capture from natural gas[J]. Molecular Systems Design & Engineering,2016,1:175-188. [70] Pardakhti Maryam, Moharreri Ehsan, Wanik David, et al. Machine learning using combined structural and chemical descriptors for prediction of methane adsorption performance of metal organic frameworks (MOFs)[J]. ACS Combinatorial Science,2017,19(10):640-645. [71] Fanourgakis G S, K Gkagkas, Tylianakis E, et al. A generic machine learning algorithm for the prediction of gas adsorption in nanoporous materials[J]. The Journal of Physical Chemistry C,2019,123(28):6080-6087. doi: 10.1021/acs.jpca.9b03290 [72] Hui W, Simmons J, Yun L, et al. Metal-organic frameworks with exceptionally high methane uptake: where and how is methane stored?[J]. Chemistry,2010,16(17):5205-5214. doi: 10.1002/chem.200902719 [73] Tsivion E, Mason J A, Gonzalez M, et al. A computational study of CH4 storage in porous framework materials with metalated linkers: connecting the atomistic character of CH4 binding sites to usable capacity[J]. Chem Sci,2016,7(7):4503-4518. [74] Czaja A, Trukhan N, Muller U. Industrial applications of metal-organic frameworks[J]. Chemical Society Reviews,2009,38(5):1284-1293. doi: 10.1039/b804680h [75] He Y, Zhou W, Qian G, et al. Methane storage in metal–organic frameworks[J]. Chemical Society Reviews,2014,43(16):5657-5678. doi: 10.1039/C4CS00032C [76] Kondo M, Yoshitomi T, Matsuzaka H, et al. Three-dimensional framework with channeling cavities for small molecules: {[m2(4,4’-bpy)3(no3)4]·xh2On (m co,ni,zn)[J]. Angewandte Chemie International Edition in English,1997,36(16):1725-1727. [77] Li H L, Eddaoudi M M, O'Keeffe M, et al. Design and synthesis of an exceptionally stable and highly porous metal-organic framework[J]. Nature,1999,402(6759):276-279. doi: 10.1038/46248 [78] Eddaoudi, M. Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage[J]. Science,2002,295(5554):469-472. doi: 10.1126/science.1067208 [79] Chae H K, Siberio-Perez D Y, Kim J, et al. A route to high surface area, porosity and inclusion of large molecules in crystals[J]. Nature,2004,427(6974):523-527. doi: 10.1038/nature02311 [80] Furukawa Hiroyasu, Ko Nakeun, Go Yong Bok, et al. Ultrahigh porosity in metal-organic frameworks[J]. Science (New York, N. Y.),2010,329(5990):424-428. doi: 10.1126/science.1192160 [81] He Y, Wei Z, Yildirim T, et al. A series of metal–organic frameworks with high methane uptake and an empirical equation for predicting methane storage capacity[J]. Energy & Environmental Science,2013,6(9):2735-2744. [82] Li B, Wen H M, Wang H, et al. A porous metal-organic framework with dynamic pyrimidine groups exhibiting record high methane storage working capacity[J]. Journal of the American Chemical Society,2014,136(17):6207-6210. doi: 10.1021/ja501810r [83] Alezi D, Belmabkhout Y, Suyetin M, et al. MOF crystal chemistry paving the way to gas storage needs: aluminum-based soc-MOF for CH4, O2, and CO2 storage[J]. Journal of the American Chemical Society,2015,137(41):13308-13318. doi: 10.1021/jacs.5b07053 [84] F Gándara, Furukawa H, Lee S, et al. High methane storage capacity in aluminum metal-organic frameworks[J]. Journal of the American Chemical Society,2014,136(14):5271-5274. doi: 10.1021/ja501606h [85] Rosi N L, Kim J, Eddaoudi M, et al. Rod packings and metal-organic frameworks constructed from rod-shaped secondary building units[J]. Journal of the American Chemical Society,2005,127(5):1504-1518. doi: 10.1021/ja045123o [86] Ma S, Sun D, Simmons J M, et al. Metal-organic framework from an anthracene derivative containing nanoscopic cages exhibiting high methane uptake[J]. Journal of the American Chemical Society,2008,130(3):1012-1016. doi: 10.1021/ja0771639 [87] Zheng B, D Tian, Zhang L, et al. Investigation of methane adsorption in strained IRMOF-1[J]. The Journal of Physical Chemistry C,2019,123(40):24592-24597. doi: 10.1021/acs.jpcc.9b06960 [88] Kong G, Han Z, He Y, et al. Expanded organic building units for the construction of highly porous metal–organic frameworks (pages 14886–14894)[J]. Hokkai-Gakuen University, The Journal of Economics,2006,54(44):35-52. [89] Purewal J J, Liu D, Yang J, et al. Increased volumetric hydrogen uptake of MOF-5 by powder densification[J]. International Journal of Hydrogen Energy,2012,37(3):2723-2727. doi: 10.1016/j.ijhydene.2011.03.002 [90] Hui W, Yildirim T, Zhou W. Exceptional mechanical stability of highly porous zirconium metal–organic framework uio-66 and its important implications[J]. Journal of Physical Chemistry Letters,2013,4(6):925-930. doi: 10.1021/jz4002345 [91] Yang P, Krungleviciute V, Eryazici I, et al. Methane storage in metal-organic frameworks: current records, surprise findings, and challenges[J]. Journal of the American Chemical Society,2013,135(32):11887-11894. doi: 10.1021/ja4045289 [92] Tian T, Zeng Z, Vulpe D, et al. A sol–gel monolithic metal–organic framework with enhanced methane uptake[J]. Nature Materials,2018,17(2):174-179. doi: 10.1038/nmat5050 [93] Jeong N C, Samanta B, Chang Y L, et al. Coordination-chemistry control of proton conductivity in the iconic metal-organic framework material hkust-1[J]. Journal of the American Chemical Society,2012,134(1):51-54. doi: 10.1021/ja2110152 [94] HuiMin Wen, Bin Li, Libo Li, et al. A metal-organic framework with optimized porosity and functional sites for high gravimetric and volumetric methane storage working capacities[J]. Advanced Materials,2018,30(16):1704792-1704792. [95] Jiang J, Furukawa H, Zhang Y B, et al. high methane storage working capacity in metal-organic frameworks with acrylate links[J]. Journal of the American Chemical Society,2016,138(32):10244-10251. doi: 10.1021/jacs.6b05261 [96] Mason J A, Oktawiec J, Taylor M K, et al. Methane storage in flexible metal-organic frameworks with intrinsic thermal management[J]. Nature,2015,527(7578):357-361. doi: 10.1038/nature15732 [97] Jiao-Min, Lin, ChunTing, et al. A Metal-organic framework with a pore size/shape suitable for strong binding and close packing of methane[J]. Angewandte Chemie,2015,55(15):4674-4678. [98] Yang P, Srinivas G, Wilmer C E, et al. Simultaneously high gravimetric and volumetric methane uptake characteristics of the metal–organic framework NU-111[J]. Chemical Communications,2013,49(29):2992-2994. doi: 10.1039/c3cc40819a [99] Chen Z, Li P, Anderson R, et al. Balancing volumetric and gravimetric uptake in highly porous materials for clean energy[J]. Science,2020,368(6488):297-303. doi: 10.1126/science.aaz8881 [100] Mason J A, Veenstra M, Long J R. ChemInform abstract: evaluating metal-organic frameworks for natural gas storage[J]. Cheminform,2014,45(16):32-51. [101] H Li, Li L, Lin R B, et al. Porous Metal-organic frameworks for gas storage and separation: status and challenges[J]. EnergyChem,2019,1(1):10006-10006. [102] Chen Cheng-Xia, Wei Zhang-Wen, Jiang Ji-Jun, et al. Dynamic spacer installation for multirole metal-organic frameworks: a new direction toward multifunctional MOFs achieving ultrahigh methane storage working capacity[J]. Journal of the American Chemical Society,2017,139(17):6034-6037. [103] Yan Y, Kolokolov D I, Silva I D, et al. Porous metal–organic polyhedral frameworks with optimal molecular dynamics and pore geometry for methane storage[J]. Journal of the American Chemical Society,2017,139(38):13349-13360. doi: 10.1021/jacs.7b05453 [104] Bourrelly S, Llewellyn P L, Serre C, et al. Different adsorption behaviors of methane and carbon dioxide in the isotypic nanoporous metal terephthalates MIL-53 and MIL-47[J]. Journal of the American Chemical Society,2005,127(39):13519-13521. doi: 10.1021/ja054668v [105] Llewellyn Philip L, Bourrelly Sandrine, Serre Christian, et al. High uptakes of CO2 and CH4 in mesoporous metal organic frameworks MIL-100 and MIL-101[J]. Langmuir,2008,24(14):7245-7250. doi: 10.1021/la800227x [106] Hong D Y, Hwang Y K, Serre C, et al. Porous chromium terephthalate MIL‐101 with coordinatively unsaturated sites: surface functionalization, encapsulation, sorption and catalysis[J]. Advanced Functional Materials,2010,19(10):1537-1552. [107] Rosi, N. L. Hydrogen storage in microporous metal-organic frameworks[J]. Science,2003,300(5622):1127-1129. doi: 10.1126/science.1083440 [108] Zhou W, Wu H, Hartman M R, et al. Hydrogen and methane adsorption in metal-organic frameworks: A high-pressure volumetric study[J]. The Journal of Physical Chemistry C,2007,111(44):16131-16137. doi: 10.1021/jp074889i [109] J Bai, Zhang M, Zhou W, et al. Fine tuning of MOF‐505 analogues to reduce low-pressure methane uptake and enhance methane working capacity[J]. Angewandte Chemie,2017,129(38):11584-11588. doi: 10.1002/ange.201704974 [110] R. D. Kennedy, V. Krungleviciute, D. J. Clingerman. Carborane-based metal-organic framework with high methane and hydrogen storage capacities[J]. Chemistry of Materials,2013,25(17):3539-3543. doi: 10.1021/cm4020942 [111] Dan Z, Yuan D, Sun D, et al. Stabilization of metal-organic frameworks with high surface areas by the incorporation of mesocavities with microwindows[J]. Journal of the American Chemical Society,2009,131(26):9186-9188. doi: 10.1021/ja901109t [112] D Yuan, Dan Z, D Sun, et al. An isoreticular series of metal–organic frameworks with dendritic hexacarboxylate ligands and exceptionally high gas-uptake capacity[J]. Angewandte Chemie International Edition,2010,49(31):5357-5361. doi: 10.1002/anie.201001009 [113] Bin Li, Huimin Wen, Hailong Wang, et al. Porous metal-organic frameworks with Lewis basic nitrogen sites for high-capacity methane storage[J]. Energy & Environmental Science,2015,8(8):2504-2511. [114] Duan X, Wu C, Xiang S, et al. Novel microporous metal–organic framework exhibiting high acetylene and methane storage capacities[J]. Inorganic Chemistry,2015,54(9):4377-4381. doi: 10.1021/acs.inorgchem.5b00194 [115] C. Özgen Karacan, Felicia A. Ruiz, Michael Cotè, et al. Coal mine methane: A review of capture and utilization practices with benefits to mining safety and to greenhouse gas reduction[J]. International Journal of Coal Geology,2011,86(2-3):121-156. doi: 10.1016/j.coal.2011.02.009 [116] Bongaarts J. Intergovernmental panel on climate change special report on global warming of 1.5°C switzerland: IPCC, 2018[J]. Population and Development Review,2019,45(1):251-252. doi: 10.1111/padr.12234 [117] Ramón A. Alvarez, Daniel Zavala-Araiza, David R. Lyon, et al. Assessment of methane emissions from the U. S. oil and gas supply chain[J]. Science,2018,361(6398):186-188. [118] IEA, Methane emissions from oil and gas, comparison of IEA and other estimates[Z]. IEA, Paris. 2020.08. 30 https://www.iea.org/data-and-statistics/charts/methane-emissions-from-oil-and-gas-comparison-of-iea-and-other-estimates. [119] IEA, Oil and gas sector methane emissions, historical and in the sustainable development scenario, 2000-2030[Z], IEA, Paris. https://www.iea.org/data-and-statistics/charts/oil-and-gas-sector-methane-emissions-historical-and-in-the-sustainable-development-scenario-2000-2030 [120] Melse R, Aw V D W. Biofiltration for mitigation of methane emission from animal husbandry[J]. Environmental Science & Technology,2005,39(14):5460-5468. [121] Girard M, Ramirez A A, Buelna G, et al. Biofiltration of methane at low concentrations representative of the piggery industry-Influence of the methane and nitrogen concentrations[J]. Chemical Engineering Journal,2011,168(1):151-158. doi: 10.1016/j.cej.2010.12.054 [122] Stuart, A, Dever, et al. Passive drainage and biofiltration of landfill gas: results of australian field trial[J]. Waste Management,2011,31(5):1029-1048. doi: 10.1016/j.wasman.2010.10.026 [123] Gebert J, Groengroeft A. Performance of a passively vented field-scale biofilter for the microbial oxidation of landfill methane[J]. Waste Manag,2006,26(4):399-407. doi: 10.1016/j.wasman.2005.11.007 [124] Nikiema J, Brzezinski R, Heitz M. Elimination of methane generated from landfills by biofiltration: a review[J]. Reviews in Environmental Science & Bio/technology,2007,6(4):261-284. [125] Berger J, Fornes L V, Ott C. Methane oxidation in a landfill cover with capillary barrier[J]. Waste Manag,2005,25(4):369-373. doi: 10.1016/j.wasman.2005.02.005 [126] Du P C, Strauss J M, Emt S, et al. Empirical model for methane oxidation using a composted pine bark biofilter[J]. Fuel,2003,82(11):1359-1365. doi: 10.1016/S0016-2361(03)00040-1 [127] Climate and Clean Air Coalition. CCAC O&G methane partnership: technical guidance document[R]. Paris, France, 2017. [128] Shindell D, Fuglestvedt J S, Collins W J. The social cost of methane: theory and applications[J]. Faraday Discussions,2017,200:429-451. doi: 10.1039/C7FD00009J [129] Chaffee A L, Knowles G P, Liang Z, et al. CO capture by adsorption: Materials and process development[J]. international journal of greenhouse gas control,2007,1(1):11-188. doi: 10.1016/S1750-5836(07)00031-X [130] Mcdonald T M, Lee W R, Mason J A, et al. Capture of carbon dioxide from air and flue gas in the alkylamine-appended metal-organic framework mmen-Mg2(dobpdc)[J]. Journal of the American Chemical Society,2012,134(16):7056-7065. doi: 10.1021/ja300034j [131] Moon S H, Shim J W. A novel process for CO2/CH4 gas separation on activated carbon fibers--electric swing adsorption[J]. J Colloid Interface,2006,298(2):523-528. doi: 10.1016/j.jcis.2005.12.052 [132] Plaza M G, S García, Rubiera F, et al. Post-combustion CO2 capture with a commercial activated carbon: Comparison of different regeneration strategies[J]. Chemical Engineering Journal,2010,163(1–2):41-47. [133] Kim J, Maiti A, Lin L C, et al. New materials for methane capture from dilute and medium-concentration sources[J]. Nature Communications,2013,4:1694. doi: 10.1038/ncomms2697 [134] Myrsini K. Antoniou, Evmorfia K. Diamanti, Apostolos Enotiadis, et al. Methane storage in zeolite-like carbon materials[J]. Microporous and Mesoporous Materials,2014,188:16-22. doi: 10.1016/j.micromeso.2013.12.030 [135] Bae J S, Su S, Yu X X. Enrichment of ventilation air methane (VAM) with carbon fiber composites[J]. Environmental Science & Technology,2014,48(10):6043-6049. [136] Beckner M, Dailly A. A pilot study of activated carbon and metal–organic frameworks for methane storage[J]. Applied Energy,2016,162(JAN. 15):506-514. [137] J Sreńscek-Nazzal, W Kamińska, Michalkiewicz B, et al. Production, characterization and methane storage potential of KOH-activated carbon from sugarcane molasses[J]. Industrial Crops & Products,2013,47:153-159. [138] Ali, Bakhtyari, Masoud, et al. Pure and binary adsorption equilibria of methane and nitrogen on zeolite 5A[J]. Journal of Chemical & Engineering Data,2014,59(3):626-639. [139] Makal T A, Li J R, Lu W, et al. Methane storage in advanced porous materials[J]. Chemical Society Reviews,2012,41(23):7761-7779. doi: 10.1039/c2cs35251f [140] Baudot A. CH4/N2 Separation[M]. 2016. [141] Kuznicki S M, Bell V A, Nair S, et al. A titanosilicate molecular sieve with adjustable pores for size-selective adsorption of molecules[J]. Nature,2001,412(6856):720-724. [142] Yang, Ralph T. Adsorbents: fundamentals and applications[J]. Belgeler Com,2003,2004:xii,404. [143] Jayaraman A, Hernandez-Maldonado A J, Yang R T, et al. Clinoptilolites for nitrogen/methane separation[J]. Chemical Engineering Science,2004,59(12):2407-2417. doi: 10.1016/j.ces.2003.10.030 [144] Liu, Xiao-Wei, Hu, Jiang-Liang, Sun, Tian-Jun, et al. Template-based synthesis of a formate metal-organic framework/activated carbon fiber composite for high-performance methane adsorptive separation[J]. Chemistry-An Asian Journal,2016,11(21):3014-3017. doi: 10.1002/asia.201601134 [145] Yang, R. Principle and application of adsorbent [M]. Higher Education Press, 2010.. [146] Rege S, Yang R. A simple parameter for selecting an adsorbent for gas separation by pressure swing adsorption[J]. Separation Science & Technology,2001,36(15):3355-3365. [147] Bae Y S, Snurr R Q. Development and evaluation of porous materials for carbon dioxide separation and capture[J]. Cheminform,2011,50(49):11586-11596. [148] Li Q, Ruan M, Lin B, et al. Molecular simulation study of metal organic frameworks for methane capture from low-concentration coal mine methane gas[J]. Journal of Porous Materials,2016,23(1):107-122. doi: 10.1007/s10934-015-0060-4 [149] Sumer Z, Keskin S. Adsorption- and membrane-based CH4/N2 separation performances of MOFs[J]. Industrial & Engineering Chemistry Research,2017,56(30):8713-8722. [150] Liu X, Guo Y, Tao A, et al. "Explosive" synthesis of metal-formate frameworks for methane capture: an experimental and computational study[J]. Chem Commun,2017,53(83):11437-11440. doi: 10.1039/C7CC06249D [151] Liu X W, Gu Y, Sun T J, et al. Water resistant and flexible MOF materials for highly-efficient separation of methane from nitrogen[J]. Industrial & Engineering Chemistry Research,2019,58(44):20392-20400. [152] Daofei Lv, Ying Wu, Jiayu Chen, et al. Improving CH4/N2 selectivity within isomeric Al-based MOFs for the highly selective capture of coal-mine methane[J]. AIChE Journal,2020,66(9):e16287-e16287. [153] Liangying Li, Lifeng Yang, Jiawei Wang, et al. Highly efficient separation of methane from nitrogen on a squarate-based metal-organic framework[J]. AIChE Journal,2018,64(10):3681-3689. [154] Li L, Yang J, Li J, et al. Separation of CO2/CH4 and CH4/N2 mixtures by M/DOBDC: A detailed dynamic comparison with MIL-100(Cr) and activated carbon[J]. Microporous & Mesoporous Materials,2014,198:236-246. [155] Saha D, Bao Z, Jia F, et al. Adsorption of CO2, CH4, N2O, and N2 on MOF-5, MOF-177, and zeolite 5A[J]. Environmental Science & Technology,2017,44(5):1820-1826. [156] Ren X, Sun T, Hu J, et al. Highly enhanced selectivity for the separation of CH4 over N2 on two ultra-microporous frameworks with multiple coordination modes[J]. Microporous & Mesoporous Materials,2014,186:137-145. [157] Lv D, Shi R, Chen Y, et al. Selective adsorptive separation of CO2/CH4 and CO2/N2 by a water resistant zirconium-porphyrin metal-organic framework[J]. Industrial & Engineering Chemistry Research,2018,57(36):12215-12224. [158] David E. Jaramillo, Douglas A. Reed, Henry Z. H. Jiang, et al. Selective nitrogen adsorption via backbonding in a metal-organic framework with exposed vanadium sites[J]. Nature Materials,2020,19(5):517-521. [159] Niu Z, Cui X, Pham T, et al. A metal-organic framework based methane nano-trap for the capture of coal-mine methane[J]. Angewandte Chemie International Edition,2019,58(30):10138-10141. doi: 10.1002/anie.201904507 [160] Chang M, Zhao Y, Yang Q, et al. Microporous metal–organic frameworks with hydrophilic and hydrophobic pores for efficient separation of CH4/N2 mixture[J]. Acs Omega,2019,4(11):14511-14516. doi: 10.1021/acsomega.9b01740 [161] Chang M, Zhao Y, Liu D, et al. Methane-trapping metal–organic frameworks with an aliphatic ligand for efficient CH4/N2 separation[J]. Sustainable Energy & Fuels,2019,4(1):138-142. [162] N. Tippayawong, P. Thanompongchart. Biogas quality upgrade by simultaneous removal of CO2 and H2S in a packed column reactor[J]. Energy,2010,35(12):4531-4535. [163] Gomez L F, Zacharia R, P Bénard, et al. Multicomponent adsorption of biogas compositions containing CO2, CH4 and N2 on Maxsorb and Cu-BTC using extended Langmuir and Doong–Yang models[J]. Adsorption,2015,21(5):433-443. doi: 10.1007/s10450-015-9684-6 [164] Pal A, Chand S, Das M C. A water-stable twofold interpenetrating microporous MOF for selective CO2 adsorption and separation[J]. Inorganic Chemistry,2017,56(22):13991-13997. doi: 10.1021/acs.inorgchem.7b02136 [165] Simone, Cavenati, Carlos, et al. Metal organic framework adsorbent for biogas upgrading[J]. Industrial & Engineering Chemistry Research,2008,47(16):6333-6335. [166] Alexandre F. P. Ferreira, Ana Mafalda Ribeiro, Seda Kulaç, et al. Methane purification by adsorptive processes on MIL-53(Al)[J]. Chemical Engineering Science,2015,124:79-95. doi: 10.1016/j.ces.2014.06.014 [167] Xiang S, He Y, Zhang Z, et al. Microporous metal-organic framework with potential for carbon dioxide capture at ambient conditions[J]. Nature Communications,2012,3:954. doi: 10.1038/ncomms1956 [168] Caskey S R, Wong-Foy A G, Matzger A J. Dramatic tuning of carbon dioxide uptake via metal substitution in a coordination polymer with cylindrical pores[J]. Journal of the American Chemical Society,2008,130(33):10870-10871. doi: 10.1021/ja8036096 [169] Chowdhury P, Mekala S, Dreisbach F, et al. Adsorption of CO, CO2 and CH4 on Cu-BTC and MIL-101 metal organic frameworks: Effect of open metal sites and adsorbate polarity[J]. Microporous & Mesoporous Materials,2012,152(none):246-252. [170] Nugent P, Belmabkhout Y, Burd S D, et al. Porous materials with optimal adsorption thermodynamics and kinetics for CO2 separation[J]. Nature,2013,495(7439):80-84. doi: 10.1038/nature11893 [171] Krishna R, Baten J. In silico screening of metal-organic frameworks in separation applications[J]. Physical Chemistry Chemical Physics Pccp,2011,13:10593-10616. doi: 10.1039/c1cp20282k [172] Altintas C, Keskin S. Molecular simulations of MOF membranes and performance predictions of MOF/polymer mixed matrix membranes for CO2/CH4 separations[J]. ACS AuthorChoice,2019,7(2):2739-2750. [173] Hongliang, Huang, Wenjuan, et al. Understanding the effect of trace amount of water on CO2 capture in natural gas upgrading in metal–organic frameworks: a molecular simulation study[J]. Industrial & Engineering Chemistry Research,2012,51(30):10031-10038. [174] Demir H, Cramer C J, Siepmann J I. Computational screening of metal–organic frameworks for biogas purification[J]. Molecular Systems Design & Engineering,2019,4:1125-1135. [175] J Rogacka, A Seremak, A Luna-Triguero. High-throughput screening of metal-Organic frameworks for CO2 and CH4 separation in the presence of water[J]. Chemical Engineering Journal,2020,403:126392. [176] Sadiq M M, M Rubio-Martínez, Zadehahmadi F, et al. Magnetic framework composites for low concentration methane capture[J]. Industrial & Engineering Chemistry Research,2018,57(18):6040-6047. [177] Yongchul Chung, Jeffrey Camp, Maciej Haranczyk, et al. Computation-ready, experimental metal–organic frameworks: a tool to enable high-throughput screening of nanoporous crystals[J]. Chemistry of Materials,2014,26(21):6185-6192. doi: 10.1021/cm502594j -





 下载:
下载: