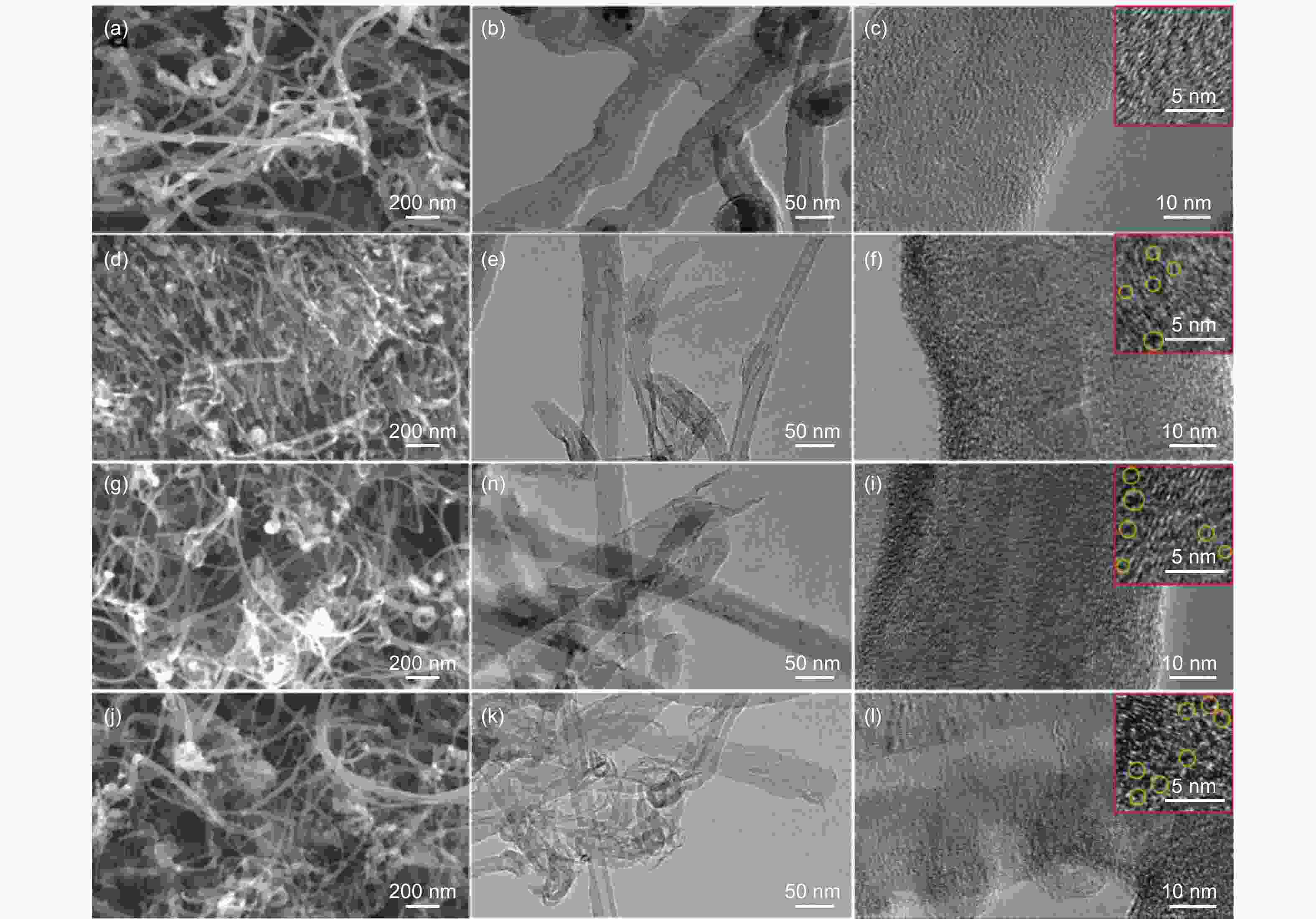
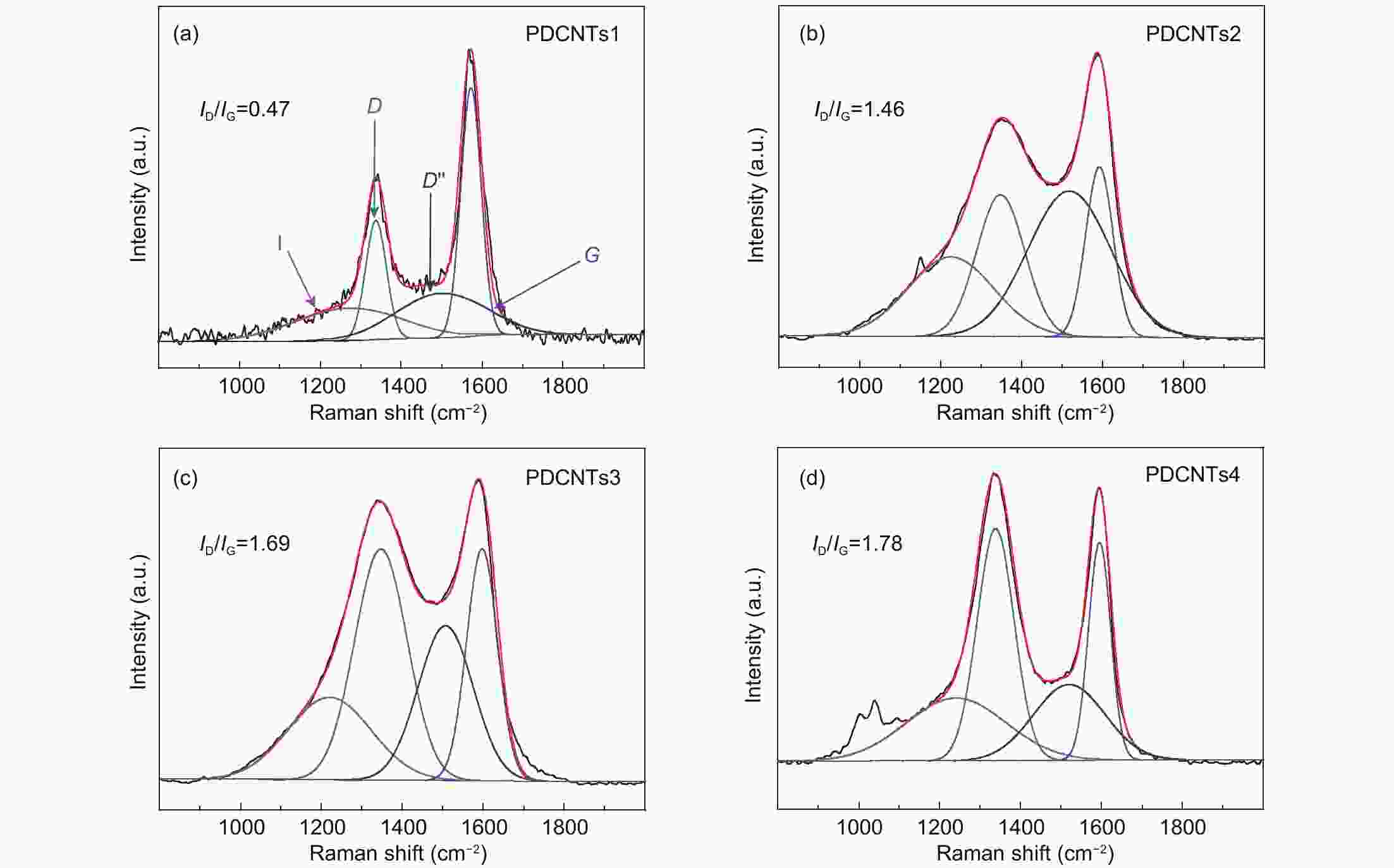
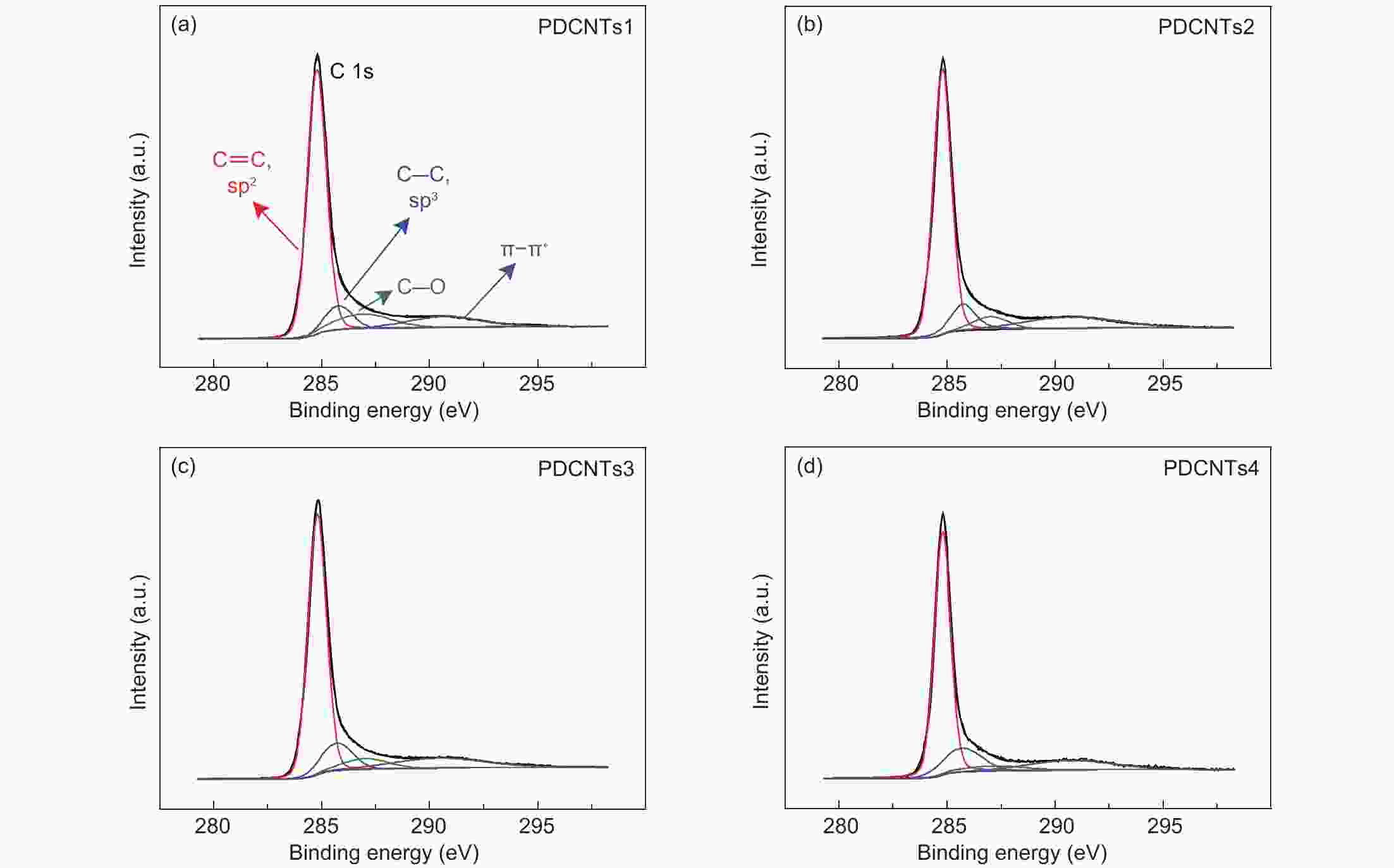
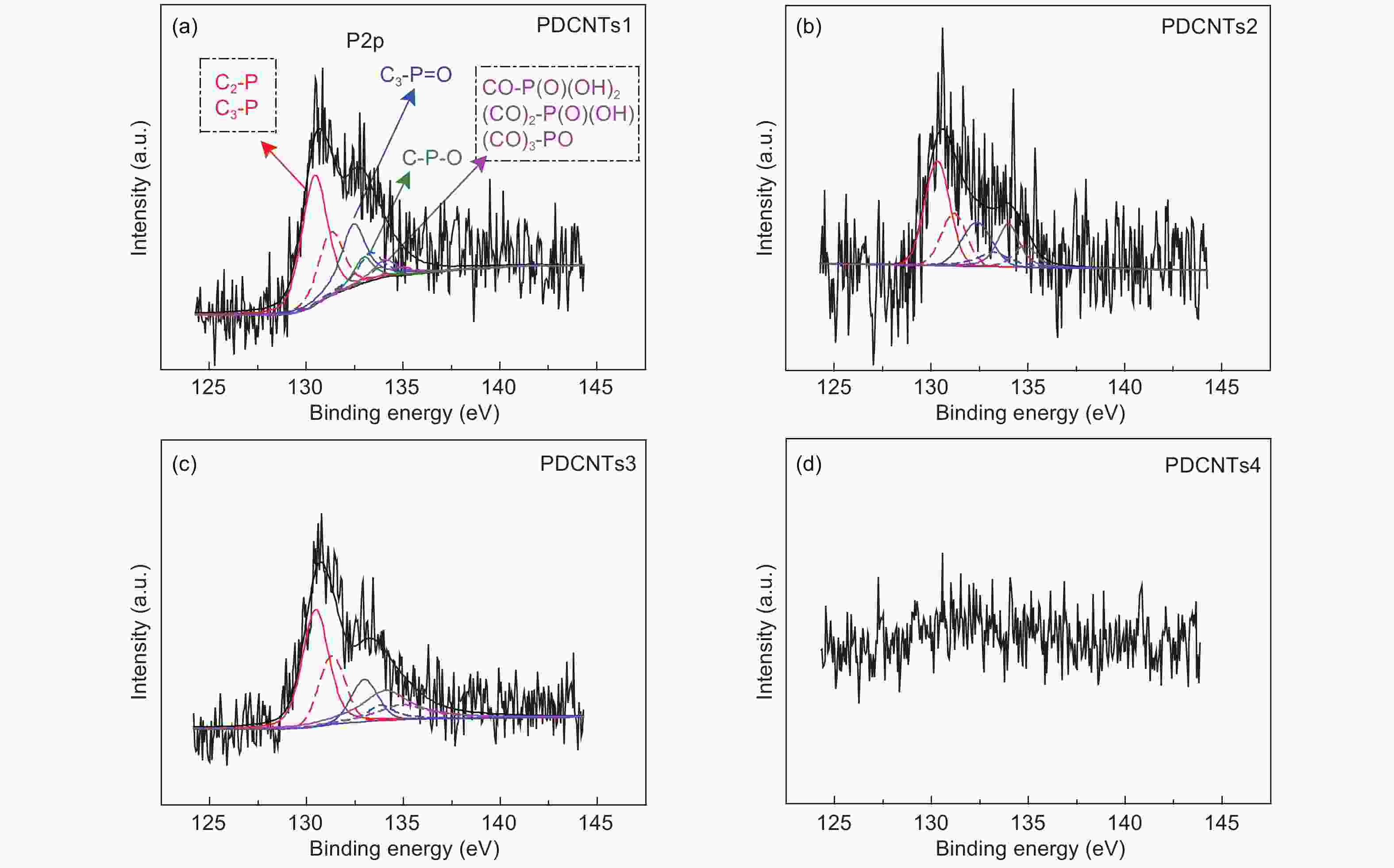
A correlation of the hydrogen evolution reaction activity to the number of defects formed by the decomposition of doped phosphorus species in carbon nanotubes
-
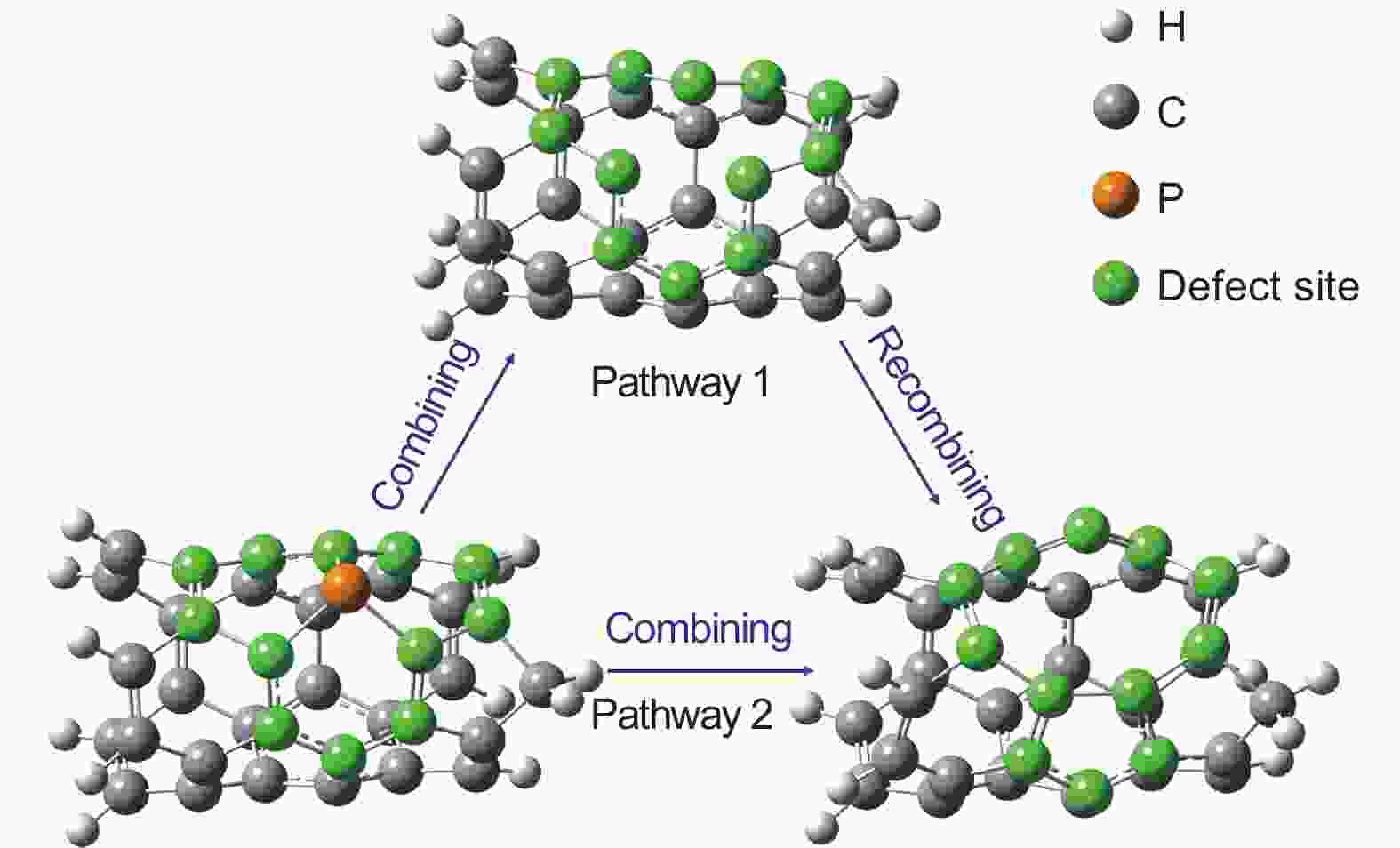
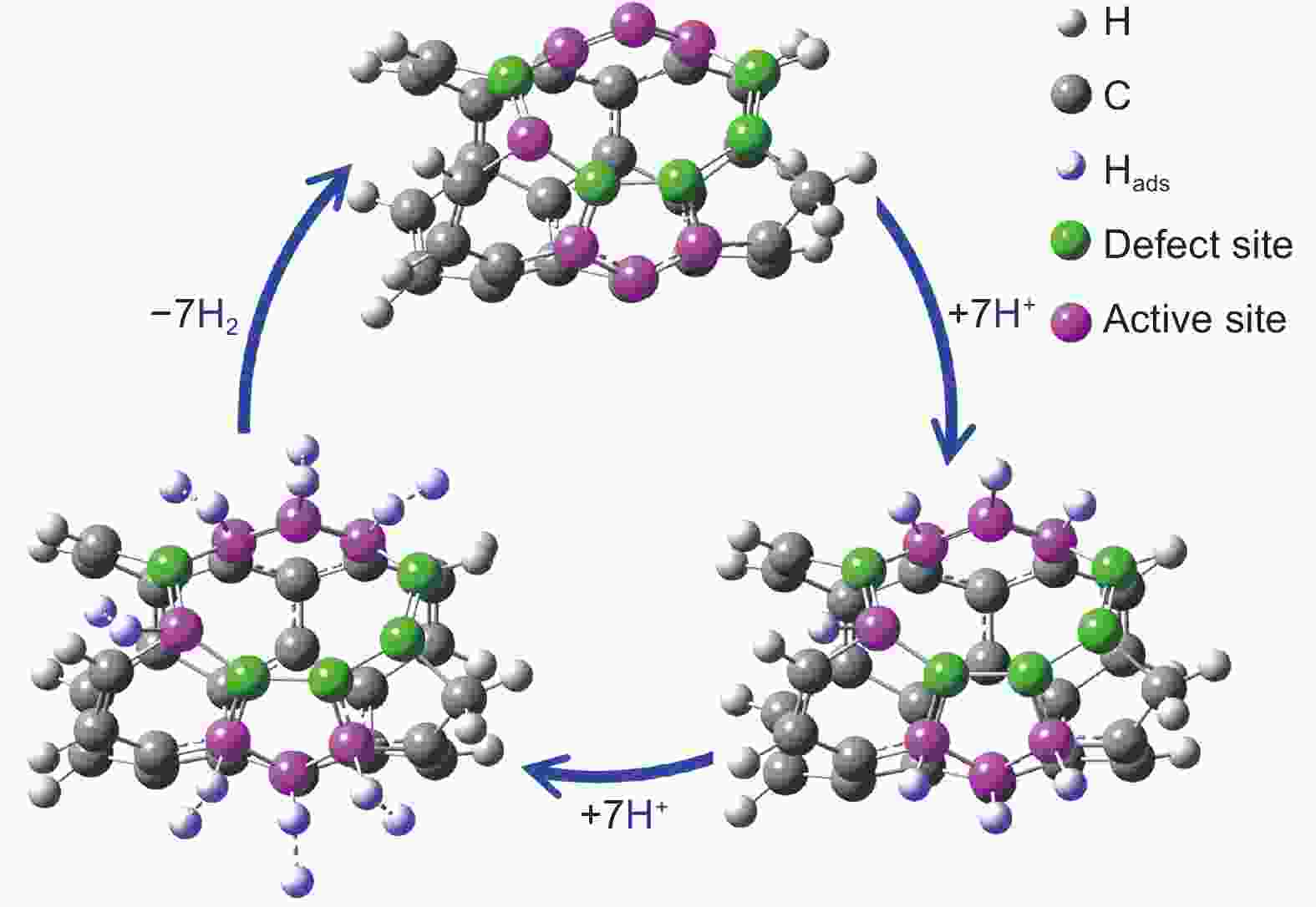
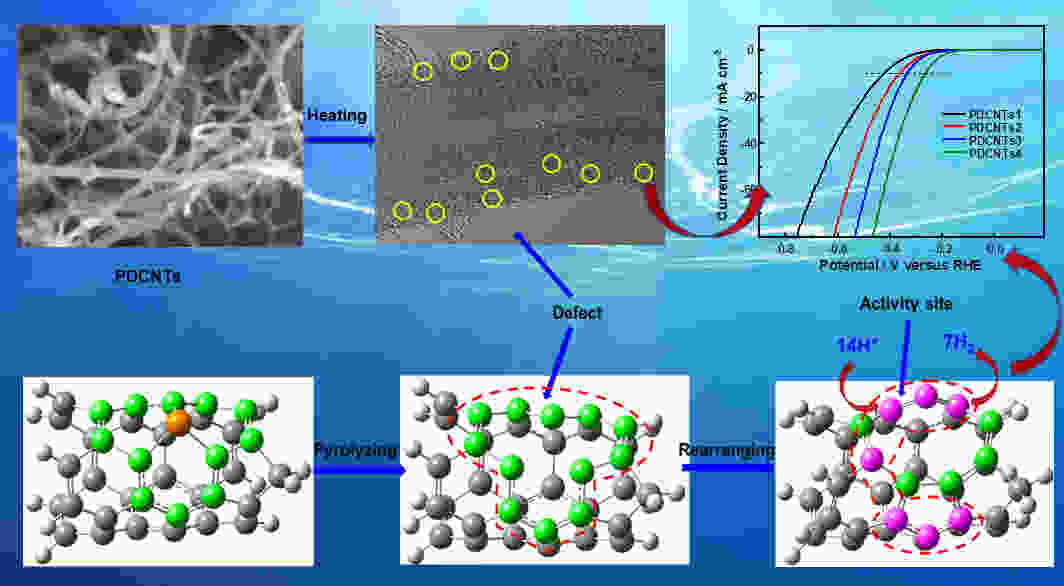
摘要: 作为一种新型碳基析氢反应催化剂,磷掺杂炭材料近年来已引起了较大关注。然而到目前为止,磷掺杂炭材料中的C―P物种对于氢析出活性的作用尚未被揭示。为了探讨碳基催化剂中C―P物种对其氢析出性能的影响,制备了4种具有不同石墨、吡啶、吡咯类磷物种分布的磷掺杂碳纳米管,并探讨了这3种磷物种的含量和氢析出活性之间的关系。结果表明在酸性介质中,一种在电流密度为10 mA cm−2 时过电位为0.266 V的磷掺杂碳纳米管展现出较高的氢析出活性。同时,密度泛函理论计算表明较高的氢析出性能主要是由石墨磷分解产生的五元环和九元环缺陷所引起,这为磷掺杂碳基催化剂表面的析氢反应提供更为深入的理解。Abstract: Phosphorus-doped carbon materials are one of the novel carbon catalysts for the hydrogen evolution reaction (HER) that have attracted considerable attention in recent years. However, the role of C―P species played in the HER activity is still not clear. Phosphorus-doped carbon nanotubes were prepared by chemical vapor deposition and annealed at 900, 1000 and 1200 °C to remove all or part of the phosporus, resulting in four samples with different amounts of substitutional-, pyridine- and pyrrole-like P species. The correlations between the HER activity and the contents of the three species were investigated. Results showed that the content of substitutional P decreased with annealing temperature and none was retained at 1200 °C. The HER activity increased with annealing temperature and the sample annealed at 1200 °C had the highest HER activity in an acid medium with an overpotential of 0.266 V at a current density of 10 mA cm−2. Density functional theory calculations showed that the pentagon- and nine-membered ring defects formed by the elimination of substitutional P mainly contributed to the HER activity.
-
Key words:
- Phosphorus-doped carbon nanotube /
- C―P species /
- Defect /
- Hydrogen evolution reaction /
- Water splitting
-
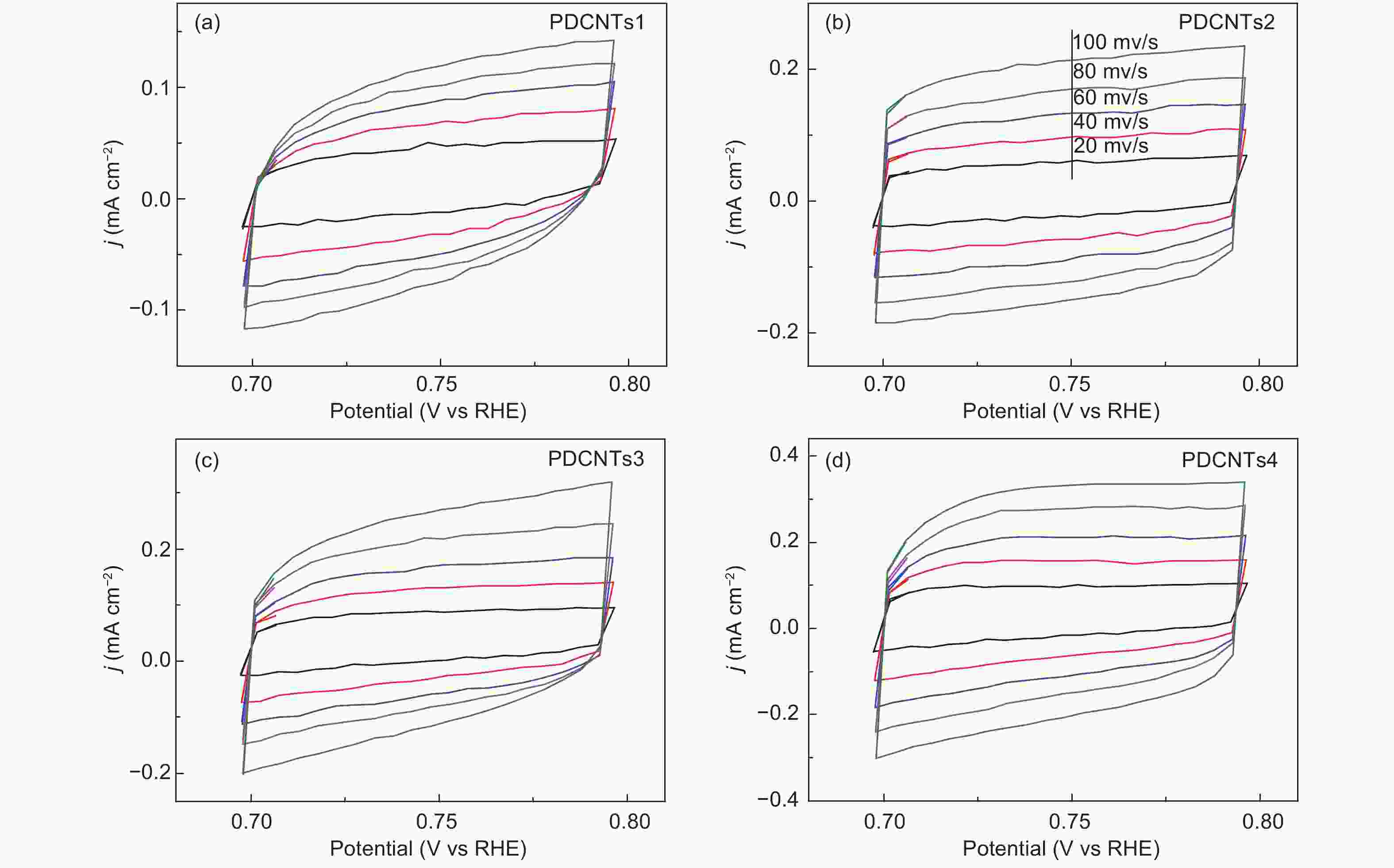
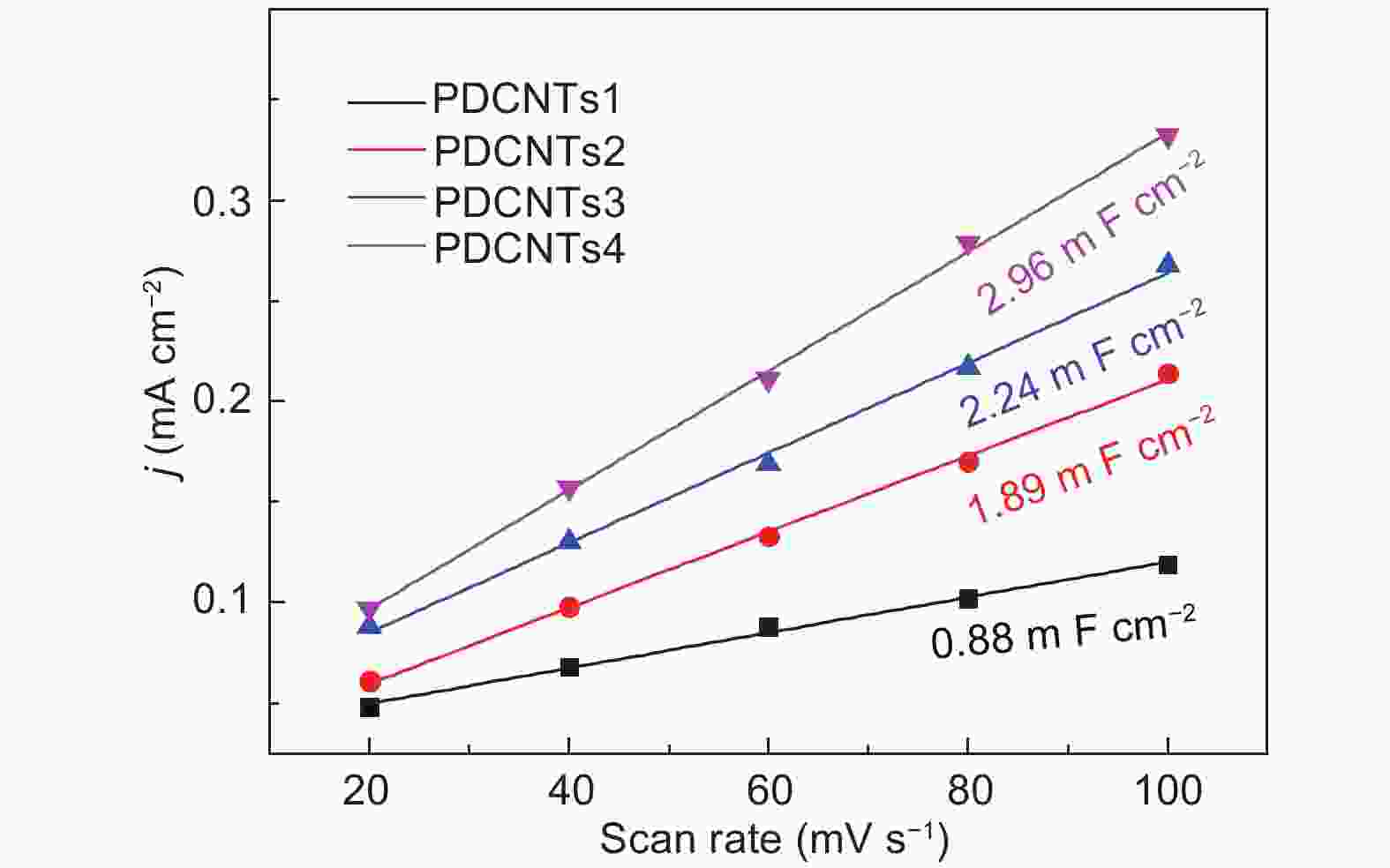
Figure 5. (a) The HER polarization curves of P-CNTs1, P-CNTs2, P-CNTs3, P-CNTs4 and 40 wt% Pt/C catalysts in a N2-saturated 0.5 mol L−1 H2SO4 solution, (b) Their corresponding overpotentials at the current density of 10 mA cm−2 and (c) tafel plots from (a), (d) The dependence of the HER current densities (at 300 mV overpotential) of P-CNTs1 (■), P-CNTs2 (■), P-CNTs3 (■), P-CNTs4 (■) on the C-P content and the values of ID/IG, (e) Impedance diagrams of P-CNTs1, P-CNTs2, P-CNTs3, and P-CNTs4, (f) The HER curves of P-CNTs4 in a 0.5 mol L−1 H2SO4 medium before and after 1000 cycles.
Table 1. Elemental contents in 4 samples from the XPS.
Samples C O P C―P C3―P=O C―O―P C―P―O P-CNTs1 93.12% 6.52% 0.191% 0.109% 0.032% 0.028% P-CNTs2 97.52% 2.20% 0.131% 0.082% 0.067% 0 P-CNTs3 97.98% 1.79% 0.119% 0.039% 0.072% 0 P-CNTs4 98.27% 1.73% 0 0 0 0 Table 2. The contents of sp2 and sp3 carbons, C=O, C―O, π-π* and the ratios of sp2/sp3 carbon from the C 1s spectra.
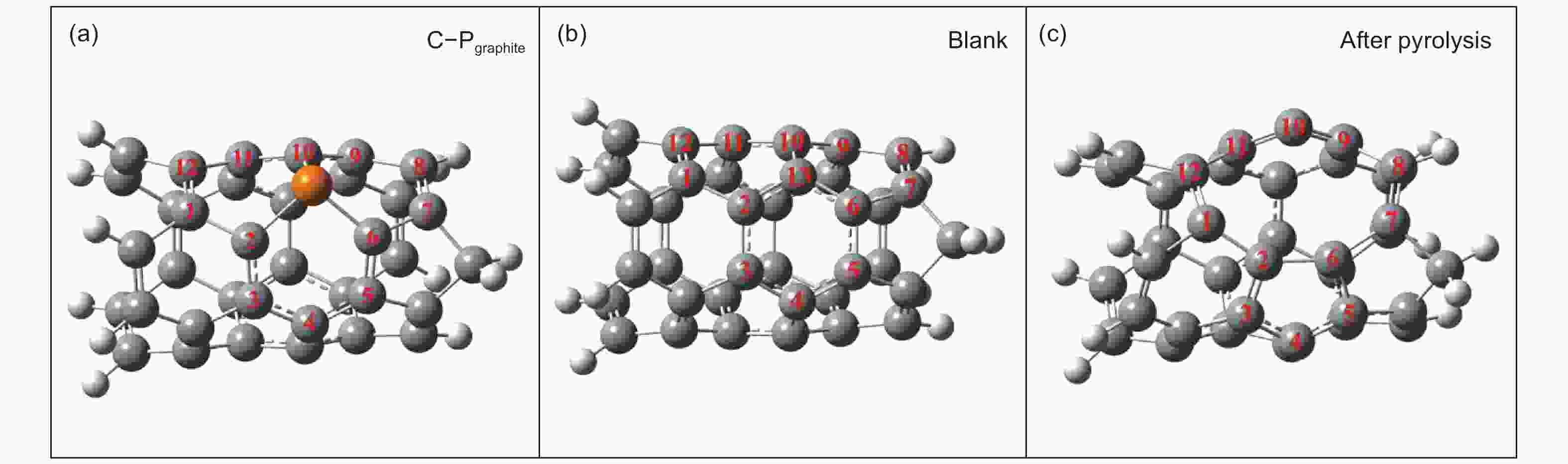
Samples sp2 sp3 sp3/sp2 C―O π-π* P-CNTs1 69.74% 8.77% 12.58 10.80% 10.69% P-CNTs2 66.82% 10.84% 16.22 6.14% 13.73% P-CNTs3 66.76% 11.66% 17.46 6.58% 12.98% P-CNTs4 66.07% 14.40% 21.79 5.08% 12.71% Table 3. The charge and spin densities (a.u.) of carbon atoms in the new formed defects after the decomposition of the C3―P graphite-like structure and those of the corresponding carbon atoms in the blank.
Carbon atom Charge density (Blank) Spin density (Blank) Charge density (After pyrolysis) Spin density
(After pyrolysis)1 −0.009 0 −0.026 0 2 0 0 0.038 0 3 0 0 −0.067 0 4 0.018 0 −0.009 0 5 −0.014 0 −0.063 0 6 −0.014 0 0.034 0 7 0.035 0 0.030 0 8 −0.153 0 −0.143 0 9 0.016 0 −0.042 0 10 0.002 0 −0.073 0 11 0.002 0 −0.046 0 12 −0.021 0 0.009 0 13 0.019 0 − − Table 4. The H+ adsorption energies (kcal mol−1) on carbon atoms in the new formed defects from the decomposition of the C3―P graphite-like structure and those of corresponding carbon atoms in the blank.
Carbon atom Adsorption energies (kcal mol−1) Pure CNT (blank) CNT (after pyrolysis) 1 −58.163 −74.825 3 −56.805 −101.009 4 −55.909 −113.664 5 −58.719 −101.588 9 −55.458 −80.907 10 −55.824 −142.826 11 −57.595 −75.465 -
[1] Liu Z W, Peng F, Wang H J, et al. Phosphorus-doped graphite layers with high electrocatalytic activity for the O2 reduction in an alkaline medium[J]. Angew Chem Int Ed,2011,50:3257-3261. doi: 10.1002/anie.201006768 [2] Liu Z W, Peng F, Wang H J, et al. Novel phosphorus-doped multiwalled nanotubes with high electrocatalytic activity for O2 reduction in alkaline medium[J]. Catal Commun,2011,16:35-38. doi: 10.1016/j.catcom.2011.08.038 [3] Liu Z W, Peng F, Wang H J, et al. Preparation of phosphorus-doped carbon nanospheres and their electrocatalytic performance for O2 reduction[J]. J Nat Gas Chem,2012,21:257-264. doi: 10.1016/S1003-9953(11)60362-9 [4] Hou H S, Shao L D, Zhang Y, et al. Large-area carbon nanosheets doped with phosphorus: A high-performance anode material for sodium-ion batteries[J]. Adv Sci,2017,4:1600243. doi: 10.1002/advs.201600243 [5] Li K, Hu Z Y, Ma J Z, et al. A 3D and stable lithium anode for high-performance lithium-iodine batteries[J]. Adv Mater,2019,31:1902399. doi: 10.1002/adma.201902399 [6] Wang J X, Xia Y, Liu Y, et al. Mass production of large-pore phosphorus-doped mesoporous carbon for fast-rechargeable lithium-ion batteries[J]. Energy Storage Materials,2019,22:147-153. doi: 10.1016/j.ensm.2019.01.008 [7] Wen Y Y, Wang B, Huang C C, et al. Synthesis of phosphorus-doped graphene and its wide potential window in aqueous supercapacitors[J]. Chem Eur J,2014,20:1-7. doi: 10.1002/chem.201390210 [8] Yang W, Yang W, Kong L N, et al. Phosphorus-doped 3D hierarchical porous carbon for high-performance supercapacitors: A balanced strategy for pore structure and chemical composition[J]. Carbon,2018,127:557-567. doi: 10.1016/j.carbon.2017.11.050 [9] Lv B J, Li P P, Liu Y, et al. Nitrogen and phosphorus co-doped carbon hollow spheres derived from polypyrrole for high-performance supercapacitor electrodes[J]. Applied Surface Science,2018,437:169-175. doi: 10.1016/j.apsusc.2017.12.171 [10] Zheng Y, Jiao Y, Li L H, et al. Toward design of synergistically active carbon-based catalysts for electrocatalytic hydrogen evolution[J]. ACS Nano,2014,8:5290-5296. doi: 10.1021/nn501434a [11] Xiao Z H, Huang X B, Xu L, et al. Edge-selectively phosphorus-doped few-layer graphene as an efficient metal-free electrocatalyst for the oxygen evolution reaction[J]. Chen Commun,2016,52:13008-13011. doi: 10.1039/C6CC07217H [12] Jiang H L, Zhu Y H, Su Y H, et al. Highly dual-doped multilayer nanoporous graphene: efficient metal-free electrocatalysts for the hydrogen evolution reaction[J]. J Mater Chem A,2015,3:12642-12645. doi: 10.1039/C5TA02792F [13] Liu Z W, Ai J, Sun M M, et al. Phosphorous-doped graphite layers with outstanding electrocatalytic activities for the oxygen and hydrogen evolution reactions in water electrolysis[J]. Adv Funct Mater,2020,30:1910741. doi: 10.1002/adfm.201910741 [14] Mcevoy N, Peltekis N, Kumar S, et al. Synthesis and analysis of thin conducting pyrolytic carbon films[J]. Carbon,2012,50:1216-1226. doi: 10.1016/j.carbon.2011.10.036 [15] Zhou Y, Ma R G, Candelaria S L, et al. Phosphorus/sulfur Co-doped porous carbon with enhanced specific capacitance for supercapacitor and improved catalytic activity for oxygen reduction reaction[J]. J Power Sources,2016,314:39-48. doi: 10.1016/j.jpowsour.2016.03.009 [16] Tian X D, Li X, Yang T, et al. Flexible carbon nanofiber mats with improved graphitic structure as scaffolds for efficient all-solid-state supercapacitor[J]. Electrochim Acta,2017,247:1060-1071. doi: 10.1016/j.electacta.2017.07.103 [17] Liu Z J, Zhao Z H, Wang Y Y, et al. In situ exfoliated, edge-rich, oxygen-functionalized graphene from carbon fibers for oxygen electrocatalysis[J]. Adv Mater,2017,29:1606207. doi: 10.1002/adma.201606207 [18] Bi Z H, Luo L, Kong Q Q, et al. Structural evolution of phosphorus species on graphene with a stabilized electrochemical interface[J]. ACS Appl Mater Interfaces,2019,11:11421-11430. doi: 10.1021/acsami.8b21903 -
 20200168 -Supporting Information.pdf
20200168 -Supporting Information.pdf

-





 下载:
下载: