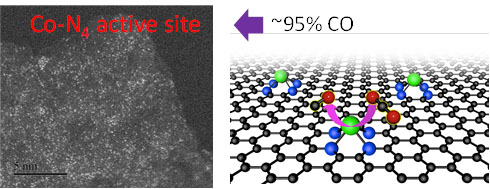
CoN4 active sites in a graphene matrix for the highly efficient electrocatalysis of CO2 reduction
-
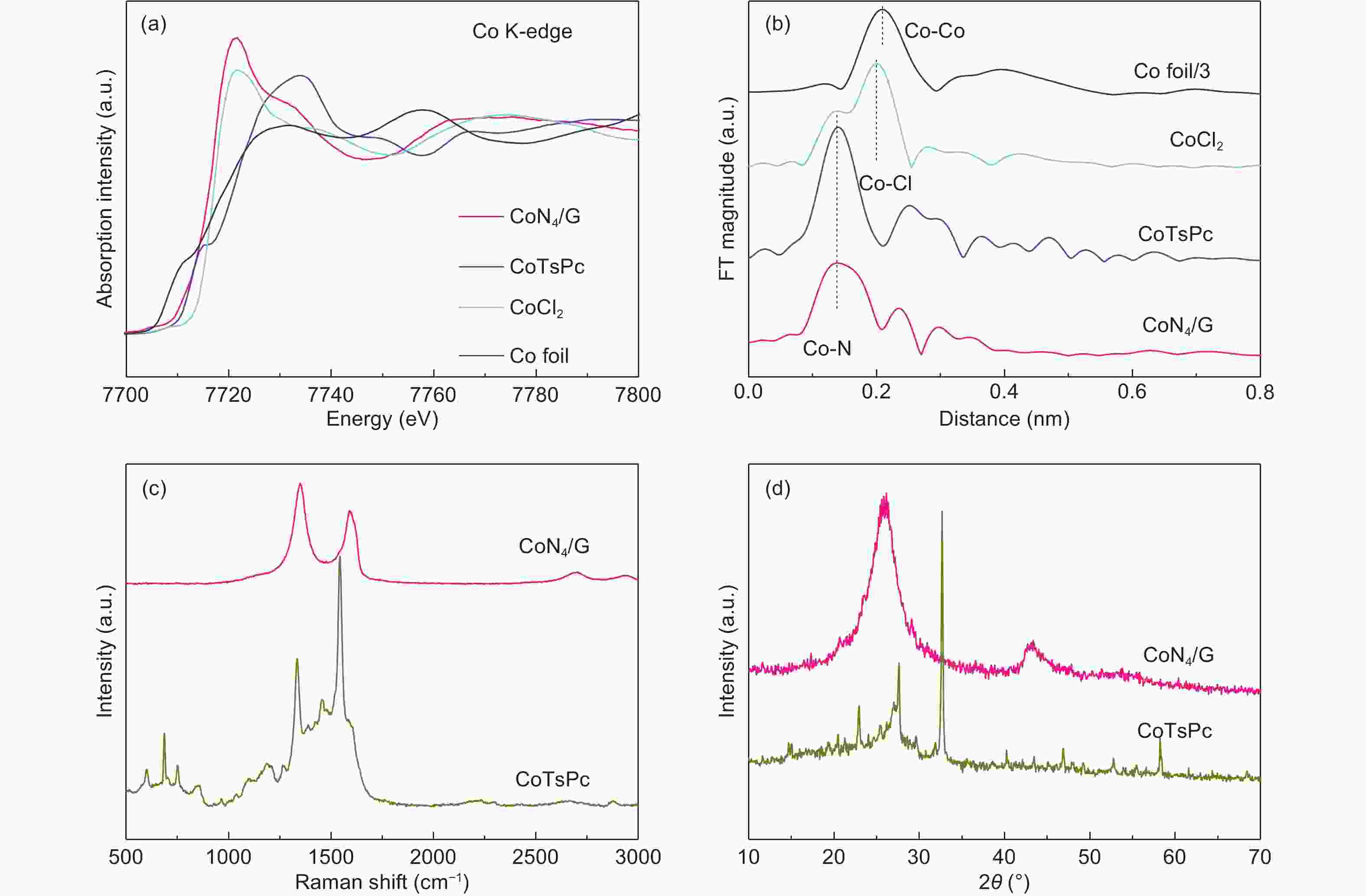
摘要: 设计高选择性、稳定和低成本的催化剂将二氧化碳电化学转化为高附加值的碳产品以缓解二氧化碳排放和能源危机仍是一个挑战。通过将单原子CoN4活性位点嵌入石墨烯基体中得到了一种强健和高效的CO2还原电催化剂。结果表明,这些高度分散的CoN4位点具有出色的CO2还原活性,在电压为−0.76 V (vs. RHE),过电位为0.65 V时,CO法拉第效率达~95%。同时,该催化剂具有优异的稳定性。本工作为设计高效CO2还原电催化剂奠定了基础。Abstract: Developing highly selective, economical and stable catalysts for the electrochemical conversion of CO2 into value-added carbon products to mitigate both CO2 emission and the energy crisis is challenging. We report an efficient and robust electrocatalyst for the CO2 reduction reaction (CO2RR) by embedding CoN4 active sites in a graphene matrix. These highly dispersed CoN4 sites show an extraordinary CO2RR activity, with a high CO Faradaic efficiency of nearly 95% at −0.76 V (vs. RHE) and remarkable durability. The corresponding overpotential is 0.65 V. Our finding could pave the way for the design at the atomic scale of highly efficient electrocatalysts for the CO2RR.
-
Key words:
- CO2 electroreduction /
- Cobalt /
- Single-atom catalysts /
- Electrochemistry /
- Graphene
-
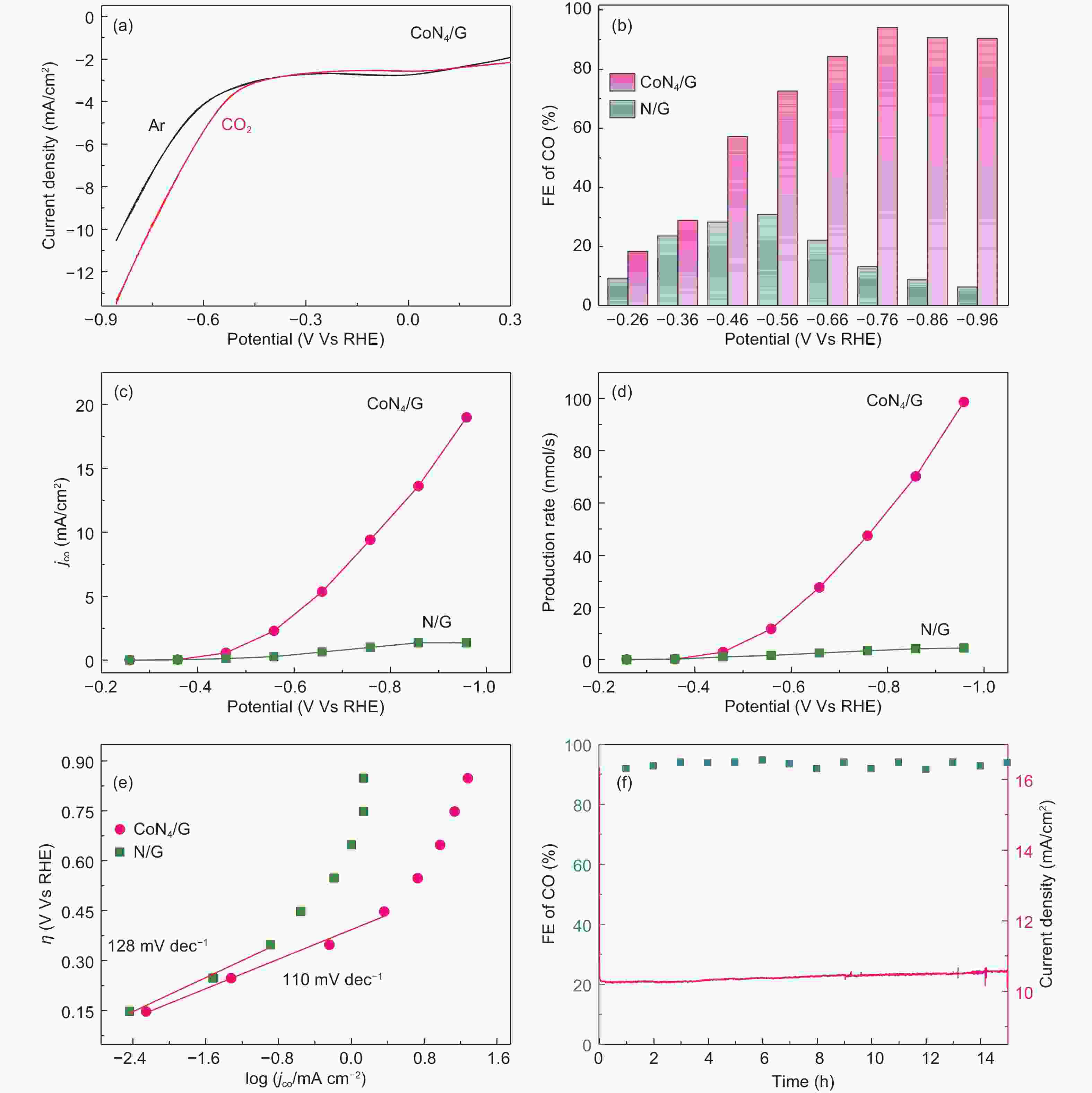
Figure 4. CO2RR catalytic performance of as-synthesized catalysts. (a) 20 mV s−1 LSV scans for the CoN4/G catalyst in KHCO3 solution. Comparison of electrocatalytic activity of CoN4/G and N/G: (b) FE of CO, (c) jCO and (d) production rates of CO at varoius applied potentials. (e) Tafel plot. (f) Long-term stability for CoN4/G catalyst at −0.76 V (vs. RHE) for 15 h.
-
[1] Benson E E, Kubiak C P, Sathrum A J, et al. Electrocatalytic and homogeneous approaches to conversion of CO2 to liquid fuels[J]. Chemical Society Reviews,2009,38(1):89-99. doi: 10.1039/B804323J [2] Chu S, Majumdar A. Opportunities and challenges for a sustainable energy future[J]. Nature,2012,488(7411):294-303. doi: 10.1038/nature11475 [3] Zhang L, Zhao Z J, Gong J L. Nanostructured materials for heterogeneous electrocatalytic CO2 reduction and their related reaction mechanisms[J]. Angewandte Chemie International Edition,2017,56(38):11326-11353. doi: 10.1002/anie.201612214 [4] Zhu D D, Liu J L, Qiao S Z. Recent advances in inorganic heterogeneous electrocatalysts for reduction of carbon dioxide[J]. Advanced Materials,2016,28(18):3423-3452. doi: 10.1002/adma.201504766 [5] Zhang Y J, Sethuraman V, Michalsky R, et al. Competition between CO2 reduction and H2 evolution on transition-metal electrocatalysts[J]. ACS Catalysis,2014,4(10):3742-3748. doi: 10.1021/cs5012298 [6] Zhang C H, Yang S Z, Wu J J, et al. Electrochemical CO2 reduction with atomic iron-dispersed on nitrogen-doped graphene[J]. Advanced Energy Materials,2018,8(19):1703487. doi: 10.1002/aenm.201703487 [7] Zhang H N, Wang J, Zhao Z, et al. The synthesis of atomic Fe embedded in bamboo-CNTs grown on graphene as a superior CO2 electrocatalyst[J]. Green Chemistry,2018,20(15):3521-3529. doi: 10.1039/C8GC01466C [8] Yuan X T, Zhang L, Li L L, et al. Ultrathin Pd-Au shells with controllable alloying degree on Pd nanocubes toward carbon dioxide reduction[J]. Journal of the American Chemical Society,2019,141(12):4791-4794. doi: 10.1021/jacs.8b11771 [9] Liu S B, Tao H B, Zeng L, et al. Shape-dependent electrocatalytic reduction of CO2 to CO on triangular silver nanoplates[J]. Journal of the American Chemical Society,2017,139(6):2160-2163. doi: 10.1021/jacs.6b12103 [10] Mistry H, Reske R, Zeng Z H, et al. Exceptional size-dependent activity enhancement in the electroreduction of CO2 over Au nanoparticles[J]. Journal of the American Chemical Society,2014,136(47):16473-16476. doi: 10.1021/ja508879j [11] Yang H B, Hung S F, Liu S, et al. Atomically dispersed Ni(I) as the active site for electrochemical CO2 reduction[J]. Nature Energy,2018,3(2):140-147. doi: 10.1038/s41560-017-0078-8 [12] Yang J, Qiu Z Y, Zhao C M, et al. In-situ thermal atomization to transfer supported metal nanoparticles to surface enriched Ni single atom catalyst[J]. Angewandte Chemie International Edition,2018,57(43):14095-14100. doi: 10.1002/anie.201808049 [13] Wang X Q, Chen Z, Zhao X Y, et al. Regulation of coordination number over single Co sites: Triggering the efficient electroreduction of CO2[J]. Angewandte Chemie International Edition,2018,57(7):1944-1948. doi: 10.1002/anie.201712451 [14] Fan Q, Hou P F, Choi C H, et al. Activation of Ni particles into single Ni-N atoms for efficient electrochemical reduction of CO2[J]. Advanced Energy Materials,2019,10(5):1903068. [15] Zhao C M, Dai X Y, Yao T, et al. Ionic exchange of metal-organic frameworks to access single nickel sites for efficient electroreduction of CO2[J]. Journal of the American Chemical Society,2017,139(24):8078-8081. doi: 10.1021/jacs.7b02736 [16] Li X G, Bi W T, Chen M L, et al. Exclusive Ni-N4 sites realize near-unity CO selectivity for electrochemical CO2 reduction[J]. Journal of the American Chemical Society,2017,139(42):14889-14892. doi: 10.1021/jacs.7b09074 [17] Zhang Y F, Yu C, Tan X Y, et al. Recent advances in multilevel nickel-nitrogen-carbon catalysts for CO2 electroreduction to CO[J]. New Carbon Materials,2021,36(1):19-33. doi: 10.1016/S1872-5805(21)60002-1 [18] Cui X J, Xiao J P, Wu Y H, et al. A graphene composite material with single cobalt active sites: A highly efficient counter electrode for dye-sensitized solar cells[J]. Angewandte Chemie International Edition,2016,55(23):6708-6712. doi: 10.1002/anie.201602097 [19] Cui X J, Li H B, Wang Y, et al. Room-temperature methane conversion by graphene-confined single iron atoms[J]. Chem,2018,4(8):1902-1910. doi: 10.1016/j.chempr.2018.05.006 [20] Geng Z G, Cao Y J, Chen W X, et al. Regulating the coordination environment of Co single atoms for achieving efficient electrocatalytic activity in CO2 reduction[J]. Applied Catalysis B: Environmental,2019,240:234-240. doi: 10.1016/j.apcatb.2018.08.075 [21] Song X K, Zhang H, Yang Y Q, et al. Bifunctional nitrogen and cobalt co-doped hollow carbon for electrochemical syngas production[J]. Advanced Science,2018,5(7):1800177. doi: 10.1002/advs.201800177 [22] Chen X, Ma D D, Chen B, et al. Metal-organic framework-derived mesoporous carbon nanoframes embedded with atomically dispersed Fe-Nx active sites for efficient bifunctional oxygen and carbon dioxide electroreduction[J]. Applied Catalysis B: Environmental,2020,267:118720. doi: 10.1016/j.apcatb.2020.118720 [23] Luo X Y, Chen Y, Mo Y. A review of charge storage in porous carbon-based supercapacitors[J]. New Carbon Materials,2021,36(1):49-68. doi: 10.1016/S1872-5805(21)60004-5 [24] Han N, Wang Y, Ma L, et al. Supported cobalt polyphthalocyanine for high-performance electrocatalytic CO2 reduction[J]. Chem,2017,3(4):652-664. doi: 10.1016/j.chempr.2017.08.002 [25] Zhu M H, Chen J C, Huang L B, et al. Covalently grafting cobalt porphyrin onto carbon nanotubes for efficient CO2 electroreduction[J]. Angewandte Chemie International Edition,2019,58(20):6595-6599. doi: 10.1002/anie.201900499 [26] Chen P Z, Zhou T P, Xing L L, et al. Atomically dispersed iron-nitrogen species as electrocatalysts for bifunctional oxygen evolution and reduction reactions[J]. Angewandte Chemie International Edition,2017,56(2):610-614. doi: 10.1002/anie.201610119 [27] Li Q H, Chen W X, Xiao H, et al. Fe isolated single atoms on S, N co-doped carbon by copolymer pyrolysis strategy for highly efficient oxygen reduction reaction[J]. Advanced Materials,2018,30(25):1800588. doi: 10.1002/adma.201800588 [28] Nabid M R, Sedghi R, Jamaat P R, et al. Catalytic oxidative polymerization of aniline by using transition-metal tetrasulfonated phthalocyanine[J]. Applied Catalysis A: General,2007,328(1):52-57. doi: 10.1016/j.apcata.2007.05.017 [29] Jeon I Y, Shin Y R, Sohn G J, et al. Edge-carboxylated graphene nanosheets via ball milling[J]. PNAS,2012,109(15):5588-5593. doi: 10.1073/pnas.1116897109 [30] Pan Y, Lin R, Chen Y J, et al. Design of single-atom Co-N5 catalytic site: A robust electrocatalyst for CO2 reduction with nearly 100% CO selectivity and remarkable stability[J]. Journal of the American Chemical Society,2018,140(12):4218-4221. doi: 10.1021/jacs.8b00814 [31] Wu J J, Liu M J, Sharma P P, et al. Incorporation of nitrogen defects for efficient reduction of CO2 via two-electron pathway on three-dimensional graphene foam[J]. Nano Letters,2016,16(1):466-470. doi: 10.1021/acs.nanolett.5b04123 [32] Wu J J, Yadav R M, Liu M J, et al. Achieving highly efficient, selective, and stable CO2 reduction on nitrogen-doped carbon nanotubes[J]. ACS Nano,2015,9(5):5364-5371. doi: 10.1021/acsnano.5b01079 [33] Xu J Y, Kan Y H, Huang R, et al. Revealing the origin of activity in nitrogen-doped nanocarbons towards electrocatalytic reduction of carbon dioxide[J]. ChemSusChem,2016,9(10):1085-1089. doi: 10.1002/cssc.201600202 [34] Jiang K, Siahrostami S, Zheng T T, et al. Isolated Ni single atoms in graphene nanosheets for high-performance CO2 reduction[J]. Energy & Environmental Science,2018,11(4):893-903. [35] Zhang H N, Li J, Xi S B, et al. A graphene-supported single-atom FeN5 catalytic site for efficient electrochemical CO2 reduction[J]. Angewandte Chemie International Edition,2019,58(42):14871-14876. doi: 10.1002/anie.201906079 [36] Liu Y S, McCrory C C L. Modulating the mechanism of electrocatalytic CO2 reduction by cobalt phthalocyanine through polymer coordination and encapsulation[J]. Nature Communications,2019,10:1683. doi: 10.1038/s41467-019-09626-8 -
 20210028-Supporting Information.pdf
20210028-Supporting Information.pdf

-





 下载:
下载: