Se with Se-C bonds encapsulated in a honeycomb 3D porous carbon as an excellent performance cathode for Li-Se batteries
-
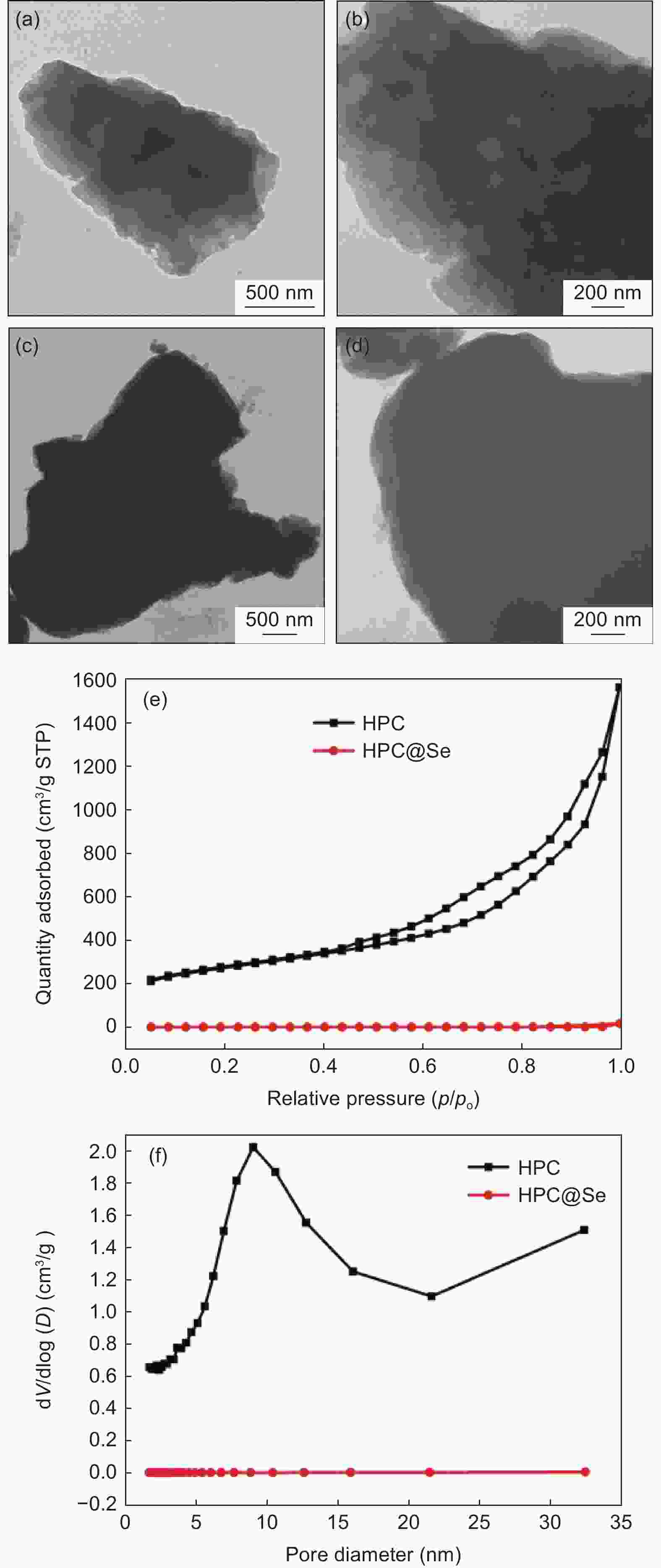
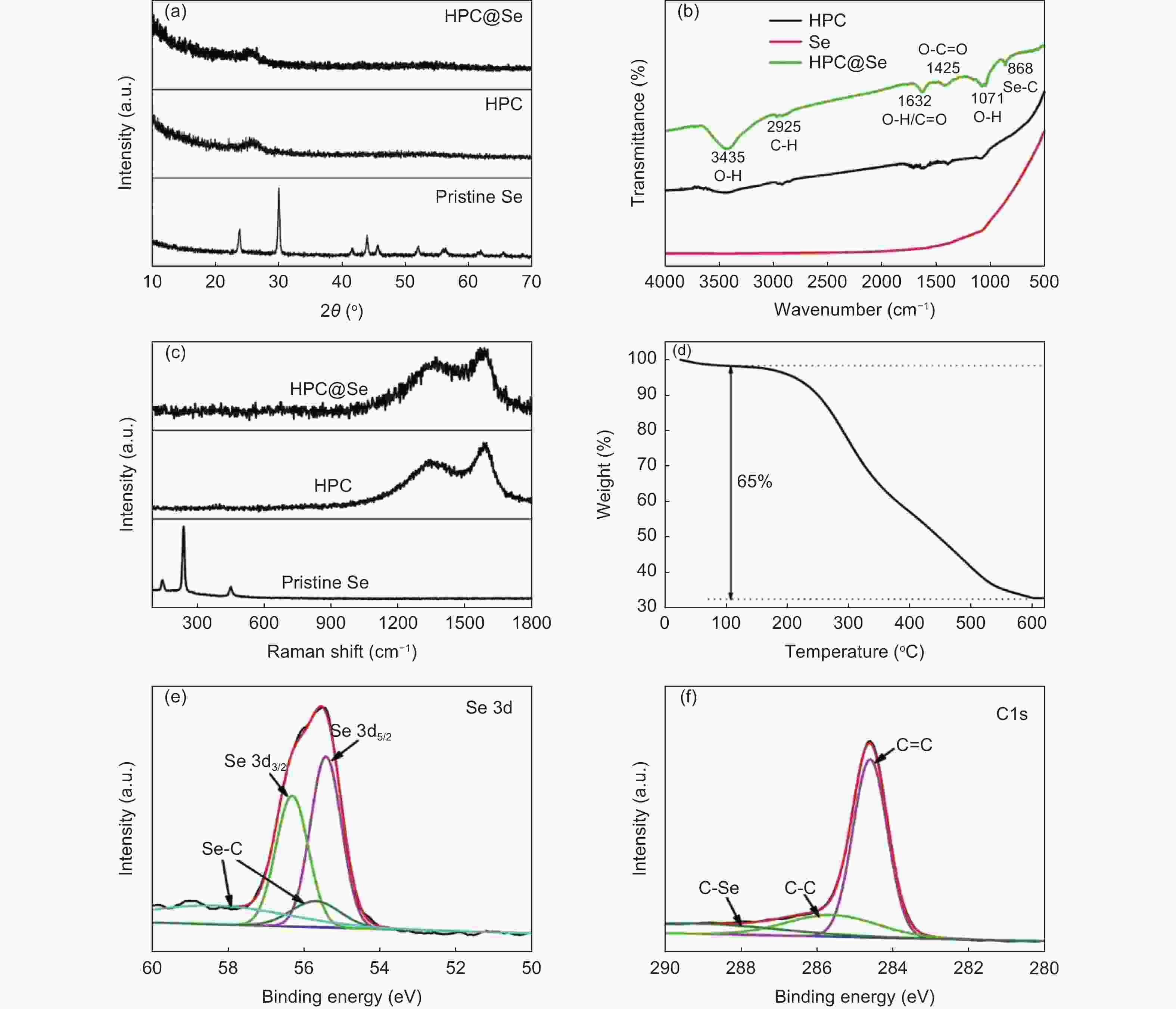
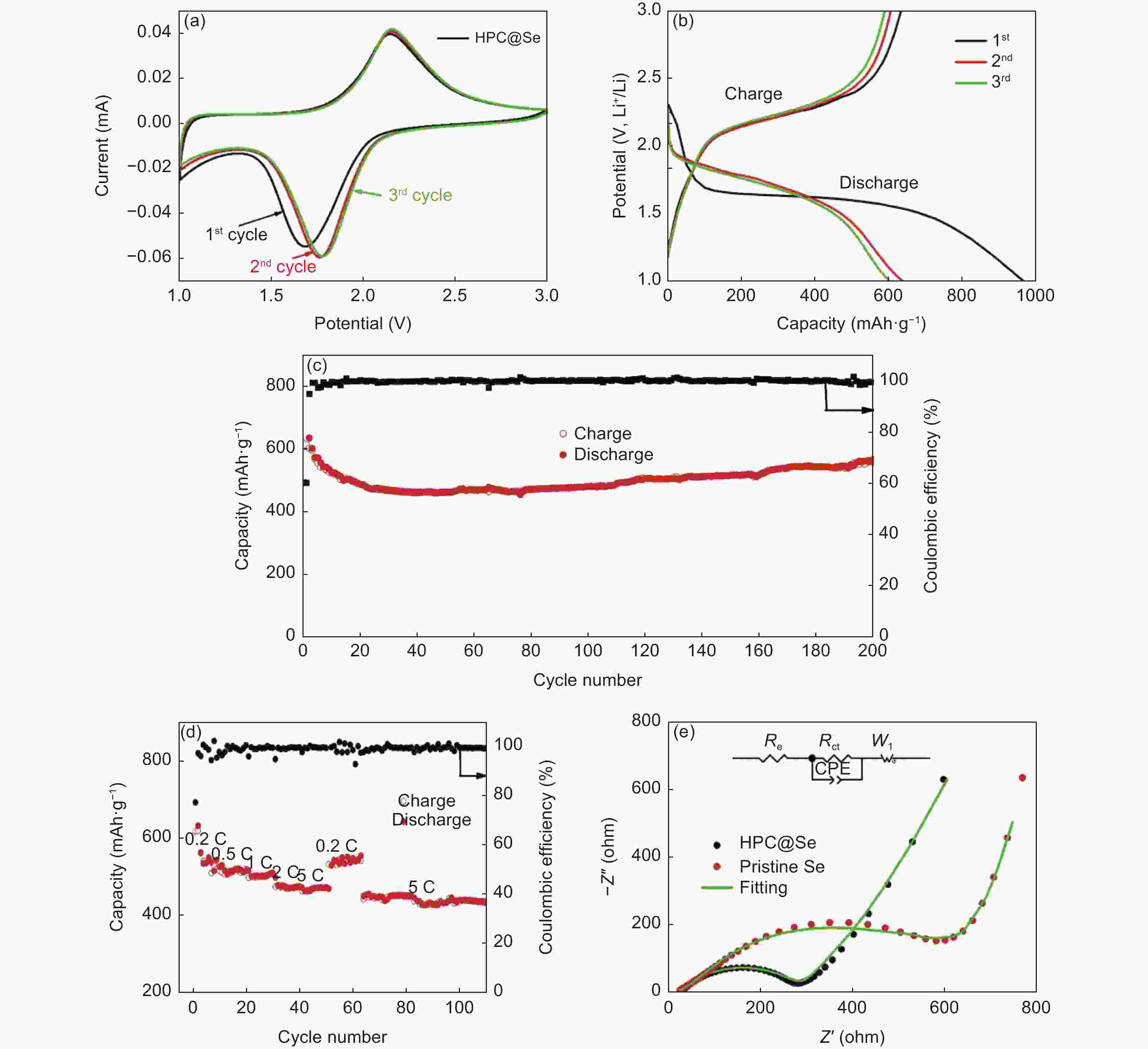
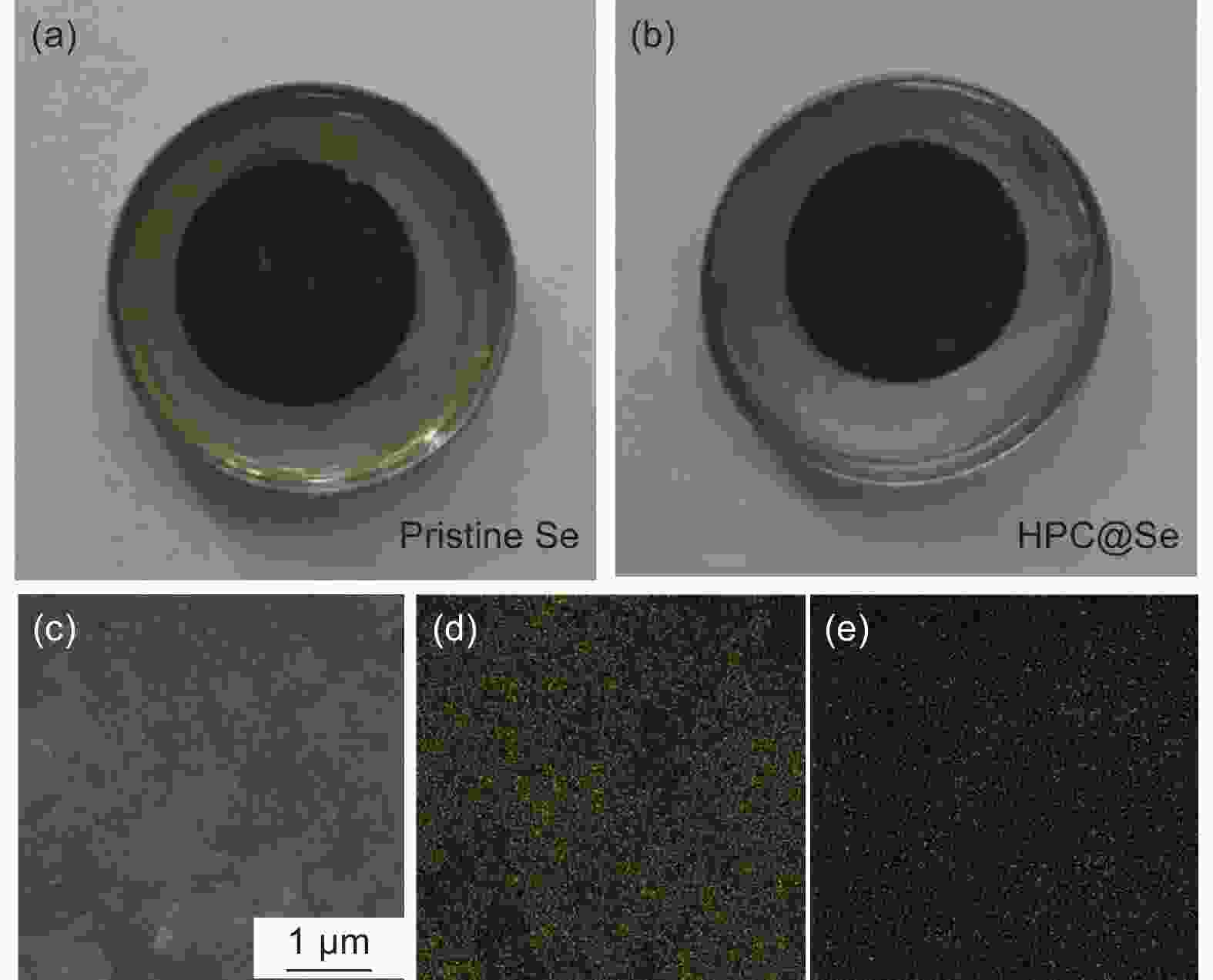
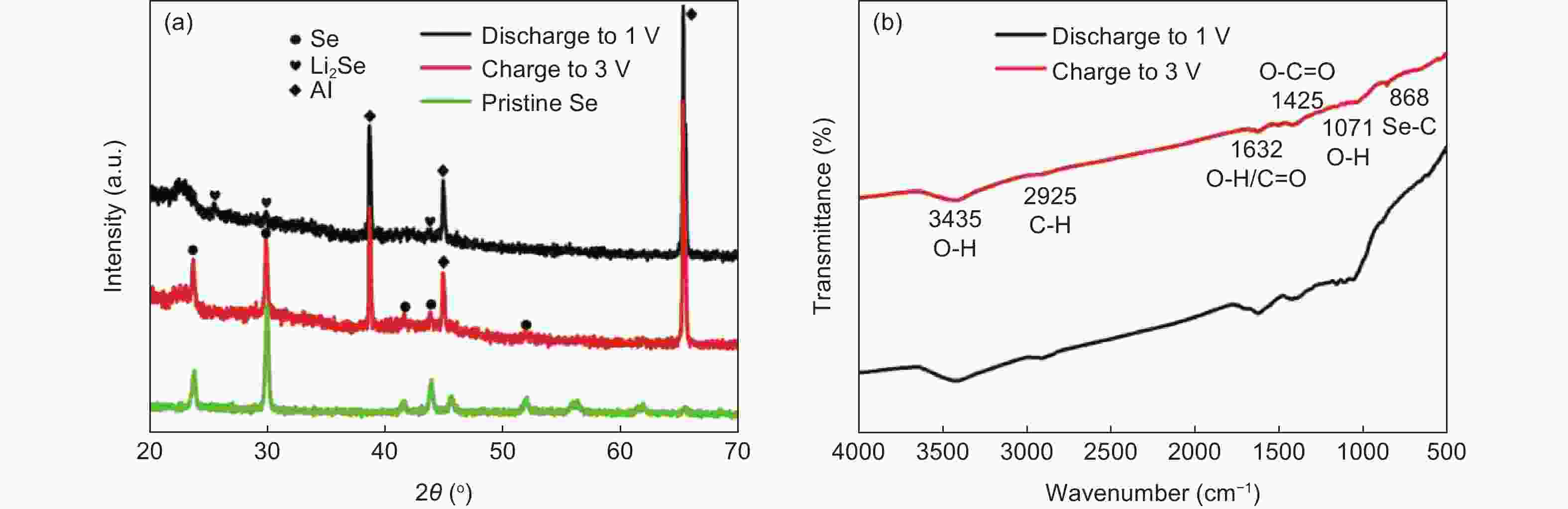
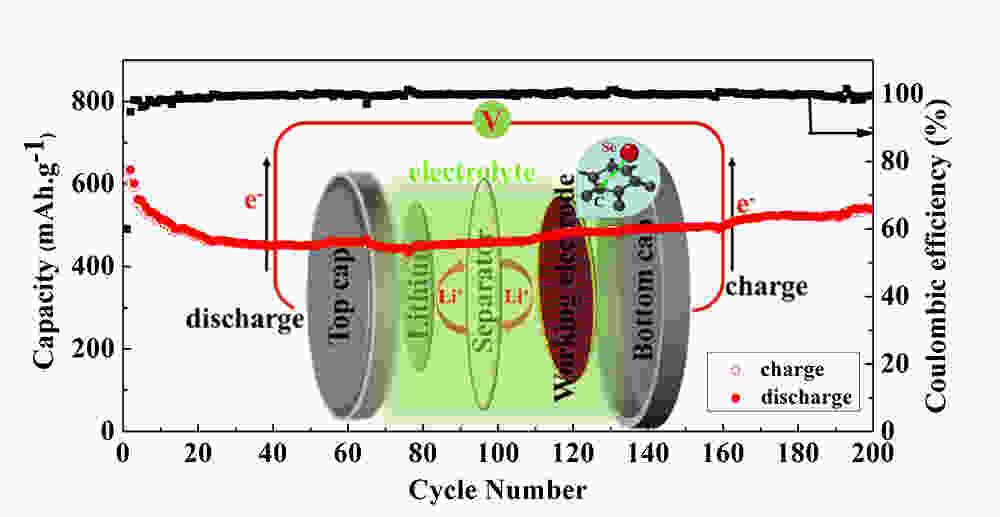
摘要: 锂-硒电池因其超高的体积能量密度和硒的高电导率而被认为是一种极具有发展前景的锂离子电池。然而,循环过程中电极严重的体积膨胀和多硒化物溶解,以及硒的低负载,阻碍了锂-硒电池应用的发展。解决这三个问题的一种行之有效的方法是将硒限制在具有丰富孔体积的碳基质中,并同时增强硒与碳的界面相互作用。通过将Se浸入酒石酸盐衍生的蜂窝状三维多孔炭中,合成出了一种具有Se―C键的蜂窝状三维多孔炭@硒(HPC@Se)的新型正极材料用于锂-Se电池。得到的蜂窝状三维多孔炭的孔体积可达1.794 cm3g−1,能够均匀包封65%硒。此外,硒与碳之间的强化学键有利于稳定硒,从而进一步缓解其巨大的体积膨胀和多硒化物的溶解,还可促进循环过程中的电荷转移。该HPC@Se正极呈现出极好的循环性能和倍率性能。在0.2 C的电流密度下,经200次循环后,其比容量可保持在561 mAhg−1(为理论比容量的83%),每次循环的比容量衰减率仅为0.058%。此外,在5 C的高电流密度下,HPC@Se正极还可以达到472.8 mAhg−1的可观容量。Abstract: Li-Se batteries have risen to prominence as promising lithium-ion batteries thanks to their ultrahigh volumetric energy density and the high electrical conductivity of Se. However, the use of Li-Se batteries is limited not only by the large volume expansion and dissolution of polyselenides in the cathodes during cycling, but also the low selenium loading. A highly effective and currently feasible approach to simultaneously tackle these problems is to position the selenium in a carbon matrix with a sufficient pore volume to accommodate the expansion while increasing the interfacial interaction between the selenium and carbon. We have synthesized a novel cathode material (Se@HPC) for Li-Se batteries of a honeycomb 3D porous carbon derived from a tartrate salt, that was impregnated with Se to produce Se-C bonds. The pore volume of the honeycomb 3D porous carbon was as high as 1.794 cm3 g−1, which allowed 65 wt% selenium to be uniformly encapsulated. Moreover, the strong chemical bonds between selenium and carbon stabilize the selenium, thus inhibiting its huge volume expansion and the dissolution of polyselenides, and promoting charge transfer during cycling. As expected, a Se@HCP cathode has excellent cyclability and a good rate performance. After 200 cycles at 0.2 C, its specific capacity remains at 561 mA h g−1, 83% of the theoretical value, and decays by only 0.058% per cycle. It also has a large capacity of 472.8 mA h g−1 under a high current density of 5 C.
-
Key words:
- Li-Se batteries /
- Cathodes /
- Honeycomb 3D porous carbon /
- High loading /
- Se―C bonds
-
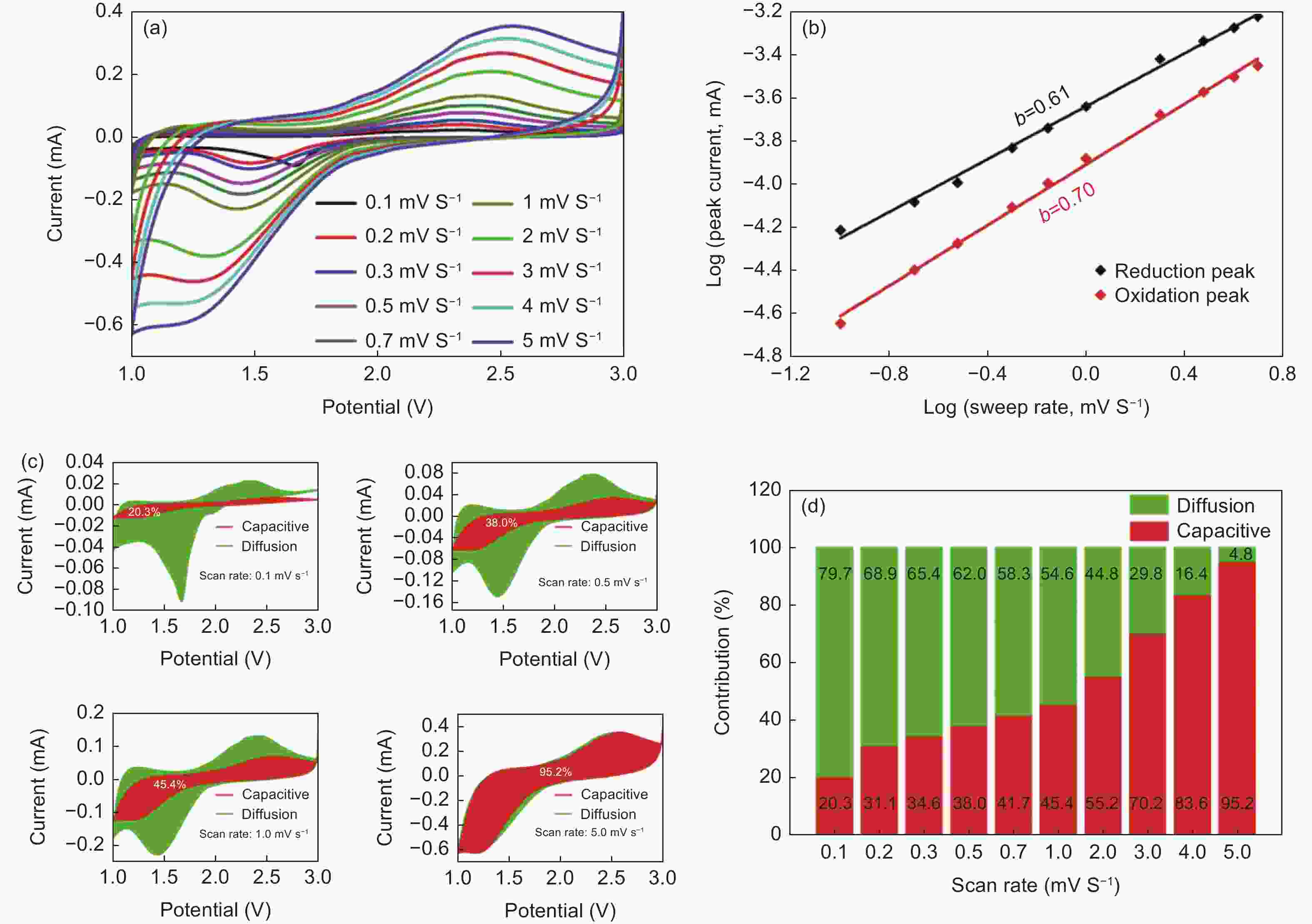
Figure 7. (a) CV profiles of HPC@Se with gradually increasing scan rates from 0.1 to 5 mV s−1, (b) b-value for the oxidation peak and reduction peak, (c) Contribution ratio of capacitance and diffusion behavior at 0.1, 0.5, 1 and 5 mV s−1 scan rates, (d) Summary of the ratio of capacitive-controlled and diffusion-controlled contribution with gradually increasing scan rates for HPC@Se electrode
Table 1. Comparison between HPC@Se (this work) and the published Se/C electrodes
Material Selenium
contentReversible capacity
(mA h g−1)Percentage of theoretical capacity
(678 mA h g−1)Current density
(C, 1C = 678 mA h g−1)Ref. HPC@Se 65 wt% 561/200 cycles 82.7% 0.2 C This work Mic/Se 44.2 wt% 400/500 cycles 59.0% 0.5 C [13] Se@LHPC 52 wt% 450/500 cycles 66.4% 0.5 C [14] Se-HPCF 50 wt% 533/50 cycles 78.6% 0.2 C [15] C/Se 54 wt% 430/250 cycles 63.4% 100 mA g−1 [16] Se@3D MIL-68 (Al)@MWCNTs 56 wt% 453/200 cycles 66.8% 0.2 C [25] Se@HPCNBs 60 wt% 560/100 cycles 82.6% 0.2 C [26] -
[1] Xie J, Li J, Mai W, et al. A decade of advanced rechargeable batteries development guided by in situ transmission electron microscopy[J]. Nano Energy,2021,83:105780. doi: 10.1016/j.nanoen.2021.105780 [2] Liang Y, Zhao C Z, Yuan H, et al. A review of rechargeable batteries for portable electronic devices[J]. InfoMat,2019,1(1):6-32. doi: 10.1002/inf2.12000 [3] Zhang X, Ju Z, Zhu Y, et al. Multiscale understanding and architecture design of high energy/power lithium-ion battery electrodes[J]. Advanced Energy Materials,2021,11(2):2000808. doi: 10.1002/aenm.202000808 [4] Wu Y, Wang W, Ming J, et al. An exploration of new energy storage system: high energy density, high safety, and fast charging lithium ion battery[J]. Advanced Functional Materials,2019,29(1):1805978. doi: 10.1002/adfm.201805978 [5] Li Q, Fung K Y, Xu L, et al. Process synthesis: Selective recovery of lithium from lithium-ion battery cathode materials[J]. Industrial & Engineering Chemistry Research,2019,58(8):3118-3130. [6] Manthiram A. A reflection on lithium-ion battery cathode chemistry[J]. Nature communications,2020,11(1):1550. doi: 10.1038/s41467-020-15355-0 [7] Huang S, Wang Z, Von Lim Y, et al. Recent advances in heterostructure engineering forlithium–sulfur batteries[J]. Advanced Energy Materials,2021,11(10):2003689. doi: 10.1002/aenm.202003689 [8] Wei P, Fan M, Chen H, et al. High-capacity graphene/sulfur/polyaniline ternary composite cathodes with stable cycling performance[J]. Electrochimica Acta,2015,174:963-969. doi: 10.1016/j.electacta.2015.06.052 [9] Wang B, Zhang J, Xia Z, et al. Polyaniline-coated selenium/carbon composites encapsulated in graphene as efficient cathodes for Li-Se batteries[J]. Nano Research,2018,11(5):2460-2469. doi: 10.1007/s12274-017-1870-2 [10] Sun J, Du Z, Liu Y, et al. State-of-the-art and future challenges in high energy lithium–selenium batteries[J]. Advanced Materials,2021,33(10):2003845. doi: 10.1002/adma.202003845 [11] Gu X, Tang T, Liu X, et al. Rechargeable metal batteries based on selenium cathodes: progress, challenges and perspectives[J]. Journal of Materials Chemistry A,2019,7(19):11566-11583. doi: 10.1039/C8TA12537F [12] Zhang J, Fan L, Zhu Y, et al. Selenium/interconnected porous hollow carbon bubbles composites as the cathodes of Li–Se batteries with high performance[J]. Nanoscale,2014,6(21):12952-12957. doi: 10.1039/C4NR03705G [13] Wang X, Tan Y, Liu Z, et al. New insight into the confinement effect of microporous carbon in Li/Se battery chemistry: a cathode with enhanced conductivity[J]. Small,2020,16(17):2000266. doi: 10.1002/smll.202000266 [14] Lu P, Liu F, Zhou F, et al. Lignin derived hierarchical porous carbon with extremely suppressed polyselenide shuttling for high-capacity and long-cycle-life lithium–selenium batteries[J]. Journal of Energy Chemistry,2021,55:476-483. doi: 10.1016/j.jechem.2020.07.022 [15] Chen X, Xu L, Zeng L, et al. Synthesis of the Se-HPCF composite via a liquid-solution route and its stable cycling performance in Li–Se batteries[J]. Dalton Transactions,2020,49(41):14536-14542. doi: 10.1039/D0DT03035J [16] Luo C, Wang J, Suo L, et al. In situ formed carbon bonded and encapsulated selenium composites for Li–Se and Na–Se batteries[J]. Journal of Materials Chemistry A,2015,3(2):555-561. doi: 10.1039/C4TA04611K [17] Zhou J, Chen M, Wang T, et al. Covalent selenium embedded in hierarchical carbon nanofibers for ultra-high areal capacity Li-Se Batteries[J]. Iscience,2020,23(3):100919. doi: 10.1016/j.isci.2020.100919 [18] Liu Y, Si L, Du Y, et al. Strongly bonded selenium/microporous carbon nanofibers composite as a high-performance cathode for lithium–selenium batteries[J]. The Journal of Physical Chemistry C,2015,119(49):27316-27321. doi: 10.1021/acs.jpcc.5b09553 [19] Jia D, Yang Z, Zhang H, et al. High performance of selenium cathode by encapsulating selenium into the micropores of chitosan-derived porous carbon framework[J]. Journal of Alloys and Compounds,2018,746:27-35. doi: 10.1016/j.jallcom.2018.02.276 [20] Bhatia P, Pandey S, Prakash R, et al. Enhanced anti-oxidant activity as a function of selenium hyperaccumulation in agaricus bisporus cultivated on Se-rich agri-residues[J]. Journal of Biologically Active Products from Nature,2014,4(5-6):354-364. doi: 10.1080/22311866.2014.961103 [21] Luo C, Xu Y, Zhu Y, et al. Selenium@ mesoporous carbon composite with superior lithium and sodium storage capacity[J]. ACS nano,2013,7(9):8003-8010. doi: 10.1021/nn403108w [22] Wang B, Li Z, Zhang J, et al. N-doped 3D interconnected carbon bubbles as anode materials for lithium-ion and sodium-ion storage with excellent performance[J]. Journal of nanoscience and nanotechnology,2019,19(11):7301-7307. doi: 10.1166/jnn.2019.16655 [23] Xiao S, Li Z, Liu J, et al. Se-C bonding promoting fast and durable Na+ storage in yolk–shell SnSe2@Se-C[J]. Small,2020,16(41):2002486. doi: 10.1002/smll.202002486 [24] Sha L, Gao P, Ren X, et al. A self-repairing cathode material for lithium–selenium batteries: Se-C chemically bonded selenium–graphene composite[J]. Chemistry–A European Journal,2018,24(9):2151-2156. doi: 10.1002/chem.201704079 [25] Li C, Wang Y, Li H, et al. Weaving 3D highly conductive hierarchically interconnected nanoporous web by threading MOF crystals onto multi walled carbon nanotubes for high performance Li–Se battery[J]. Journal of Energy Chemistry,2021,59:396-404. doi: 10.1016/j.jechem.2020.11.023 [26] Dong W D, Yu W B, Xia F J, et al. Melamine-based polymer networks enabled N, O, S Co-doped defect-rich hierarchically porous carbon nanobelts for stable and long-cycle Li-ion and Li-Se batteries[J]. Journal of Colloid and Interface Science,2021,582:60-69. doi: 10.1016/j.jcis.2020.06.071 [27] Choi W, Shin H C, Kim J M, et al. Modeling and applications of electrochemical impedance spectroscopy (EIS) for lithium-ion batteries[J]. Journal of Electrochemical Science and Technology,2020,11(1):1-13. doi: 10.33961/jecst.2019.00528 [28] Cui Y, Abouimrane A, Sun C J, et al. Li–Se battery: absence of lithium polyselenides in carbonate based electrolyte[J]. Chemical Communications,2014,50(42):5576-5579. doi: 10.1039/C4CC00934G [29] Wu F, Lee J T, Xiao Y, et al. Nanostructured Li2Se cathodes for high performance lithium-selenium batteries[J]. Nano Energy,2016,27:238-246. doi: 10.1016/j.nanoen.2016.07.012 [30] Yao W, Wu S, Zhan L, et al. Two-dimensional porous carbon-coated sandwich-like mesoporous SnO2/graphene/mesoporous SnO2 nanosheets towards high-rate and long cycle life lithium-ion batteries[J]. Chemical Engineering Journal,2019,361:329-341. doi: 10.1016/j.cej.2018.08.217 [31] Ding J, Zhou H, Zhang H, et al. Selenium impregnated monolithic carbons as free-standing cathodes for high volumetric energy lithium and sodium metal batteries[J]. Advanced Energy Materials,2018,8(8):1701918. doi: 10.1002/aenm.201701918 [32] Tian H, Tian H, Wang S, et al. High-power lithium–selenium batteries enabled by atomic cobalt electrocatalyst in hollow carbon cathode[J]. Nature communications,2020,11(1):5025. doi: 10.1038_s41467-020-18820-y -
 Supporting Information20230112.pdf
Supporting Information20230112.pdf

-





 下载:
下载: