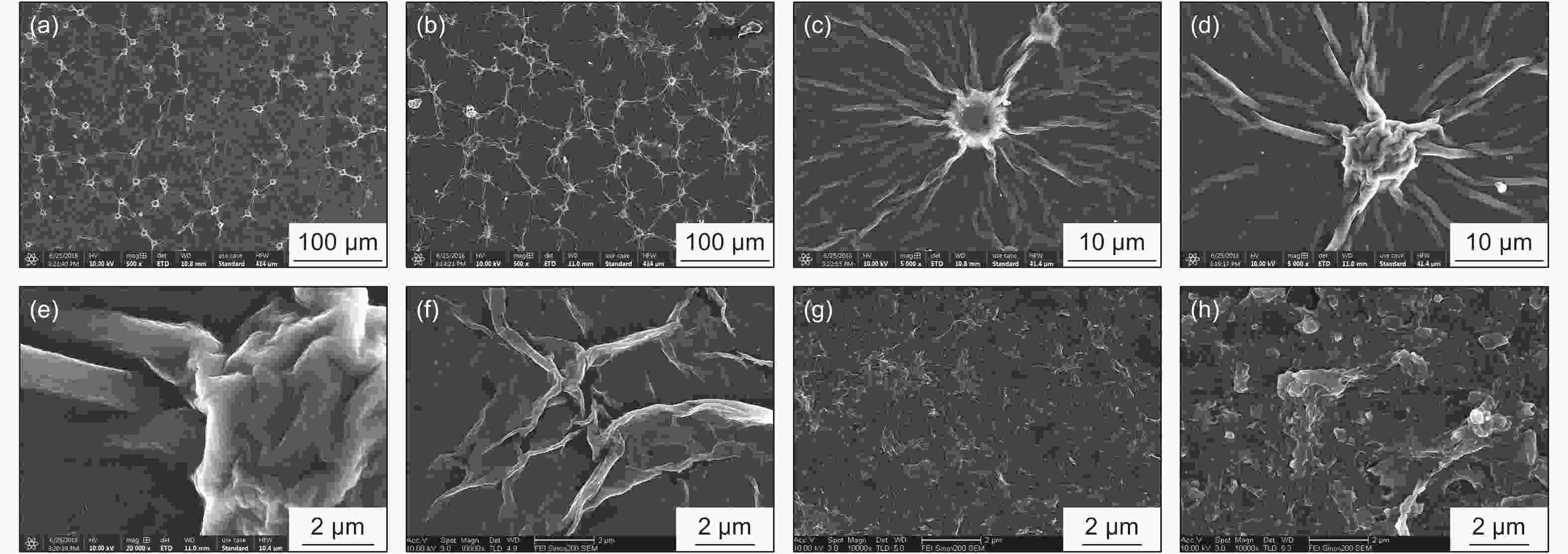
Electrochemical sensing of phenacetin on electrochemically reduced graphene oxide modified glassy carbon electrode
-
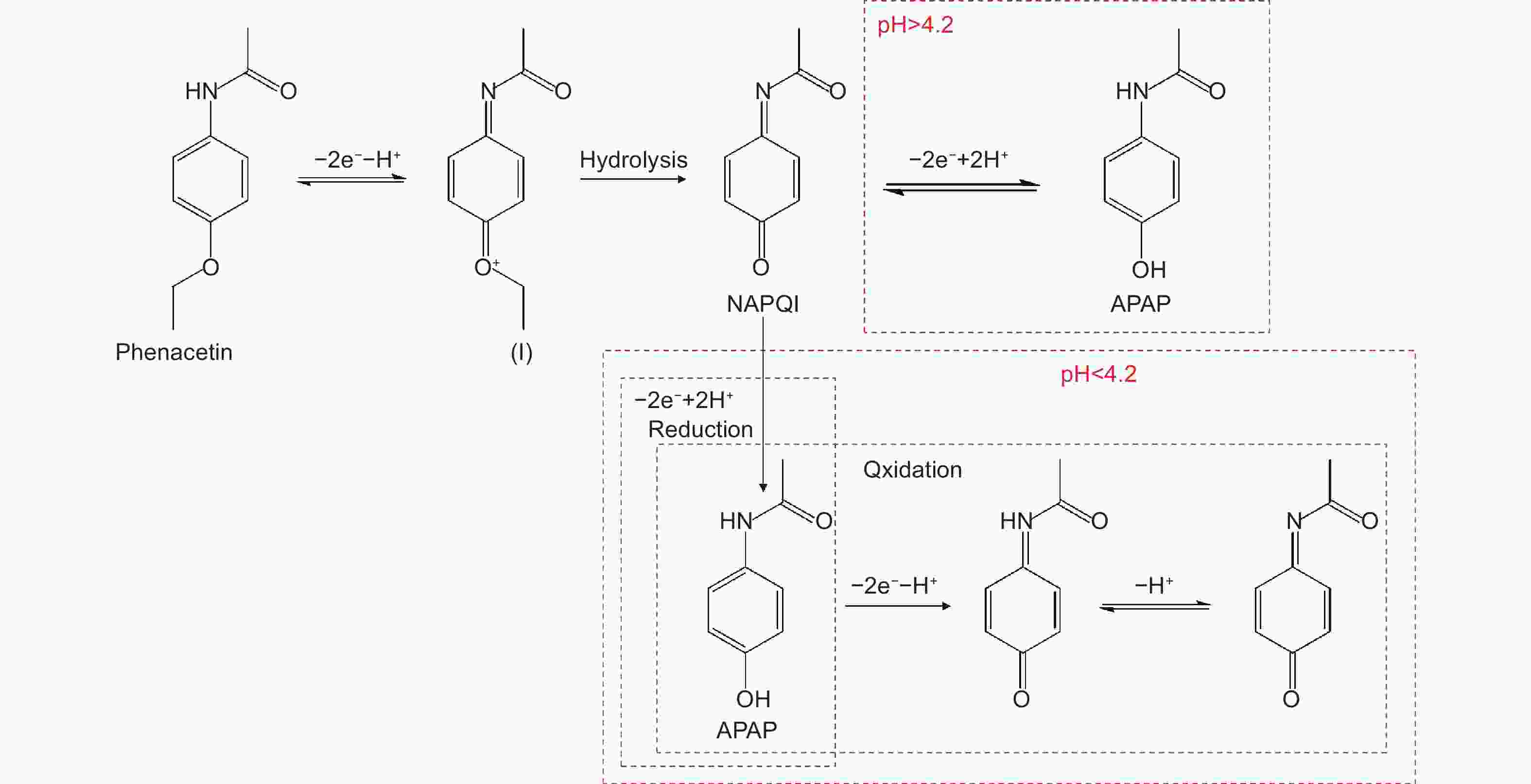
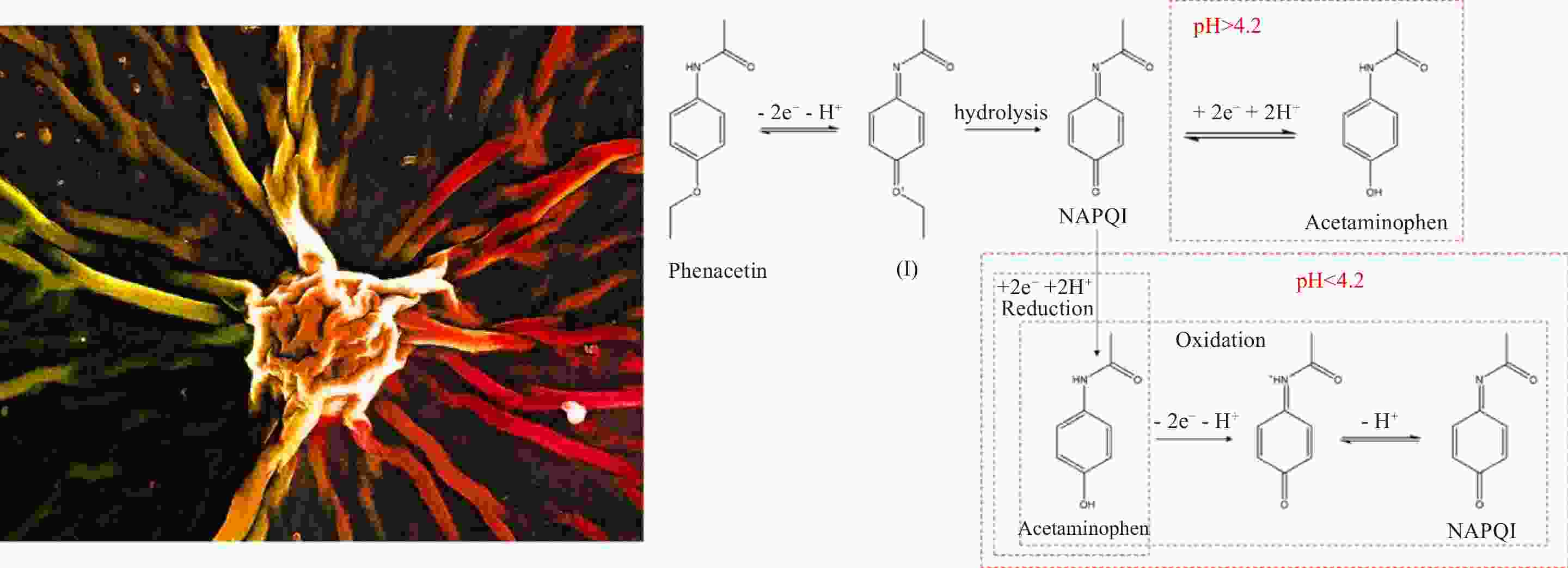
摘要: 研究了非纳西丁在还原氧化石墨烯、氮掺杂石墨烯等材料表面的电化学氧化还原行为,证明了还原氧化石墨烯具有更好的电化学响应,体现为更高的电流响应和更低的氧化还原过电位。同时,通过电化学方法对非那西丁的氧化还原反应机理进行了推断,证明了非那西丁通过氧化反应生成了一种醌-亚胺阳离子的中间体,通过水解生成N-乙酰基-对-苯醌亚胺(NAPQI),经可逆的氧化还原反应实现NAPQI与对乙酰氨基酚的互相转化。基于还原氧化石墨烯修饰电极,对非那西丁进行了定量检测,检出限为0.91 μmol L−1;证明了对乙酰氨基酚不会干扰对非那西丁的检测,但非那西丁经氧化还原反应产生的对乙酰氨基酚会影响对溶液中原本的对乙酰氨基酚的测定。
-
关键词:
- 非纳西丁 /
- 对乙酰氨基酚 /
- 电化学还原氧化石墨烯 /
- 电化学传感
Abstract: It is known that the electrochemical determination of phenacetin, a widely used analgesic, is challenging because of the interference of the electroactive intermediate, acetaminophen. Phenacetin was proven to be electroactive in 1980s, but its electrochemical determination has not been widely reported. This determination on an electrochemically reduced graphene oxide (ERGO) electrode was investigated and compared with several nitrogen-doped graphene samples. Results indicate that ERGO has a higher current response and lower oxidation potential than nitrogen-doped graphene. An ERGO electrode as a phenacetin sensor has a detection limit of 0.91 μmol L−1. The redox mechanism of phenacetin is inferred by electrochemical experiments, and the reactions under different pH values are proposed. Acetaminophen is considered to be the main intermediate and that does not interfere with the determination of phenacetin. But phenacetin obviously interferes with the response of acetaminophen, suggesting that the simultaneous detection of phenacetin and acetaminophen is not possible. Species such as Cu2+, Al3+, methanol, ethylene glycol, glucose, and ascorbic acid do not interfere with the determination of phenacetin. -
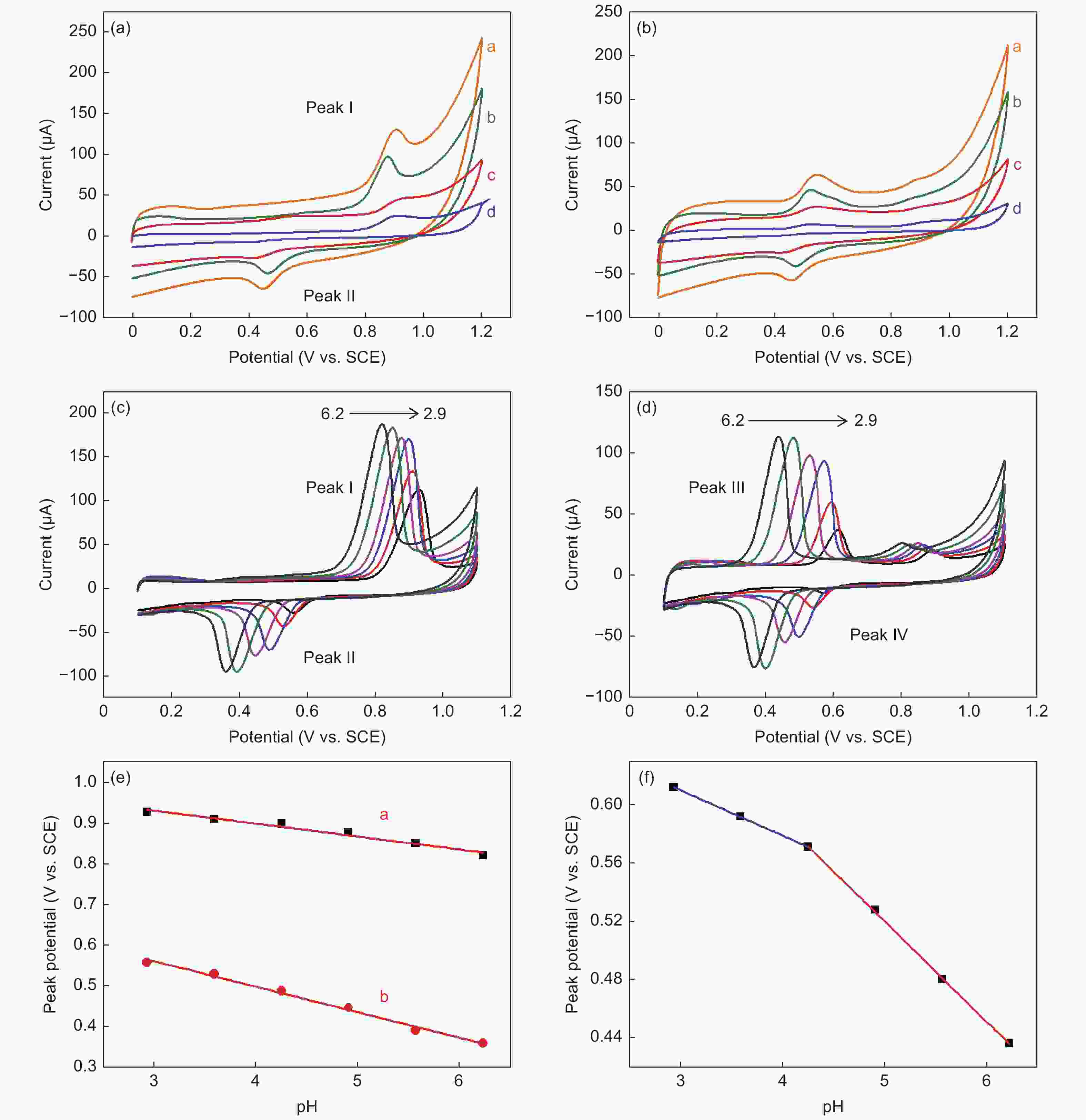
Figure 2. CVs of phenacetin (0.05 mmol L−1) on various modified electrodes (a) the 1st cycle and (b) the 2nd cycle ( curve a: NGE-A, curve b: ERGO, cure c: NGE-U, curve d: NGE-N). CVs of phenacetin (0.1 mmol L−1) on ERGO at different pH values (c) the 1st cycle and (d) the 2nd cycle. (e) Dependence of Epa (phenacetin) and Epc (NAPQI) on pH value. (f) dependence of Epa (acetaminophen) on pH value at a scan rate of 100 mV s−1
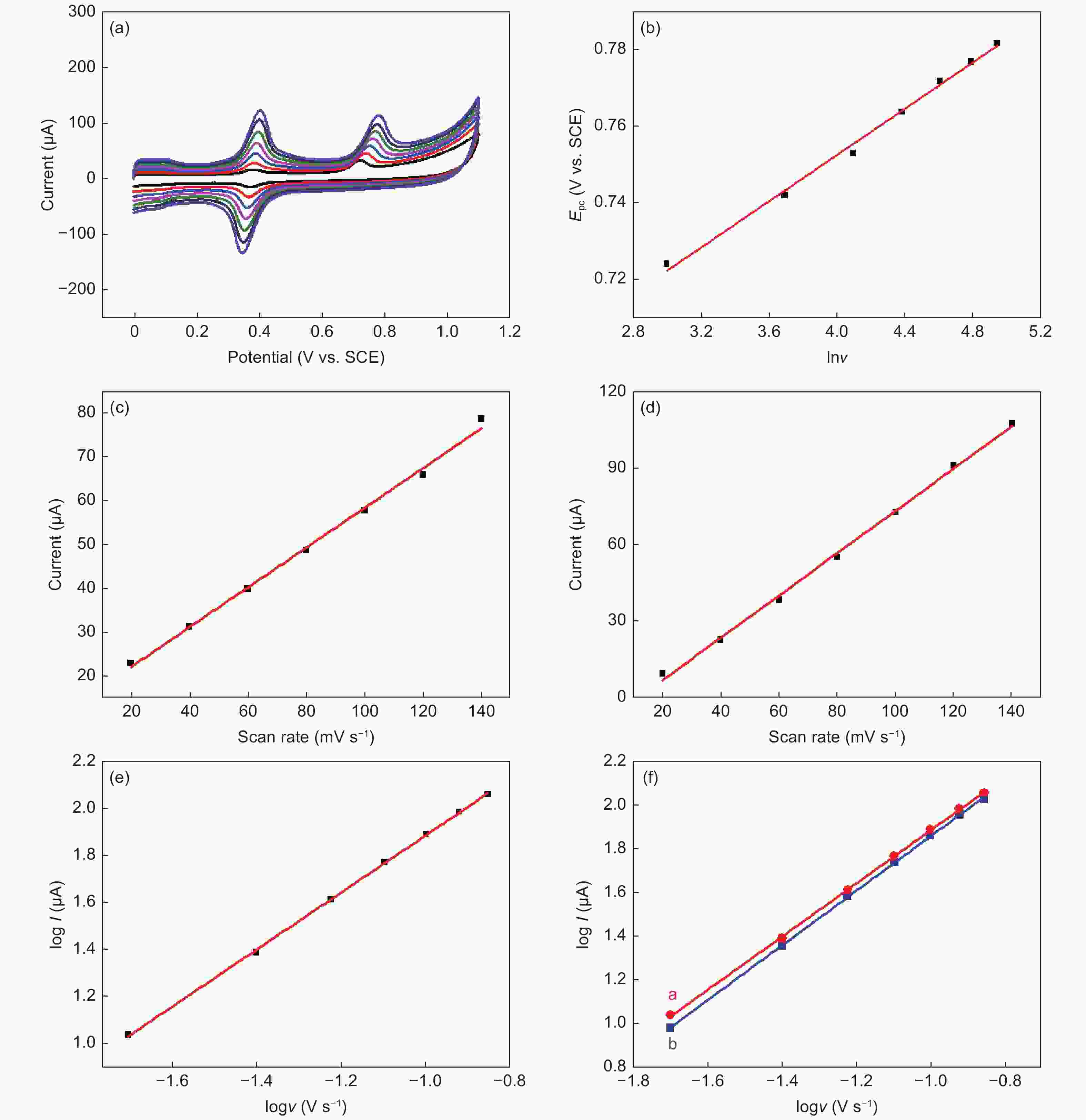
Figure 3. (a) CVs of phenacetin (0.1 mmol L−1) on ERGO at different scan rates (20-140 mV s−1). (b) Dependence of Epc on lnv. Dependence of the peak currents for (c) peak Ⅰ and (d) peak Ⅱ on the scan rate. (e) The relationship between logI and logv for phenacetin oxidation. (f) The relationship between logI and logv for NAPQI reduction (red) and acetaminophen oxidation (black)
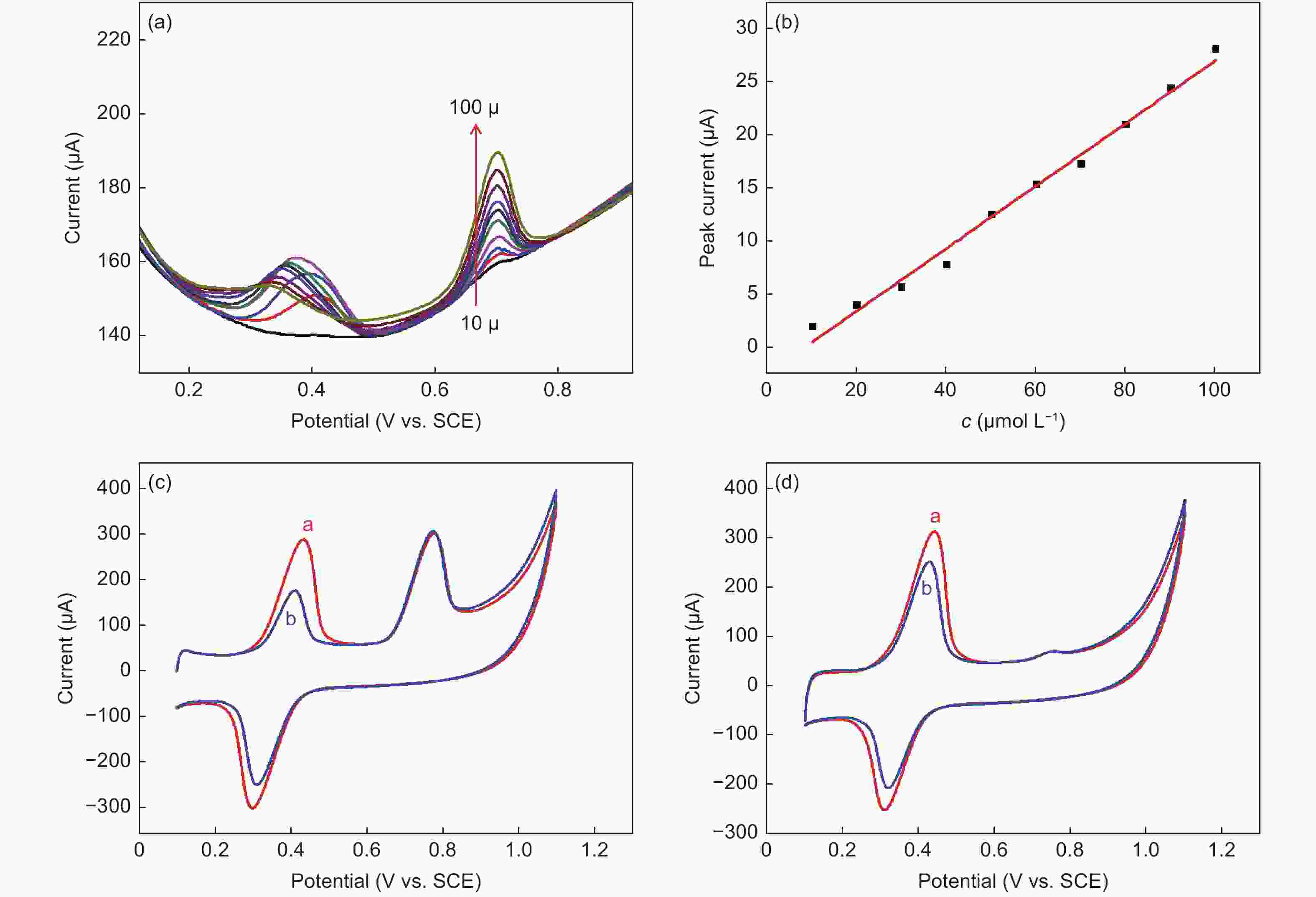
Figure 4. (a) DPVs on ERGO with successive adding of phenacetin (10-100 μmol L−1). (b) Dependence of the peak current on the phenacetin concentration. (c) The 1st CV cycle of 1 mmol L−1 phenacetin (curve b) and 1 mmol L−1 phenacetin + 1 mmol L−1 acetaminophen (curve a). (d) The 2nd CV cycle of 1 mmol L−1 phenacetin (curve b) and 1 mmol L−1 phenacetin + 1 mmol L−1 acetaminophen (curve a)
-
[1] Miner D J, Rice J R, Riggin R M, et al. Voltammetry of acetaminophen and its metabolites[J]. Analytical chemistry,1981,53(14):2258-2263. doi: 10.1021/ac00237a029 [2] Bussy U, Giraudeau P, Tea I, et al. Understanding the degradation of electrochemically-generated reactive drug metabolites by quantitative NMR[J]. Talanta,2013,116:554-558. doi: 10.1016/j.talanta.2013.07.026 [3] Nouri-Nigjeh E, Bischoff R, Bruins A P, et al. Electrochemical oxidation by square-wave potential pulses in the imitation of phenacetin to acetaminophen biotransformation[J]. Analyst,2011,136(23):5064-5067. doi: 10.1039/c1an15643h [4] Yarman A, Scheller F W. MIP‐esterase/tyrosinase combinations for paracetamol and phenacetin[J]. Electroanalysis,2016,28(9):2222-2227. doi: 10.1002/elan.201600042 [5] Jiang L, Gu S, Ding Y, et al. Facile and novel electrochemical preparation of a graphene–transition metal oxide nanocomposite for ultrasensitive electrochemical sensing of acetaminophen and phenacetin[J]. Nanoscale,2014,6(1):207-214. doi: 10.1039/C3NR03620K [6] Yin H, Meng X, Xu Z, et al. Electrochemical behavior of phenacetin on CdSe microspheres modified glassy carbon electrode and its simultaneous determination with paracetamol and 4-aminophenol[J]. Analytical Methods,2012,4(5):1445-1451. doi: 10.1039/c2ay05912f [7] Li R, Li H, Li Z, et al. Electrochemical determination of acetaminophen using a glassy carbon electrode modified with a hybrid material consisting of graphene aerogel and octadecylamine-functionalized carbon quantum dots[J]. Microchimica Acta,2018,185(2):1-9. [8] Wong A, Santos A M, Silva T A, et al. Simultaneous determination of isoproterenol, acetaminophen, folic acid, propranolol and caffeine using a sensor platform based on carbon black, graphene oxide, copper nanoparticles and PEDOT: PSS[J]. Talanta,2018,183:329-338. doi: 10.1016/j.talanta.2018.02.066 [9] Zhu W, Huang H, Gao X, et al. Electrochemical behavior and voltammetric determination of acetaminophen based on glassy carbon electrodes modified with poly (4-aminobenzoic acid)/electrochemically reduced graphene oxide composite films[J]. Materials Science and Engineering: C,2014,45:21-28. doi: 10.1016/j.msec.2014.08.067 [10] Zhang X, Wang K P, Zhang L N, et al. Phosphorus-doped graphene-based electrochemical sensor for sensitive detection of acetaminophen[J]. Analytica chimica acta,2018,1036:26-32. doi: 10.1016/j.aca.2018.06.079 [11] Cao Y, Si W, Zhang Y, et al. Nitrogen-doped graphene: effect of graphitic-N on the electrochemical sensing properties towards acetaminophen[J]. FlatChem,2018,9:1-7. doi: 10.1016/j.flatc.2018.03.002 [12] Anuar N S, Basirun W J, Ladan M, et al. Fabrication of platinum nitrogen-doped graphene nanocomposite modified electrode for the electrochemical detection of acetaminophen[J]. Sensors and Actuators B: Chemical,2018,266:375-383. doi: 10.1016/j.snb.2018.03.138 [13] Wu L, Lei W, Han Z, et al. A novel non-enzyme amperometric platform based on poly (3-methylthiophene)/nitrogen doped graphene modified electrode for determination of trace amounts of pesticide phoxim[J]. Sensors and Actuators B: Chemical,2015,206:495-501. doi: 10.1016/j.snb.2014.09.098 [14] Chen X, Zhang G, Shi L, et al. Au/ZnO hybrid nanocatalysts impregnated in N-doped graphene for simultaneous determination of ascorbic acid, acetaminophen and dopamine[J]. Materials Science and Engineering: C,2016,65:80-89. doi: 10.1016/j.msec.2016.03.106 [15] Geng D, Chen Y, Chen Y, et al. High oxygen-reduction activity and durability of nitrogen-doped graphene[J]. Energy & Environmental Science,2011,4(3):760-764. [16] Lin Z, Waller G, Liu Y, et al. Facile synthesis of nitrogen‐doped graphene via pyrolysis of graphene oxide and urea, and its electrocatalytic activity toward the oxygen‐reduction reaction[J]. Advanced Energy Materials,2012,2(7):884-888. doi: 10.1002/aenm.201200038 [17] Deng D, Pan X, Yu L, et al. Toward N-doped graphene via solvothermal synthesis[J]. Chemistry of Materials,2011,23(5):1188-1193. doi: 10.1021/cm102666r [18] Si W, Lei W, Hao Q, et al. Facile synthesis of nitrogen-doped graphene derived from graphene oxide and vitamin B3 as high-performance sensor for imidacloprid determination[J]. Electrochimica Acta,2016,212:784-790. doi: 10.1016/j.electacta.2016.07.063 [19] Wang L, Wang C, Wang H, et al. ZIF-8 nanocrystals derived N-doped carbon decorated graphene sheets for symmetric supercapacitors[J]. Electrochimica Acta,2018,289:494-502. doi: 10.1016/j.electacta.2018.09.091 [20] Wang H, Hao Q, Yang X, et al. Graphene oxide doped polyaniline for supercapacitors[J]. Electrochemistry Communications,2009,11(6):1158-1161. doi: 10.1016/j.elecom.2009.03.036 [21] Wang H, Hao Q, Yang X, et al. Effect of graphene oxide on the properties of its composite with polyaniline[J]. ACS applied materials & interfaces,2010,2(3):821-828. [22] Hao Q, Xia X, Lei W, et al. Facile synthesis of sandwich-like polyaniline/boron-doped graphene nano hybrid for supercapacitors[J]. Carbon,2015,81:552-563. doi: 10.1016/j.carbon.2014.09.090 [23] Rocha D P, Dornellas R M, Cardoso R M, et al. Chemically versus electrochemically reduced graphene oxide: Improved amperometric and voltammetric sensors of phenolic compounds on higher roughness surfaces[J]. Sensors and Actuators B: Chemical,2018,254:701-708. doi: 10.1016/j.snb.2017.07.070 [24] Lv W, Zhang C, Li Z, et al. Self-assembled 3D graphene monolith from solution[J]. The journal of physical chemistry letters,2015,6(4):658-668. doi: 10.1021/jz502655m [25] Adhikari B R, Govindhan M, Chen A. Sensitive detection of acetaminophen with graphene-based electrochemical sensor[J]. Electrochimica Acta,2015,162:198-204. doi: 10.1016/j.electacta.2014.10.028 [26] Ensafi A A, Ahmadi N, Rezaei B, et al. A new electrochemical sensor for the simultaneous determination of acetaminophen and codeine based on porous silicon/palladium nanostructure[J]. Talanta,2015,134:745-753. doi: 10.1016/j.talanta.2014.12.028 [27] Nematollahi D, Shayani-Jam H, Alimoradi M, et al. Electrochemical oxidation of acetaminophen in aqueous solutions: Kinetic evaluation of hydrolysis, hydroxylation and dimerization processes[J]. Electrochimica Acta,2009,54(28):7407-7415. doi: 10.1016/j.electacta.2009.07.077 [28] Laviron E. Surface linear potential sweep voltammetry: Equation of the peaks for a reversible reaction when interactions between the adsorbed molecules are taken into account[J]. Journal of Electroanalytical Chemistry and Interfacial Electrochemistry,1974,52(3):395-402. doi: 10.1016/S0022-0728(74)80449-3 [29] Yin H, Cui L, Ai S, et al. Electrochemical determination of bisphenol A at Mg–Al–CO3 layered double hydroxide modified glassy carbon electrode[J]. Electrochimica Acta,2010,55(3):603-610. doi: 10.1016/j.electacta.2009.09.020 -





 下载:
下载: