Electrochemical oxidation of 2D B, N-codoped carbon nanosheets to improve their pseudo-capacitance
-
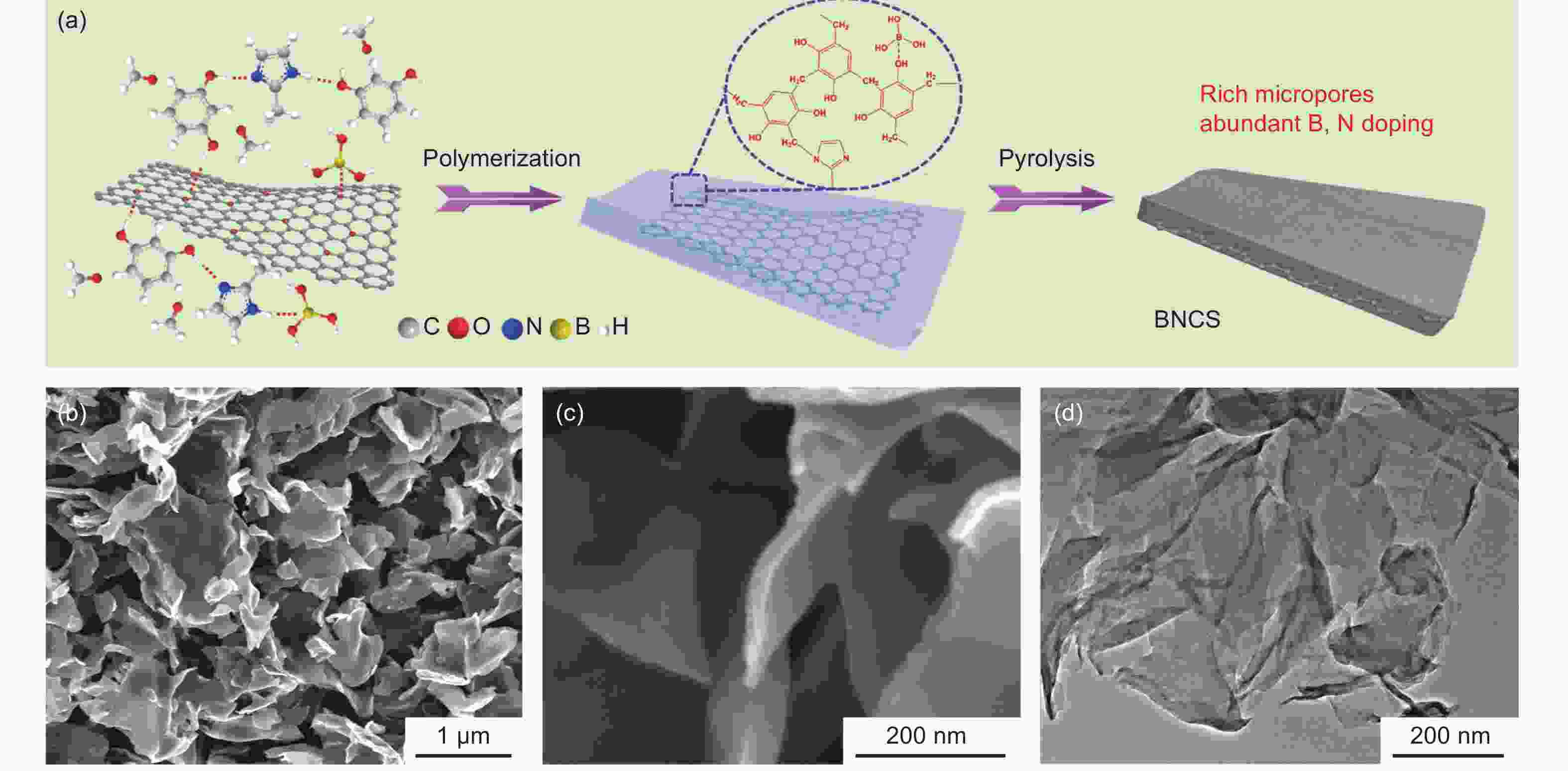
摘要: 在炭基电极材料中引入氧化还原赝电容是提升其比电容的有效手段,有望解决炭基超级电容器低能量密度的瓶颈。本文通过原位电化学氧化,在B、N掺杂二维纳米炭片电极上引入电化学活性含氧官能团,以显著提升炭基电极的赝电容,并研究了B、N掺杂炭在不同氧化工艺下的表面组成和电容性能变化。结果表明,B、N掺杂可以提升氧化电极的电子传输和电荷转移,有效促进电化学氧化效果,提高电极的赝电容。此外,相比于恒压氧化工艺,循环伏安氧化方法可以有效提升炭电极的氧化深度和总氧含量,并且也有利于选择性地生成以电化学活性的醌基为主的含氧官能团。制备的氧化电极在1 A·g−1电流密度下显示出601.5 F·g−1的高比电容,并在20 A·g−1下仍保持74.8%,显示出良好的倍率性能。此外,氧化电极还表现出优异的循环稳定性,在5 A·g−1下8000次循环后保持了初始电容的92.6%。Abstract: Introducing redox pseudocapacitance could effectively improve the specific capacitance of carbon-based electrode materials, and is a promising way to overcome the low energy density of carbon-based supercapacitors. An in-situ electrochemical oxidation method was used to electrochemically generate active oxygen-containing functional groups for B, N co-doped carbon nanosheets to significantly increase the pseudocapacitance. Results show that the degree of oxidation, the pseudocapacitance, and the charge transfer rate of the oxidized carbon nanosheets were effectively increased by co-doping with B and N. Compared with the constant potential oxidation method, the cyclic voltammetry oxidation method was more effective in increasing the total oxygen content of the oxidized electrode and to selectively generate electrochemically active quinone groups. The oxidized electrode had a high specific capacitance of 601.5 F g−1 at a current density of 1 A g−1, retaining 74.8% of the original value at 20 A g−1, revealing a favorable rate capability. The oxidized electrode also had excellent cycle stability, retaining 92.6% of the initial capacitance after 8 000 cycles at 5 A g−1.
-
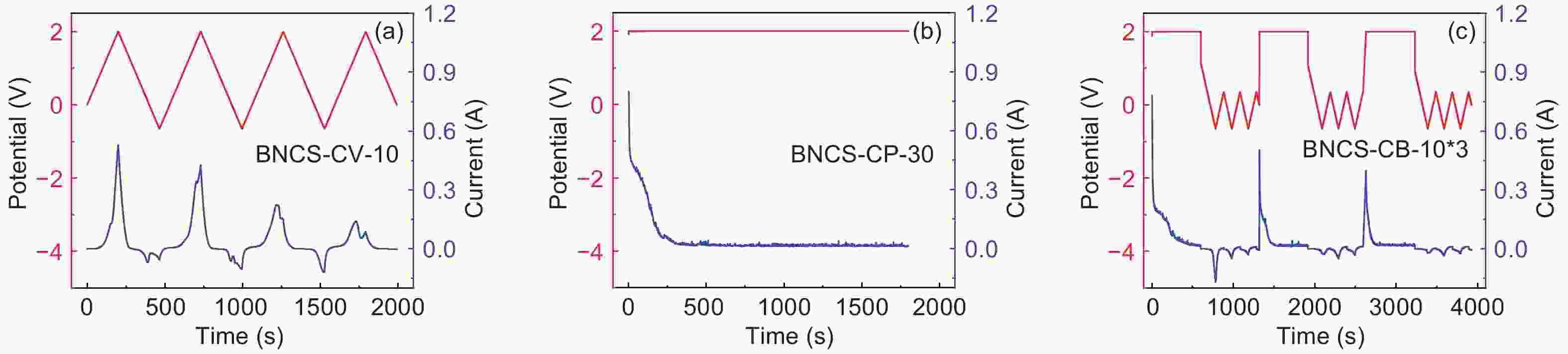
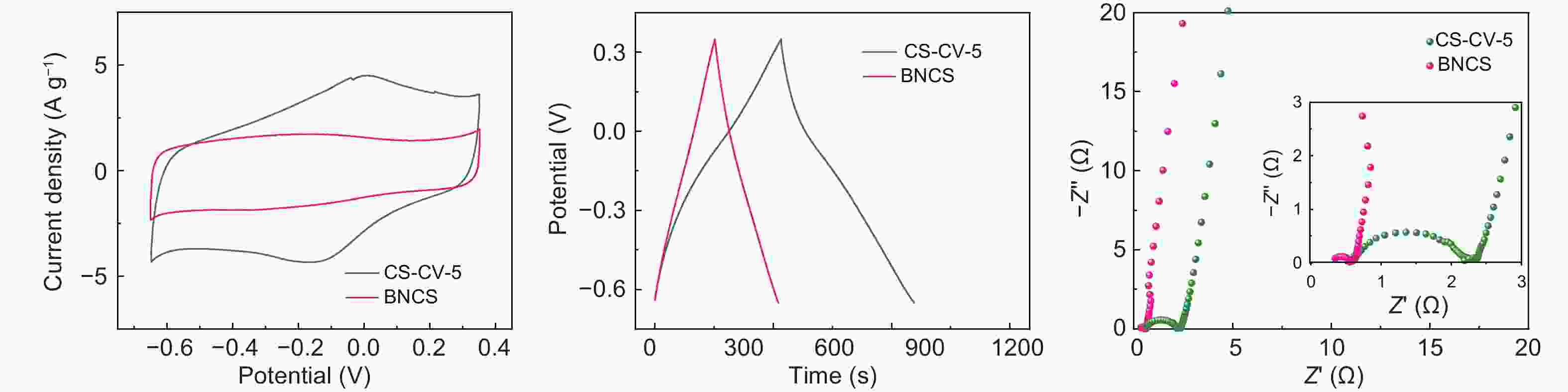
图 3 不同氧化条件制备的BNCS氧化电极在三电极体系下的1 mol L−1 H2SO4中的电化学测试:BNCS-CV-5、BNCS-CV-10、BNCS-CV-20氧化电极(a) 在10 mV·s−1下的CV曲线、(b) 1 A·g−1下的GC曲线和(c) 电化学阻抗谱;BNCS-CP-10、BNCS-CP-30和BNCS-CB-10*3氧化电极(d) 在10 mV·s−1下的CV曲线和(e) 1 A·g−1下的GC曲线和(f) 电化学阻抗谱
Figure 3. Electrochemical evaluation of oxidized BNCS electrodes prepared under different oxidation conditions measured in a three-electrode system in 1 mol L−1 H2SO4: (a) CV curves at 10 mV·s−1, (b) GC curves at 1 A·g−1 and (c) EIS curves of BNCS-CV-5, BNCS-CV-10 and BNCS-CV-20 electrodes; (d) CV curves at 10 mV·s−1, (e) GC curves at 1 A·g−1 and (f) EIS curves of BNCS-CP-10, BNCS-CP-30 and BNCS-CB-10*3 electrodes.
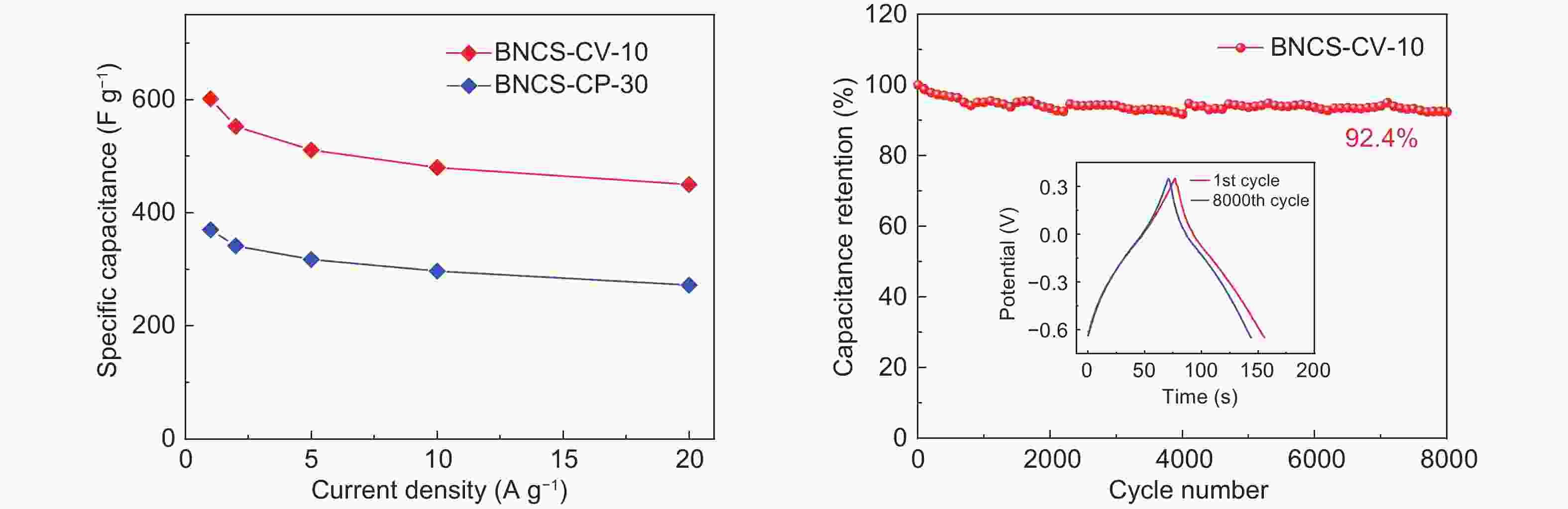
图 6 氧化电极在三电极体系下1 mol L−1 H2SO4中的倍率与循环测试:(a) BNCS-CV-10和BNCS-CP-30电极0.5~20 A·g−1下的倍率性能测试;(b) BNCS-CV-10电极在5 A·g−1下的循环稳定性测试
Figure 6. Rate and cycling tests of oxidized electrodes measured in a three-electrode system in 1 mol L−1 H2SO4:(a) Rate performances of BNCS-CV-10 and BNCS-CP-30 electrodes from 0.5 to 20 A·g−1. (b) Cycling performance of BNCS-CV-10 electrode at 5 A·g−1.
表 1 BNCS、BNCS-CV-10和BNCS-CP-30电极元素分析与XPS表征测定的元素含量
Table 1. Elemental contents of the BNCS, BNCS-CV-10 and BNCS-CP-30 electrodes obtained by elemental analysis and XPS.
Sample Elemental analysis (wt.%) XPS (at.%) C H N O C B N O F BNCS 90.2 1.2 1.2 7.4 80.85 1.45 3.41 7.09 7.2 BNCS-CV-10 83.1 1.0 1.1 14.9 70.98 0.51 3.97 17.96 6.58 BNCS-CP-30 85.4 0.9 1.2 12.6 73.58 0.6 2.77 16.27 6.77 表 2 BNCS、BNCS-CV-10和BNCS-CP-30电极C 1s、O 1s、N 1s分峰拟合结果中各类官能团相对含量
Table 2. The relative contents of functional groups of the BNCS, BNCS-CV-10 and BNCS-CP-30 electrodes calculated by the XPS C 1s, O 1s, N 1s fitting peak area.
Sample C 1s/at. % O 1s/at. % N 1s/at. % ―COOH C=O C―O/C―N ―COOH C―OH C=O N―Q N―5 N―6 BNCS 3.8 2.7 15.1 32.8 7.2 59.9 21.3 37.8 40.9 BNCS-CV-10 6.6 7.4 15.3 29.0 21.4 49.6 14.7 78.4 6.9 BNCS-CP-30 7.6 4.2 18.3 40.9 38.0 21.1 16.0 71.0 13.0 -
[1] Choudhary N, Li C, Moore J, Nagaiah N, et al. Asymmetric supercapacitor electrodes and devices[J]. Advanced Materials,2017,29(21):1605336. doi: 10.1002/adma.201605336 [2] Raza W, Ali F Z, Raza N, et al. Recent advancements in supercapacitor technology[J]. Nano Energy,2018,52:441-473. doi: 10.1016/j.nanoen.2018.08.013 [3] Nomura K, Nishihara H, Kobayashi N, et al. 4.4 V supercapacitors based on super-stable mesoporous carbon sheet made of edge-free graphene walls[J]. Energy & Environmental Science,2019,12(5):1542-1549. [4] Borenstein A, Hanna O, Attias R, et al. Carbon-based composite materials for supercapacitor electrodes: a review[J]. Journal of Materials Chemistry A,2017,5(25):12653-12672. doi: 10.1039/C7TA00863E [5] Chen X L, Paul R, Dai L M. Carbon-based supercapacitors for efficient energy storage[J]. National Science Review,2017,4(3):453-489. doi: 10.1093/nsr/nwx009 [6] Fleischmann S, Mitchell J B, Wang R C, et al. Pseudocapacitance: from fundamental understanding to high power energy storage materials[J]. Chemical Reviews,2020,120(14):6738-6782. doi: 10.1021/acs.chemrev.0c00170 [7] Lee J S M, Briggs M E, Hu C C, et al. Controlling electric double-layer capacitance and pseudocapacitance in heteroatom-doped carbons derived from hypercrosslinked microporous polymers[J]. Nano Energy,2018,46:277-289. doi: 10.1016/j.nanoen.2018.01.042 [8] Li Z, Xu Z W, Wang H L, et al. Colossal pseudocapacitance in a high functionality-high surface area carbon anode doubles the energy of an asymmetric supercapacitor[J]. Energy & Environmental Science,2014,7(5):1708-1718. [9] Xu Z X, Zhuang X D, Yang C Q, et al. Nitrogen-doped porous carbon superstructures derived from hierarchical assembly of polyimide nanosheets[J]. Advanced Materials,2016,28(10):1981-1987. doi: 10.1002/adma.201505131 [10] Enterria M, Pereira M F R, Martins J I, et al. Hydrothermal functionalization of ordered mesoporous carbons: The effect of boron on supercapacitor performance[J]. Carbon,2015,95:72-83. doi: 10.1016/j.carbon.2015.08.009 [11] Lin T Q, Chen I W, Liu F X, et al. Nitrogen-doped mesoporous carbon of extraordinary capacitance for electrochemical energy storage[J]. Science,2015,350(6267):1508-1513. doi: 10.1126/science.aab3798 [12] Zhang Y, Qu T T, Xiang K, et al. In situ formation/carbonization of quinone-amine polymers towards hierarchical porous carbon foam with high faradaic activity for energy storage[J]. Journal of Materials Chemistry A,2018,6(5):2353-2359. doi: 10.1039/C7TA09644E [13] Song Z Y, Miao L, Li L C, et al. A universal strategy to obtain highly redox-active porous carbons for efficient energy storage[J]. Journal of Materials Chemistry A,2020,8(7):3717-3725. doi: 10.1039/C9TA13520K [14] Zhou M, Li X Y, Zhao H, et al. Combined effect of nitrogen and oxygen heteroatoms and micropores of porous carbon frameworks from Schiff-base networks on their high supercapacitance[J]. Journal of Materials Chemistry A,2018,6(4):1621-1629. doi: 10.1039/C7TA08366A [15] Wang Y G, Song Y F, Xia Y Y. Electrochemical capacitors: mechanism, materials, systems, characterization and applications[J]. Chemical Society Reviews,2016,45(21):5925-5950. doi: 10.1039/C5CS00580A [16] Liu B, Liu Y J, Chen H B, et al. Oxygen and nitrogen co-doped porous carbon nanosheets derived from perilla frutescens for high volumetric performance supercapacitors[J]. Journal of Power Sources,2017,341:309-317. doi: 10.1016/j.jpowsour.2016.12.022 [17] Sanchez-Sanchez A, Izquierdo M T, Mathieu S, et al. Outstanding electrochemical performance of highly N- and O-doped carbons derived from pine tannin[J]. Green Chemistry,2017,19(11):2653-2665. doi: 10.1039/C7GC00491E [18] Liu M R, Zhang K J, Si M Y, et al. Three-dimensional carbon nanosheets derived from micro-morphologically regulated biomass for ultrahigh-performance supercapacitors[J]. Carbon,2019,153:707-716. doi: 10.1016/j.carbon.2019.07.060 [19] Park J H, Lee H J, Cho J Y, et al. Highly exfoliated and functionalized single-walled carbon nanotubes as fast-charging, high-capacity cathodes for rechargeable lithium-ion batteries[J]. ACS Applied Material & Interfaces,2020,12(1):1322-1329. [20] Liu T Y, Davijani A A B, Sun J Y, et al. Hydrothermally oxidized single-walled carbon nanotube networks for high volumetric electrochemical energy storage[J]. Small,2016,12(25):3423-3431. doi: 10.1002/smll.201600974 [21] Tabti Z, Ruiz-Rosas R, Quijada C, et al. Tailoring the surface chemistry of activated carbon cloth by electrochemical methods[J]. ACS Applied Material & Interfaces,2014,6(14):11682-11691. [22] Wang W, Liu W Y, Zeng Y X, et al. A novel exfoliation strategy to significantly boost the energy storage capability of commercial carbon cloth[J]. Advanced Materials,2015,27(23):3572-3578. doi: 10.1002/adma.201500707 [23] Berenguer R, Nishihara H, Itoi H, et al. Electrochemical generation of oxygen-containing groups in an ordered microporous zeolite-templated carbon[J]. Carbon,2013,54:94-104. doi: 10.1016/j.carbon.2012.11.007 [24] Berenguer R, Marco-Lozar J P, Quijada C, et al. A comparison between oxidation of activated carbon by electrochemical and chemical treatments[J]. Carbon,2012,50(3):1123-1134. doi: 10.1016/j.carbon.2011.10.025 [25] Wang Y, Chang Z, Zhang Z C, et al. A facile approach to improve electrochemical capacitance of carbons by in situ electrochemical oxidation[J]. ACS Applied Material & Interfaces,2019,11(6):5999-6008. doi: 10.1021/acsami.8b19071 [26] Hu Y R, Dong X L, Zhuang H K, et al. Introducing electrochemically active oxygen species to boost the pseudocapacitance of carbon-based supercapacitor[J]. ChemElectroChem,2021,8(16):3073-3079. doi: 10.1002/celc.202100641 -





 下载:
下载: