The formation of uniform graphene-polyaniline hybrids using a completely miscible cosolvent that have an excellent electrochemical performance
-
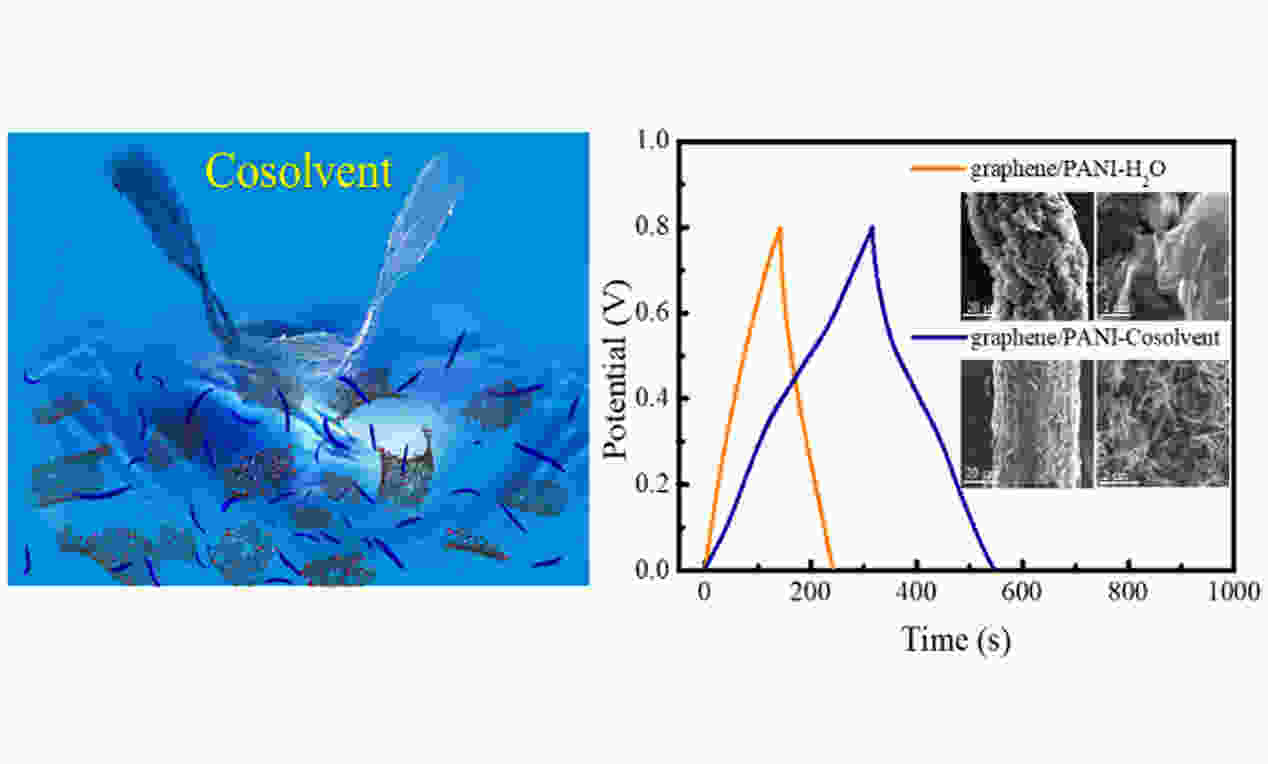
摘要: 石墨烯/聚苯胺复合材料的电化学性能很大程度上由其微观结构形貌和聚苯胺在石墨烯片层上的分布情况决定。在制备该复合材料时,两者的均相分布对于解决石墨烯片层的堆叠以及聚苯胺纳米结构的团聚至关重要。常见的聚苯胺/石墨烯均相复合材料制备方法,如逐层自组装或电化学原位聚合等,流程复杂、难以实现大规模生产。本文采用N, N-二甲基甲酰胺和水组成的互溶共溶剂成功地实现了石墨烯和聚苯胺的均相复合,通过共溶剂这一方式解决了上述所面临的问题。使用共溶剂的复合纤维展现出均相分布的微观结构,且聚苯胺均匀分布在石墨烯片层上,而相对比于只用水作为溶剂制备的复合纤维并未展现出均相复合的微观结构,使用共溶剂得到的复合纤维显示出优异的电化学性能。更重要的是通过共溶剂这一简单有效的策略,有望实现大规模生产两种成分均相分布且性能优异的石墨烯/聚苯胺复合材料。Abstract: The electrochemical properties of graphene-polyaniline (PANI) hybrids are largely determined by their microstructures and the distribution of PANI on the graphene network. Uniform hybridization of each component is critical to avoid the re-stacking of the graphene sheets and the agglomeration of PANI nanoassemblies during the use of the hybrids. Conventional strategies, such as layer-by-layer assembly or electrochemical in-situ polymerization, involve intricate procedures, making it difficult to achieve the large-scale production of the hybrids. We report a completely miscible cosolvent consisting of N, N-dimethylformamide and water that solves this problem and was used to produce graphene-PANI hybrid flexible fibers. It was found that the composite fiber had a homogeneous microstructure with PANI nanoassemblies uniformly distributed on the graphene sheets, and had outstanding electrochemical properties, much better than the counterpart fabricated using only water as the solvent. The work proposes a universal but simple strategy to achieve the mass production of graphene-PANI hybrids or similar materials with uniform hybridization of the two components.
-
Key words:
- Graphene /
- Polyaniline /
- Cosolvent /
- Hybridization
-
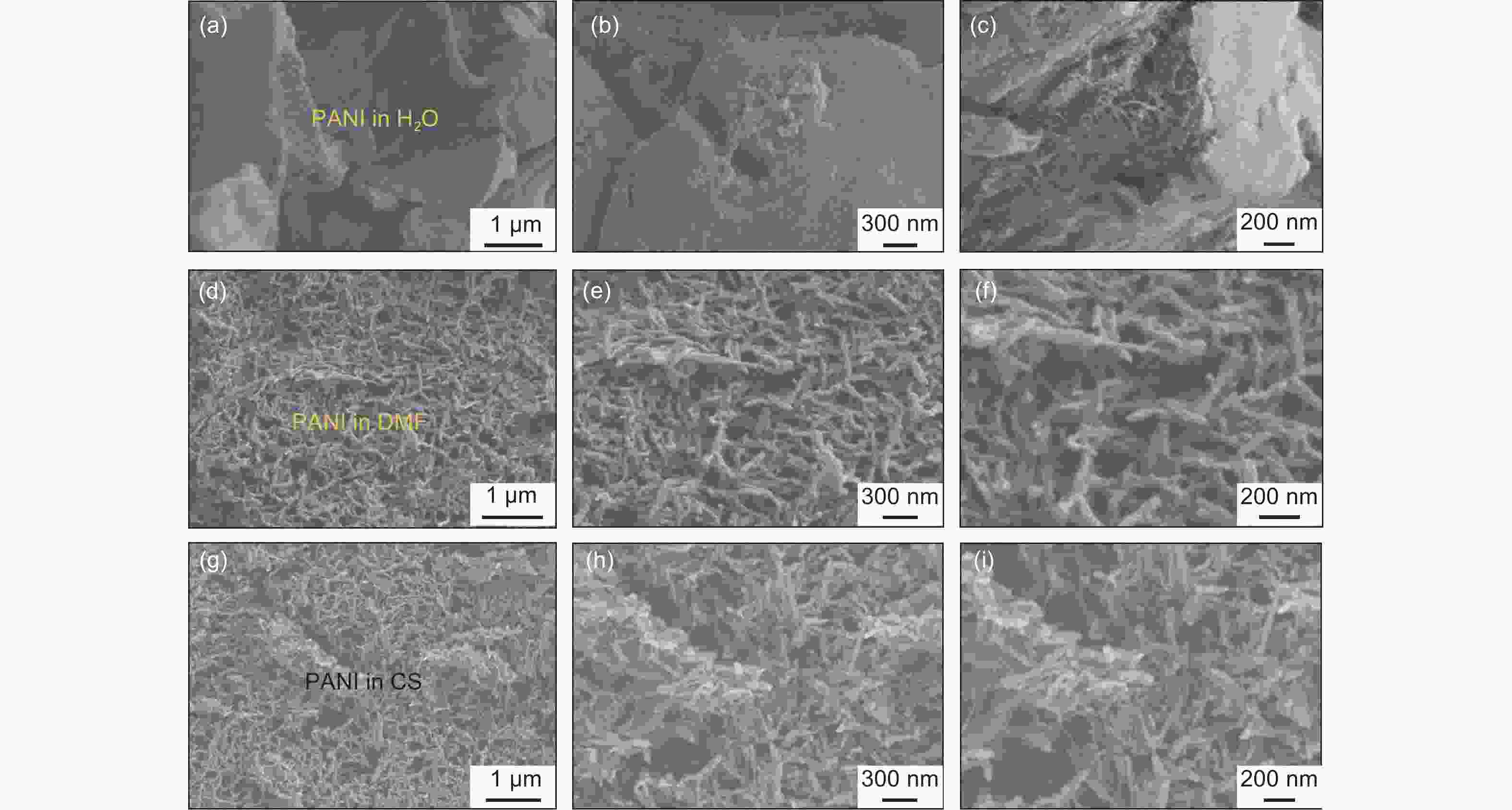
Figure 1. Photos of (a) GO suspensions, (b) PANI suspensions, (c) GO/PANI gel in a H2O/DMF cosolvent, and (d) the as-spun flexible GN/PANI composite fiber. (e) Raman spectra and (f) XRD patterns of PANI, pure GN fiber, and GN/PANI-CS fiber. (g) SEM image of GN/PANI-CS fiber showing the uniform diameter of the fiber. (h) Higher magnification SEM image and (i) TEM image of GN/PANI-CS fiber, further exhibiting the uniformly distributed PANI nanoneedles.
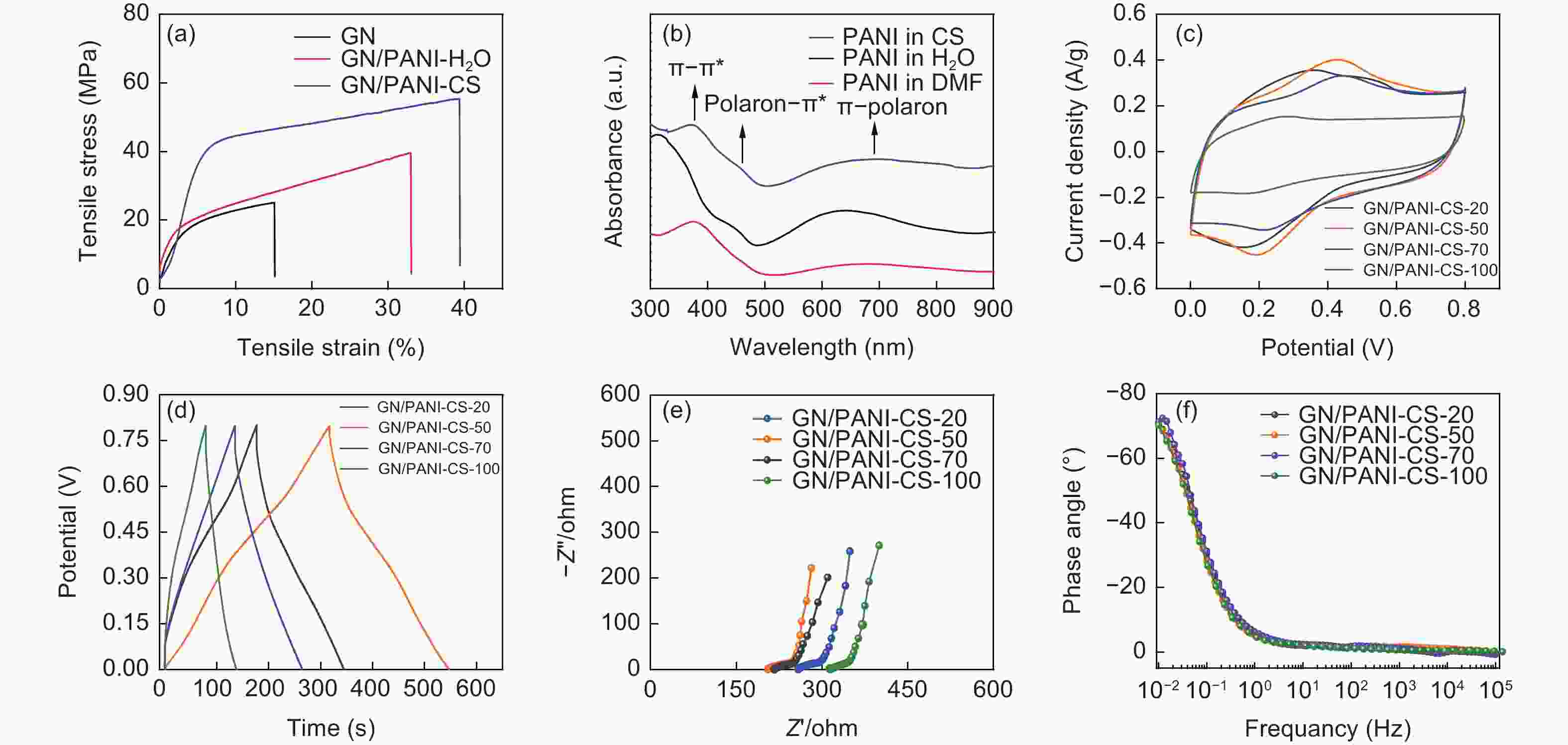
Figure 3. (a) Stress-strain curves of pure GN fiber, GN/PANI-H2O, and GN/PANI-CS. (b) UV-vis spectra of PANI dispersed in H2O (black), DMF (red), and H2O/DMF cosolvent (blue). (c) CV curves at 5 mV s−1, (d) GCD profiles at 0.2 A g−1, (e) Nyquist plots and (f) Bode plots of GN/PANI-CS fiber supercapacitors with different mass ratios of GO to PANI.
Figure 4. (a) CV curves at 5 mV s−1, (b) GCD curves at 0.2 A g−1, (c) rate performance, (d) Nyquist plots, and (e) Bode plots of GN/PANI-H2O and GN/PANI-CS. (f) CV curves at different scan rates. (g) GCD profiles at various current densities of GN/PANI-CS. (h) Ragone plot of this work and other previously-reported literatures. (i) Cyclic performance at 1 A g−1 of GN/PANI-CS.
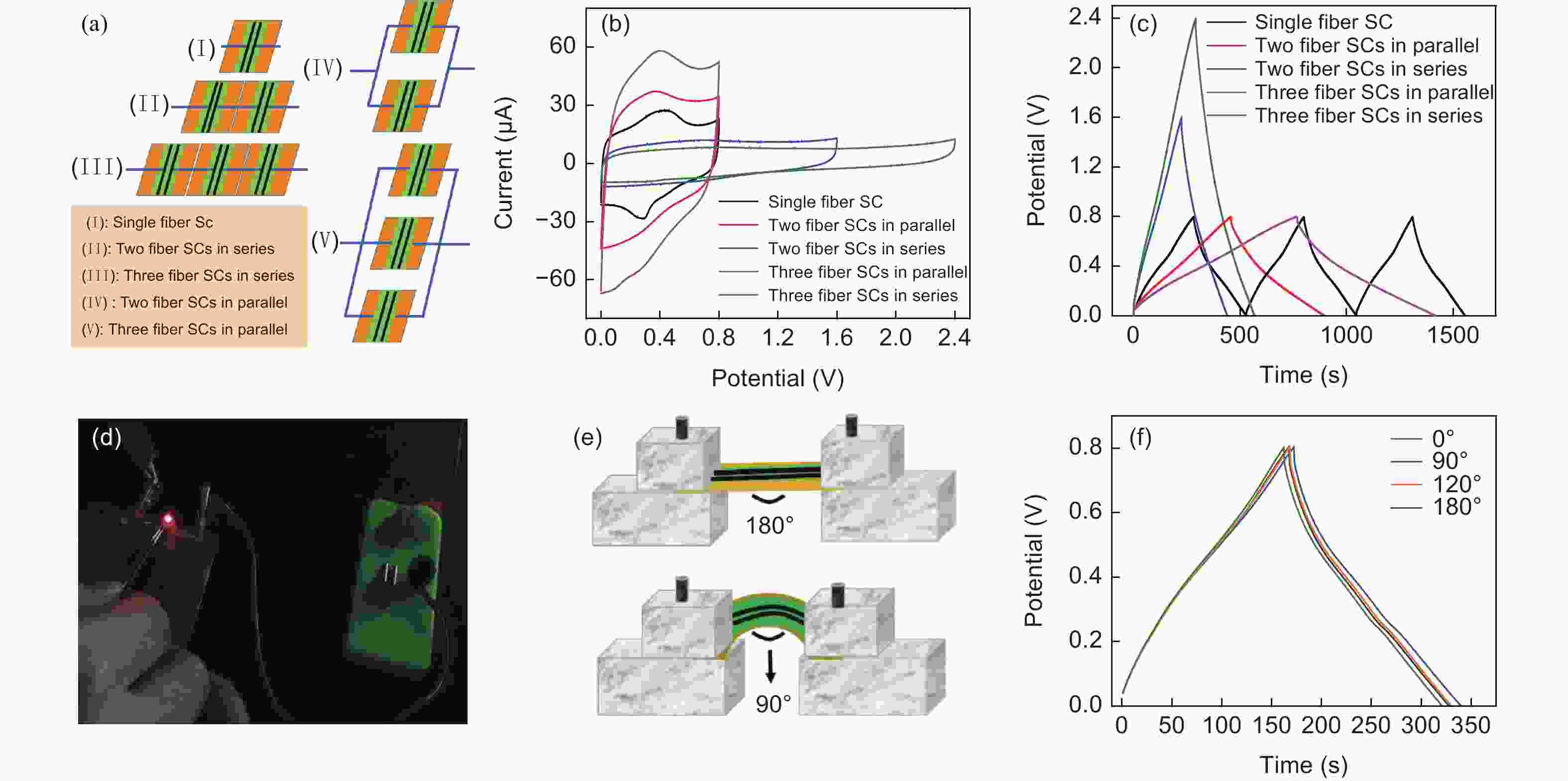
Figure 5. (a) Cartoons of devices of a single, two and three graphene/PANI-CS fiber supercapacitors connected in series or in parallel. (b) CV curves of a single, two, and three GN/PANI-CS fiber-based devices connected in parallel or in series at 2 mV s−1. (c) The GCD curves of single, two and three GN/PANI-CS fiber supercapacitors connected in parallel or in series at 0.1 A g−1. (d) Optical photo of a LED with a rated voltage of 1.5 V powered by a tandem device assembled by four fiber supercapacitors in series. (e) Cartoons of the fiber supercapacitor under bending conditions. (f) GCD profiles of a fiber supercapacitor at different bending angles.
-
[1] Zhang S, Pan N. Supercapacitors performance evaluation[J]. Advanced Energy Materials,2015,5(6):1401401. doi: 10.1002/aenm.201401401 [2] WANG Shuai, GUO Yu-zhe, WANG Fang-xiao, et al. Research progress on metal and covalent organic framework-based materials for high-performance supercapacitors[J]. New Carbon Materials,2022,37(1):109-135. [3] Tang X N, Zhu S K, Ning J, et al. Charge storage mechanisms of manganese dioxide-based supercapacitors: A review[J]. New Carbon Materials,2021,36(4):702-710. doi: 10.1016/S1872-5805(21)60082-3 [4] Cheng L, Li X J, Li J, et al. Construction of three-dimensional all-carbon C60/graphene hybrids and their use as electrodes for high performance supercapacitors[J]. New Carbon Materials,2020,35(6):684-695. doi: 10.1016/S1872-5805(20)60522-4 [5] Novoselov K S, Geim A K, Morozov S V, et al. Electric field effect in atomically thin carbon films[J]. Science,2004,306(5696):666-669. doi: 10.1126/science.1102896 [6] Castro Neto A H, Guinea F, Peres N M R, et al. The electronic properties of graphene[J]. Reviews of Modern Physics,2009,81(1):109-162. doi: 10.1103/RevModPhys.81.109 [7] Lee C, Wei X D, Kysar J W, et al. Measurement of the elastic properties and intrinsic strength of monolayer graphene[J]. Science,2008,321(5887):385-388. doi: 10.1126/science.1157996 [8] Qu G, Cheng J, Li X, et al. A fiber supercapacitor with high energy density based on hollow graphene/conducting polymer fiber electrode[J]. Advanced Materials,2016,28(19):3605-3605. doi: 10.1002/adma.201670128 [9] Zhu Z H, Xiong W, Hu X, et al. Flexible fiber-shaped supercapacitor utilizing hierarchical NiCo2O4@Polypyrrole core-shell nanowires on hemp-derived carbon[J]. Journal of Materials Chemistry A,2015,3(33):17209-17216. doi: 10.1039/C5TA04201A [10] Chen Q, Meng Y, Hu C, et al. MnO2 -modified hierarchical graphene fiber electrochemical supercapacitor[J]. Journal of Power Sources,2014,247(3):32-39. [11] Zhai S, Wang C, Karahan H E, et al. Nano-RuO2-decorated holey graphene composite fibers for nicro-supercapacitors with ultrahigh energy dnsity[J]. Small,2018,14(29):1800582. doi: 10.1002/smll.201800582 [12] Zhu Y L, Wang Y X, Gao C, et al. CoMoO4-N-doped carbon hybrid nanoparticles loaded on a petroleum asphalt-based porous carbon for lithium storage[J]. New Carbon Materials,2020,35(4):358-370. doi: 10.1016/S1872-5805(20)60494-2 [13] Gao F, Qin S H, Zang Y H, et al. Highly efficient formation of Mn3O4-graphene oxide hybrid aerogels for use as the cathode material of high performance lithium ion batteries[J]. New Carbon Materials,2020,35(2):121-130. doi: 10.1016/S1872-5805(20)60479-6 [14] Pandolfo A G, Hollenkamp A F. Carbon properties and their role in supercapacitors[J]. Journal of Power Sources,2006,157(1):11-27. doi: 10.1016/j.jpowsour.2006.02.065 [15] Wang Y, Shi Z, Huang Y, et al. Supercapacitor devices based on graphene materials[J]. The Journal of Physical Chemistry C,2009,113(30):13103-13107. doi: 10.1021/jp902214f [16] Liu C, Yu Z, Neff D, et al. Graphene-based supercapacitor with an ultrahigh energy Ddensity[J]. Nano Letters,2010,10(12):4863-4868. doi: 10.1021/nl102661q [17] Wei F, Zhang H F, He X J, et al. Synthesis of porous carbons from coal tar pitch for high-performance supercapacitors[J]. New Carbon Materials,2019,34(2):132-139. doi: 10.1016/S1872-5805(19)60006-5 [18] Gao F, Zang Y H, Wang Y, et al. A review of the synthesis of carbon materials for energy storage from biomass and coal/heavy oil waste[J]. New Carbon Materials,2021,36(1):34-48. doi: 10.1016/S1872-5805(21)60003-3 [19] Peng C, Hu D, Chen G Z. Theoretical specific capacitance based on charge storage mechanisms of conducting polymers: Comment on ‘Vertically oriented arrays of polyaniline nanorods and their super electrochemical properties’[J]. Chemical Communications,2011,47(14):4105-4107. doi: 10.1039/c1cc10675a [20] Huang J, Virji S, Weiller B H, et al. Polyaniline nanofibers: Facile synthesis and chemical sensors[J]. Journal of the American Chemical Society,2003,125(2):314-315. doi: 10.1021/ja028371y [21] Kim J, Cote L J, Kim F, et al. Graphene oxide sheets at interfaces[J]. Journal of the American Chemical Society,2010,132(23):8180-8186. doi: 10.1021/ja102777p [22] Shao J J, Lv W, Guo Q, et al. Hybridization of graphene oxide and carbon nanotubes at the liquid/air interface[J]. Chemical Communications,2012,48(31):3706-3708. doi: 10.1039/C1CC16838J [23] Cote L J, Cruz-Silva R, Huang J. Flash reduction and patterning of graphite oxide and its polymer composite[J]. Journal of the American Chemical Society,2009,131(31):11027-11032. doi: 10.1021/ja902348k [24] Chen C M, Huang J Q, Zhang Q, et al. Annealing a graphene oxide film to produce a free standing high conductive graphene film[J]. Carbon,2012,50(2):659-667. doi: 10.1016/j.carbon.2011.09.022 [25] Pei S, Zhao J, Du J, et al. Direct reduction of graphene oxide films into highly conductive and flexible graphene films by hydrohalic acids[J]. Carbon,2010,48(15):4466-4474. doi: 10.1016/j.carbon.2010.08.006 [26] Shao J J, Lv W, Yang Q H. Self-assembly of graphene oxide at interfaces[J]. Advanced Materials,2014,26(32):5586-5612. doi: 10.1002/adma.201400267 [27] Chen C, Yang Q H, Yang Y, et al. Self-assembled free-standing graphite oxide membrane[J]. Advanced Materials,2009,21(29):3007-3011. doi: 10.1002/adma.200803726 [28] Wu D Y, Zhou W H, He L Y, et al. Micro-corrugated graphene sheet enabled high-performance all-solid-state film supercapacitor[J]. Carbon,2020,160:156-163. doi: 10.1016/j.carbon.2020.01.019 [29] Xu Z, Zheng B, Chen J, et al. Highly efficient synthesis of neat graphene nanoscrolls from graphene oxide by well-controlled lyophilization[J]. Chemistry of Materials,2014,26(23):6811-6818. doi: 10.1021/cm503418h [30] Xu Z, Gao C. Graphene chiral liquid crystals and macroscopic assembled fibres[J]. Nature Communications,2011,2(1):571. doi: 10.1038/ncomms1583 [31] Sarker A K, Hong J D. Layer-by-layer self-assembled multilayer films composed of graphene/polyaniline bilayers: High-energy electrode materials for supercapacitors[J]. Langmuir,2012,28(34):12637-12646. doi: 10.1021/la3021589 [32] Zhou S, Zhang H, Zhao Q, et al. Graphene-wrapped polyaniline nanofibers as electrode materials for organic supercapacitors[J]. Carbon,2013,52:440-450. doi: 10.1016/j.carbon.2012.09.055 [33] Feng X M, Li R M, Ma Y W, et al. One-step electrochemical synthesis of graphene/polyaniline composite film and its applications[J]. Advanced Functional Materials,2011,21(15):2989-2996. doi: 10.1002/adfm.201100038 [34] Zhang Q, Li Y, Feng Y, et al. Electropolymerization of graphene oxide/polyaniline composite for high-performance supercapacitor[J]. Electrochimica Acta,2013,90:95-100. doi: 10.1016/j.electacta.2012.11.035 [35] Kim F, Luo J, Cruz-Silva R, et al. Self-propagating domino-like reactions in oxidized graphite[J]. Advanced Functional Materials,2010,20(17):2867-2873. doi: 10.1002/adfm.201000736 [36] Chen X, Xiang T, Li Z, et al. A planar graphene-based film supercapacitor formed by liquid-air interfacial assembly[J]. Advanced Materials Interfaces,2017,4(9):1601127. doi: 10.1002/admi.201601127 [37] Huang J, Kaner R B. A general chemical route to polyaniline nanofibers[J]. Journal of the American Chemical Society,2004,126(3):851-855. doi: 10.1021/ja0371754 [38] Xu Z, Sun H, Zhao X, et al. Ultrastrong fibers assembled from giant graphene oxide sheets[J]. Advanced Materials,2013,25(2):188-193. doi: 10.1002/adma.201203448 [39] Zhang X, Zhang J, Wang R, et al. Cationic surfactant directed polyaniline/CNT nanocables: Synthesis, characterization, and enhanced electrical properties[J]. Carbon,2004,42(8):1455-1461. [40] Nobrega M M, Ceppatelli M, Temperini M L A, et al. Pressure-induced reactivity in the emeraldine salt and base forms of polyaniline probed by FTIR and Raman[J]. The Journal of Physical Chemistry C,2014,118(47):27559-27566. doi: 10.1021/jp509154j [41] Potphode D D, Sivaraman P, Mishra S P, et al. Polyaniline/partially exfoliated multi-walled carbon nanotubes based nanocomposites for supercapacitors[J]. Electrochimica Acta,2015,155:402-410. doi: 10.1016/j.electacta.2014.12.126 [42] Morávková Z, Trchová M, Dybal J, et al. The interaction of thin polyaniline films with various H-phosphonates: Spectroscopy and quantum chemical calculations[J]. Journal of Applied Polymer Science,2018,135(38):46728. doi: 10.1002/app.46728 [43] Casado J, Hernández V, López Navarrete J T. Vibrational Raman shifts and aromaticity: The case of oligothiophenes[J]. The Chemical Record,2015,15(6):1110-1118. doi: 10.1002/tcr.201500025 [44] Gao H, Ji B, Jäger I L, et al. Materials become insensitive to flaws at nanoscale: Lessons from nature[J]. Proceedings of the National Academy of Sciences,2003,100(10):5597. doi: 10.1073/pnas.0631609100 [45] Huang Y F, Lin C W. Facile synthesis and morphology control of graphene oxide/polyaniline nanocomposites via in-situ polymerization process[J]. Polymer,2012,53(13):2574-2582. doi: 10.1016/j.polymer.2012.04.022 [46] Yue J, Wang Z H, Cromack K R, et al. Effect of sulfonic acid group on polyaniline backbone[J]. Journal of the American Chemical Society,1991,113(7):2665-2671. doi: 10.1021/ja00007a046 [47] Xu J, Wang K, Zu S, et al. Hierarchical nanocomposites of polyaniline nanowire arrays on graphene oxide sheets with synergistic effect for energy storage[J]. ACS Nano,2010,4(9):5019-5026. doi: 10.1021/nn1006539 [48] Yang N, Zhai J, Wan M, et al. Layered nanostructures of polyaniline with graphene oxide as the dopant and template[J]. Synthetic Metals,2010,160(15):1617-1622. [49] Xia H, Narayanan J, Cheng D, et al. Formation of ordered arrays of oriented polyaniline nanoparticle nanorods[J]. The Journal of Physical Chemistry B,2005,109(26):12677-12684. doi: 10.1021/jp0503260 [50] Li D, Li Y, Feng Y, et al. Hierarchical graphene oxide/polyaniline nanocomposites prepared by interfacial electrochemical polymerization for flexible solid-state supercapacitors[J]. Journal of Materials Chemistry A,2015,3(5):2135-2143. doi: 10.1039/C4TA05643D [51] Sun G, Xiao Z, Lin R, et al. Hybrid fibers made of molybdenum disulfide, reduced graphene oxide, and multi-walled carbon nanotubes for solid-state, flexible, asymmetric supercapacitors[J]. Angewandte Chemie International Ed in English,2015,127(15):4734-4739. doi: 10.1002/ange.201411533 [52] Dhawale D S, Vinu A, Lokhande C D. Stable nanostructured polyaniline electrode for supercapacitor application[J]. Electrochimica Acta,2011,56(25):9482-9487. doi: 10.1016/j.electacta.2011.08.042 [53] Wu Q, Xu Y, Yao Z, et al. Supercapacitors based on flexible graphene/polyaniline nanofiber composite films[J]. ACS Nano,2010,4(4):1963-1970. doi: 10.1021/nn1000035 -
 20210194-支撑材料.pdf
20210194-支撑材料.pdf

-





 下载:
下载: