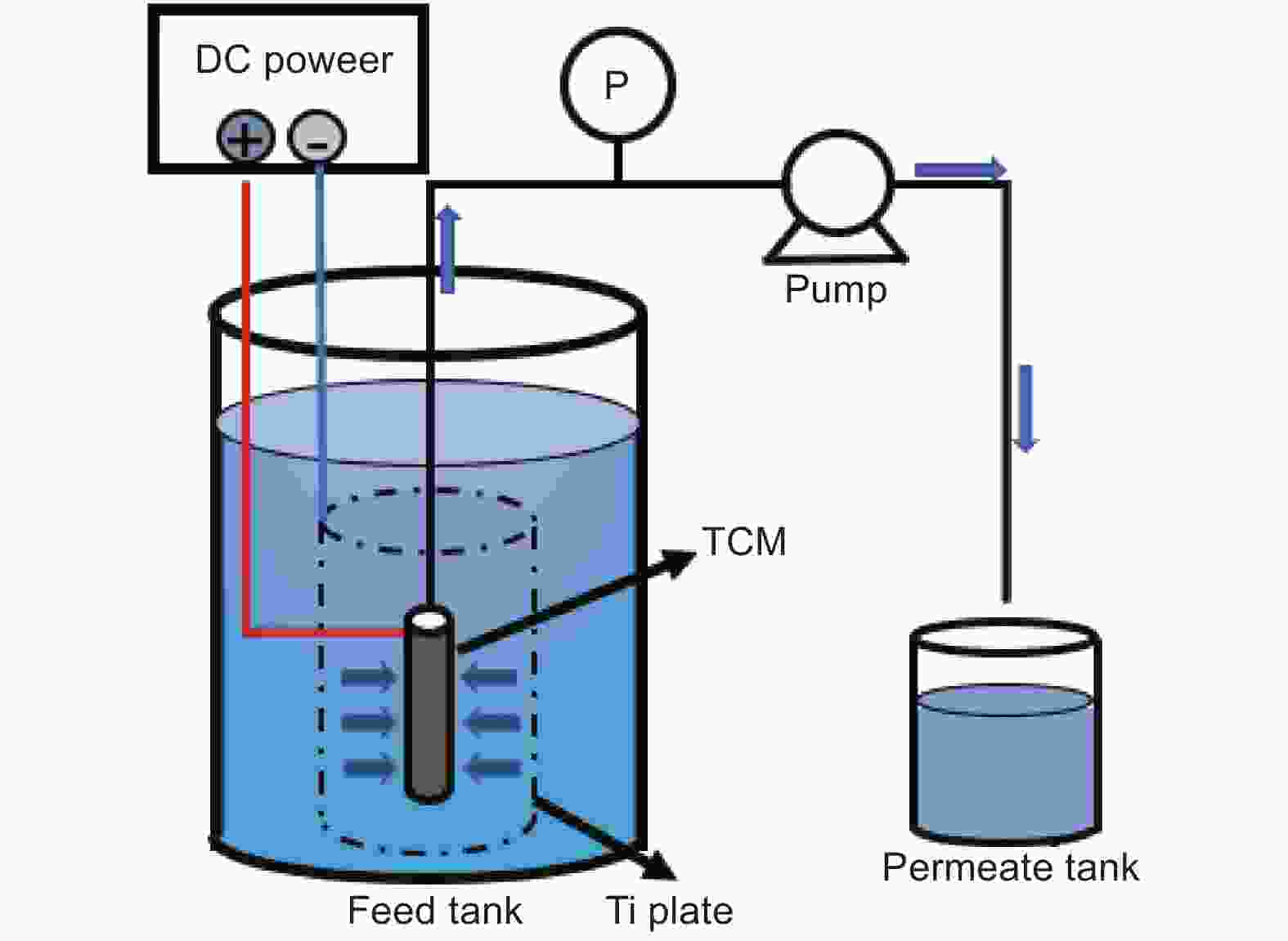
Preparation and performance of electrocatalytic carbon membranes for treating micro-polluted water
-
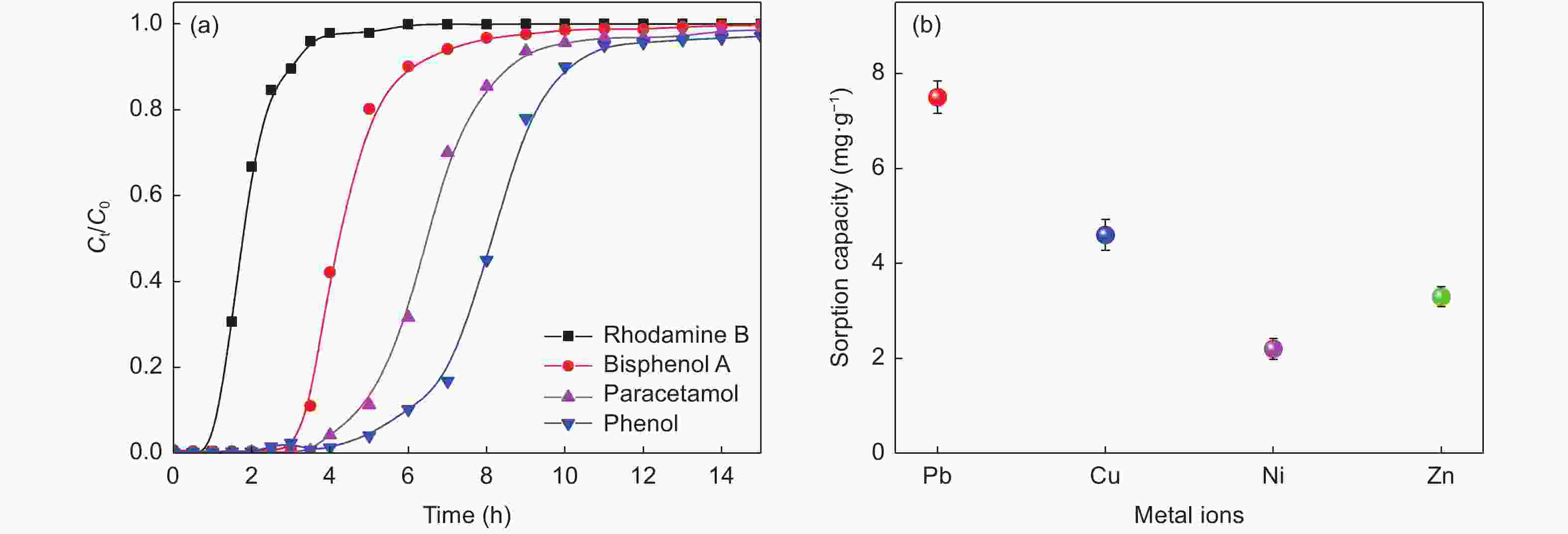
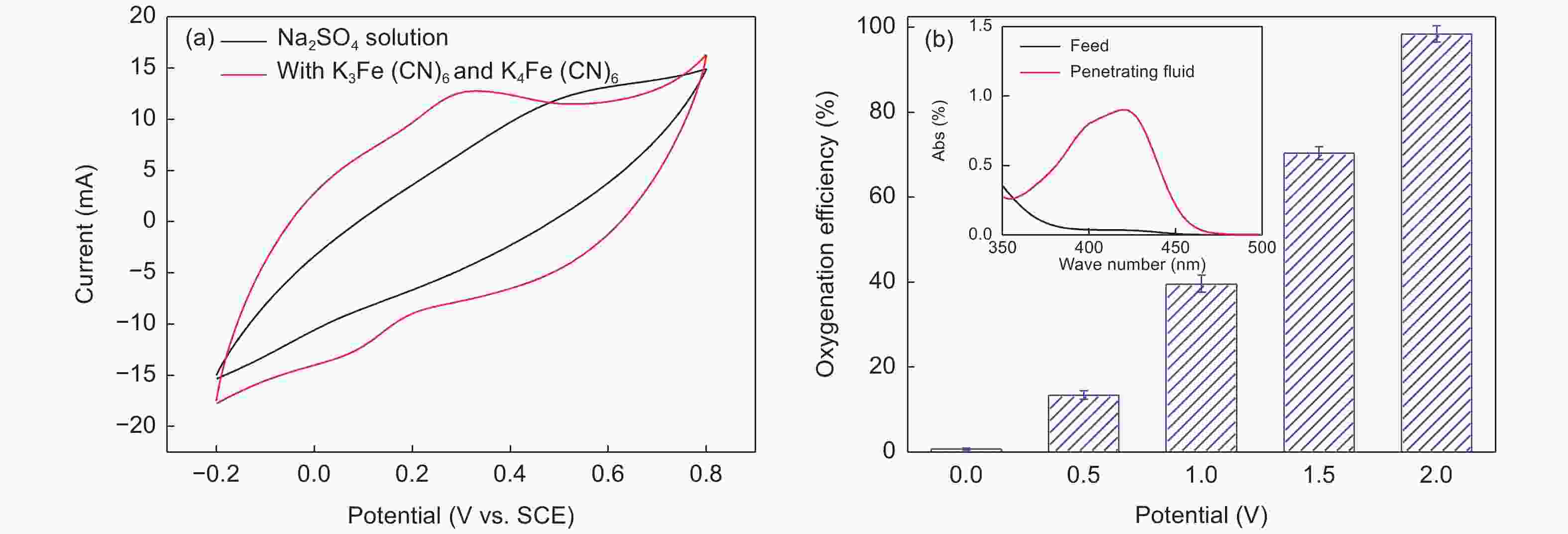
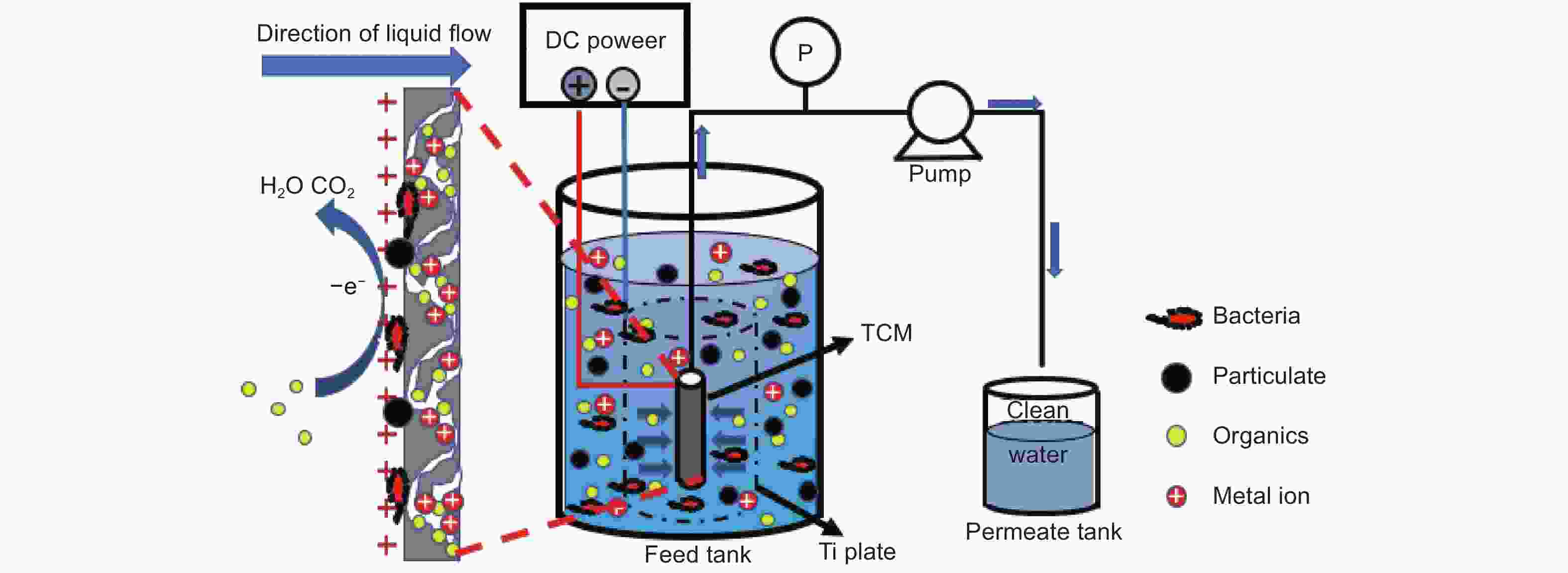
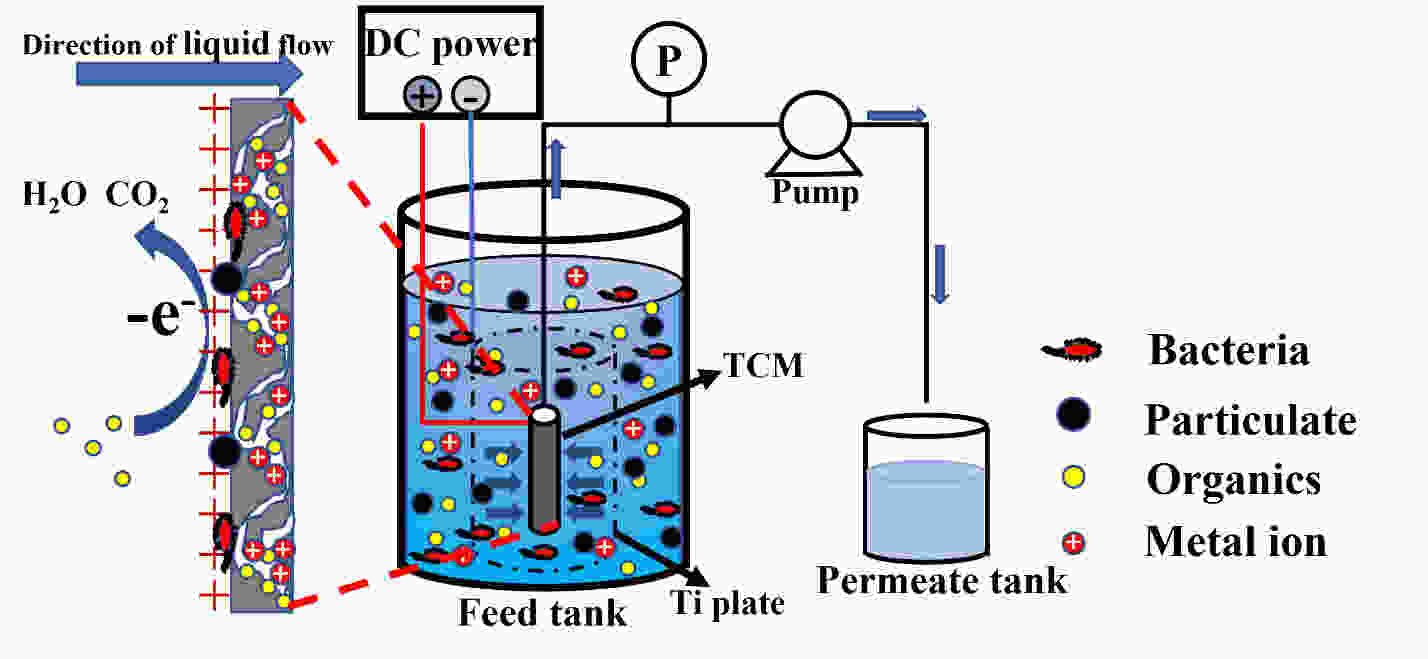
摘要: 以椰壳活性炭为原料,设计制备一种具有吸附、电催化氧化、膜过滤三重功能的电催化炭膜(TCM),通过调节原料配比、炭化温度以及原料活性炭的粒径实现对TCM性能的调控。采用SEM、XRD、拉曼和氮吸附等技术对TCM的形貌与结构进行表征,并以TCM为阳极构建电催化膜反应器(ECMR),考察其水处理性能。结果表明,TCM具有发达的孔道结构和较高的比表面积,整体呈现出大孔-介孔-微孔的多级孔道结构,并具有良好的机械强度与导电性;改变活性炭的粒径可以有效调控TCM的孔道结构。TCM对水中微污染有机物和重金属离子均具有较高的吸附量;在外加2 V电压电场下,对水中亚铁氰化钾的氧化率为98.4%,表现出良好的电催化氧化活性;在低压电场的作用下处理真实微污染水时,炭膜的三重功能协同作用使其展现出优异的综合处理性能,其中COD、UV254、浊度以及细菌的去除率分别达到了94.3%、90.5%、96.3%和100%,重金属离子几乎完全去除,出水水质得到显著地改善,并且水渗透通量有所提升,具有良好的抗污染性能。Abstract: Porous carbon membranes (PCMs) with three functions of adsorption, electrocatalytic oxidation and membrane filtration were prepared from coconut shell activated carbon using carboxymethyl cellulose (CMC,10 wt%) and benzoxazine resin (BR, 10 wt%-40 wt%) as the binder components. The morphology and microstructure of PCMs were characterized by SEM, XRD, Raman and nitrogen adsorption. An electrocatalytic membrane reactor (ECMR) was constructed using PCMs as the anode materials to investigate their water treatment performance. Results show that the PCMs have well-developed hierarchical macro, meso and micropores, whose macropore size decreases with the particle size of the activated carbon. The mechanical strength and electrical conductivity of the PCMs increased with BR content and carbonization temperature. The water flux decreased as the average particle size of the activated carbon decreased and the iodine value decreased with decreasing BR content. The PCM performed best with an excellent comprehensive performance in adsorption, electrocatalytic oxidation and filtration when it was prepared from an activated carbon of average particle size of 37.9 μm using a BR content of 30 wt% and a carbonization temperature of 950 ℃. For the micro-polluted Lingshui River water in Dalian, the removal of COD, UV254, turbidity and bacteria with the ECMR reached 94.3%, 90.5%, 96.3% and 100%, respectively, and heavy metal ions (Pb2+, Cu2+, Zn2+, Ni2+ ) were removed to levels below the detection limits, and the anti-fouling performance was good. The excellent performance in treating micro-polluted water is ascribed to the combined effects of adsorption, electrocatalytic oxidation and filtration.
-
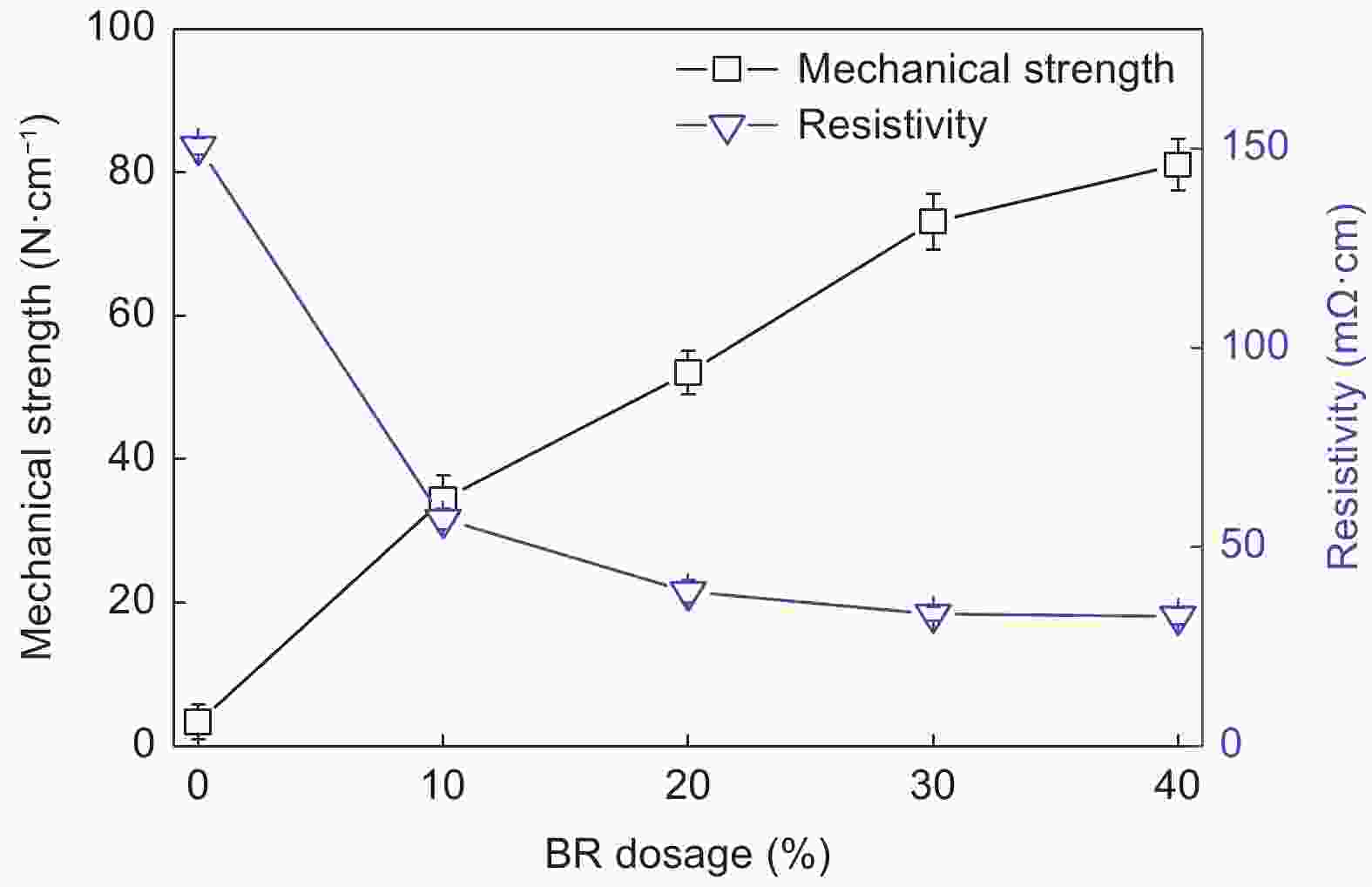
表 1 不同BR添加量TCMs的孔结构性能与碘值
Table 1. Pore structure and Iodine absorb of TCMs with different BR dosages.
BR dosage Average pore size/μm Porosity Water flux (L·m−2·h−1·MPa−1) Iodine absorb /mg·g−1 0% - - - 803.2 10% - 62.7% 11431 696.9 20% 1.19 60.5% 9931 585.7 30% 1.08 58.7% 9448 526.7 40% 1.00 58.3% 9012 342.1 表 2 不同炭化温度TCMs的孔结构性能与碘值
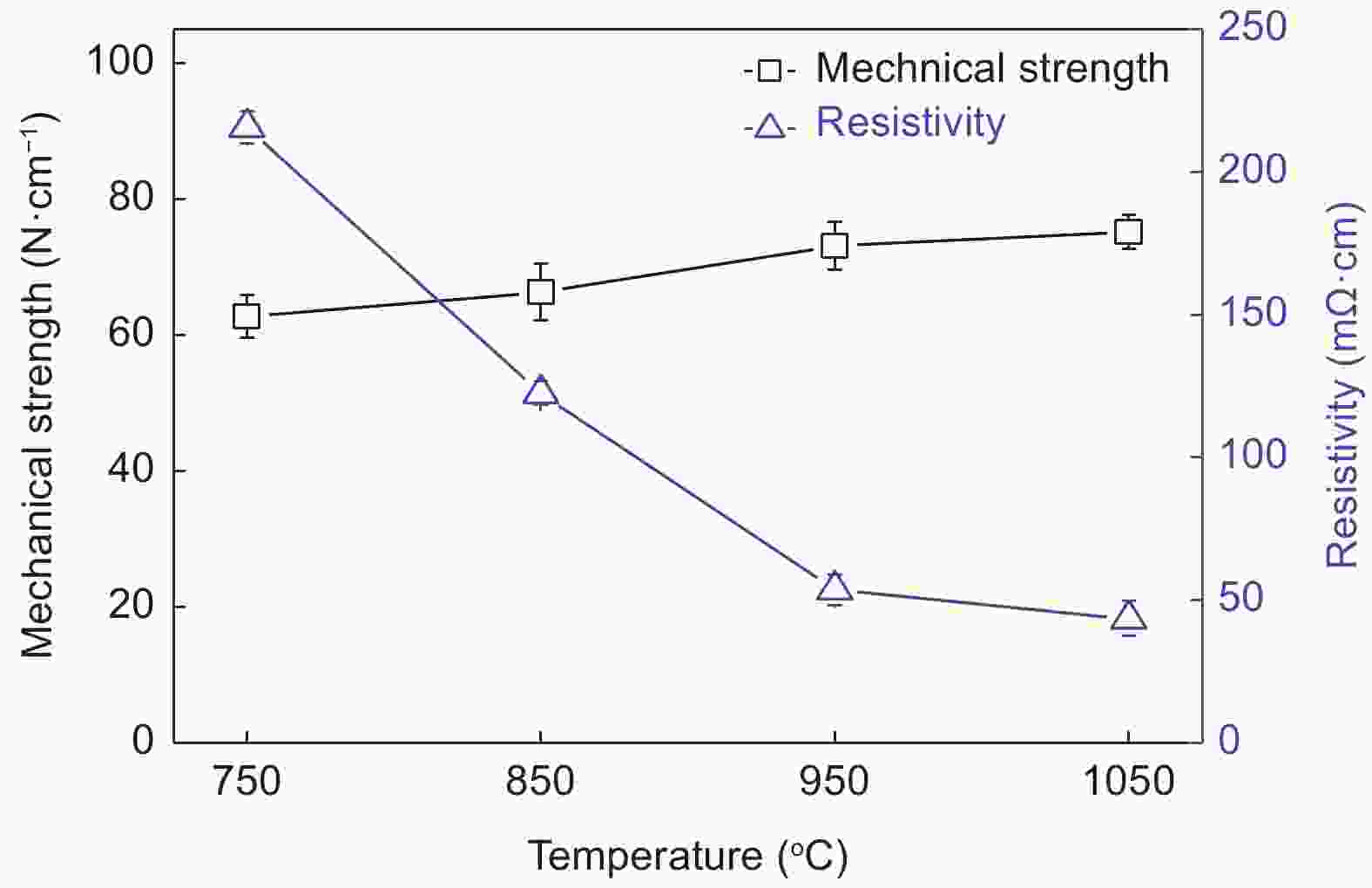
Table 2. Pore structure and Iodine absorb of TCMs at different carbonization temperatures.
Temper-atur (°C) Average pore size (μm) Porosity Water flux (L·m−2·h−1·MPa−1) Iodine absorb (mg·g−1) 750 0.99 56.6% 9118 474.2 850 1.03 57.0% 9283 481.0 950 1.08 58.7% 9448 526.7 1050 1.12 59.4% 9899 551.4 表 3 不同粒径活性炭制得TCMs的孔结构性能与碘值
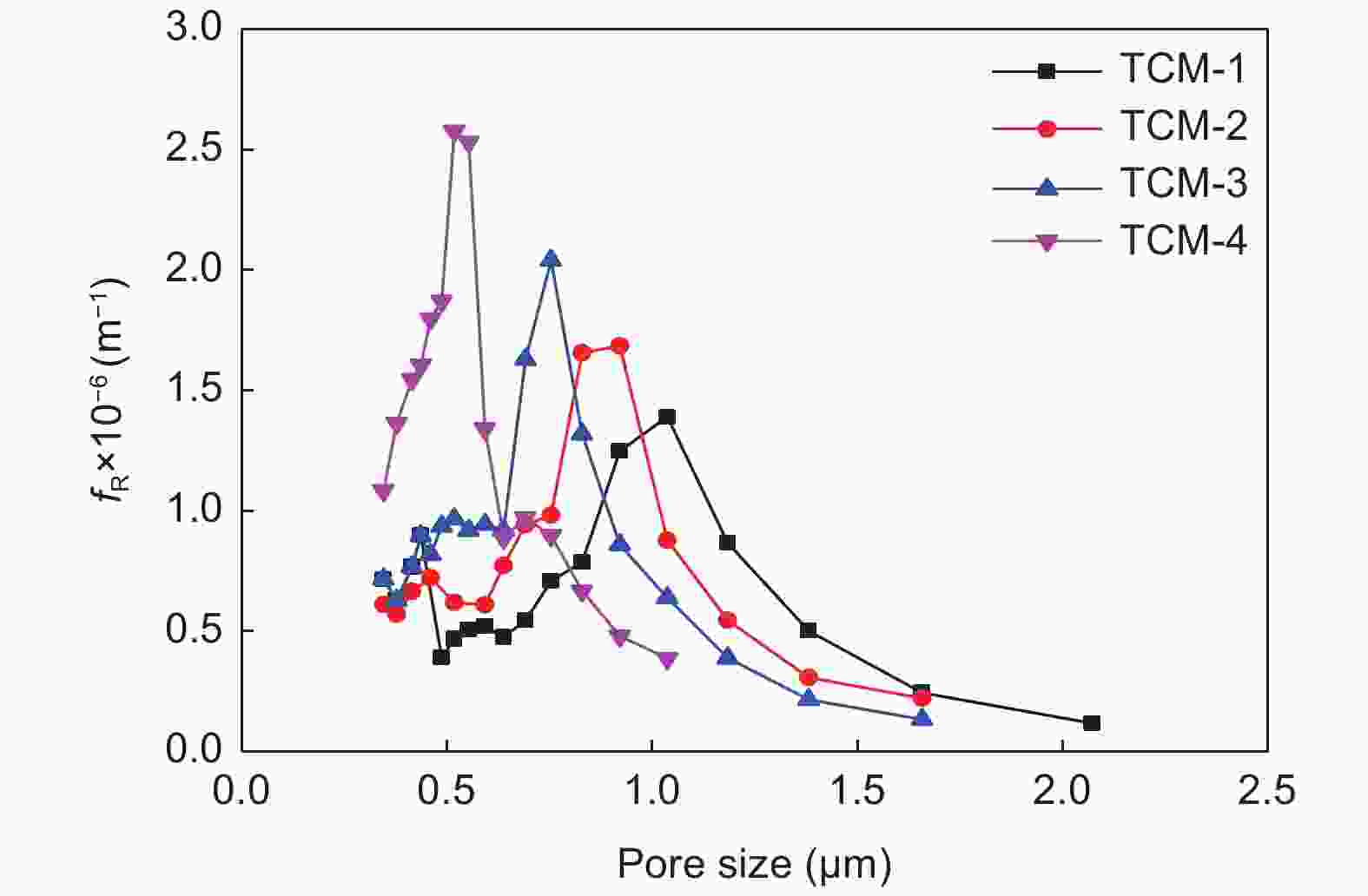
Table 3. Pore structure and Iodine absorb of TCMs prepared by AC with different particle sizes.
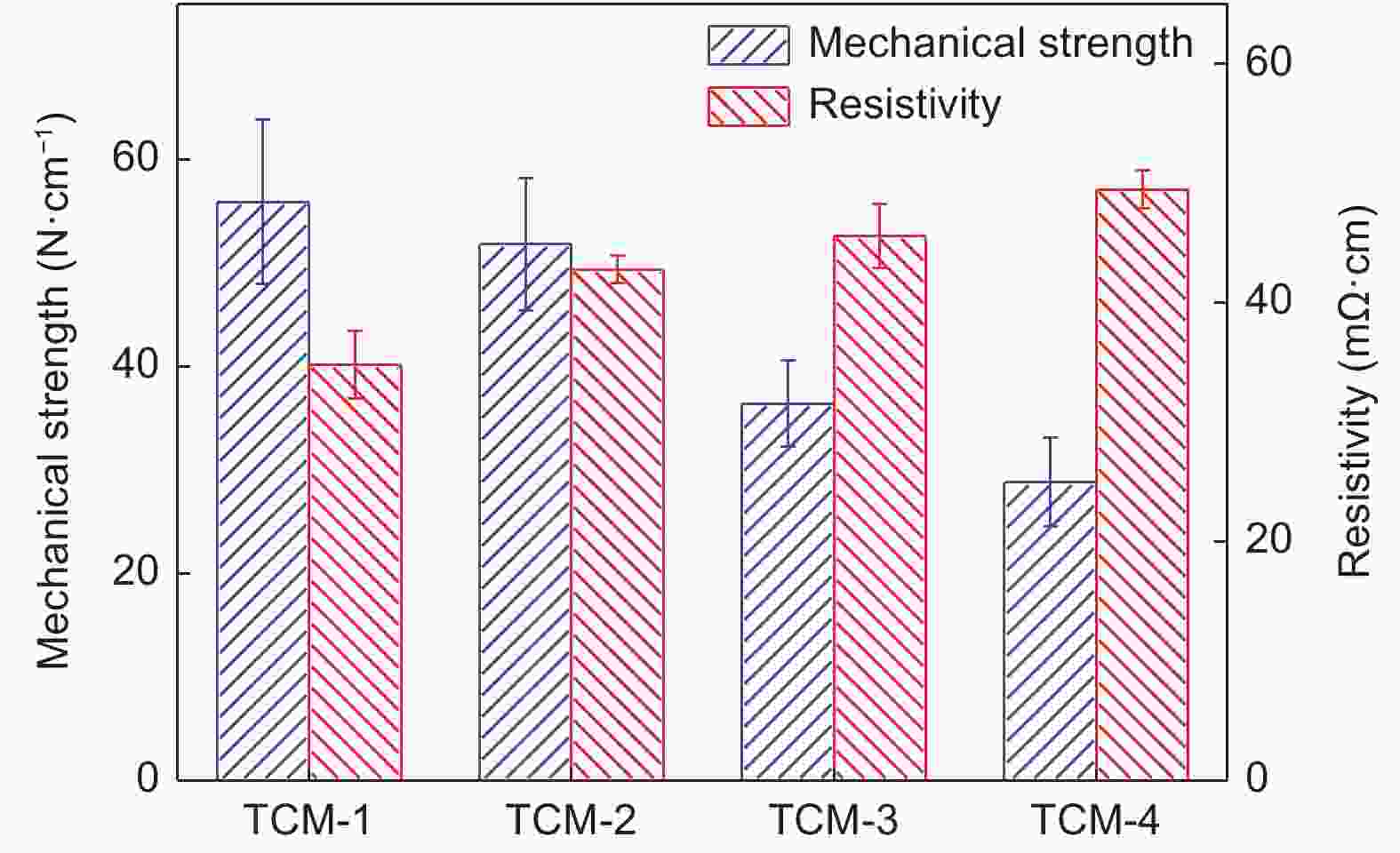
Sample Average pore size (μm) Porosity Water flux (L·m−2·h−1·MPa−1) Iodine absorb (mg·g−1) TCM-1 1.08 58.7% 9448 526.7 TCM-2 0.87 58.4% 5923 480.9 TCM-3 0.75 58.2% 5073 483.8 TCM-4 0.54 57.9% 1536 461.5 表 4 AC与TCM的微介孔结构性能
Table 4. Micropore and mesopore structure properties of AC and TCM.
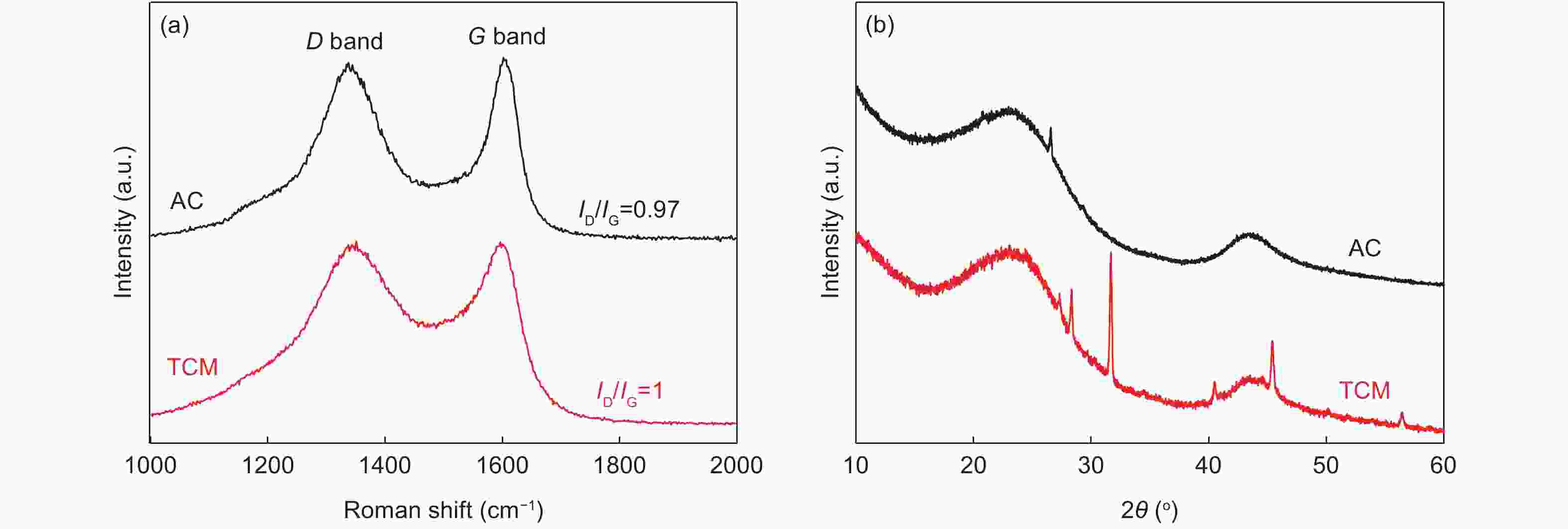
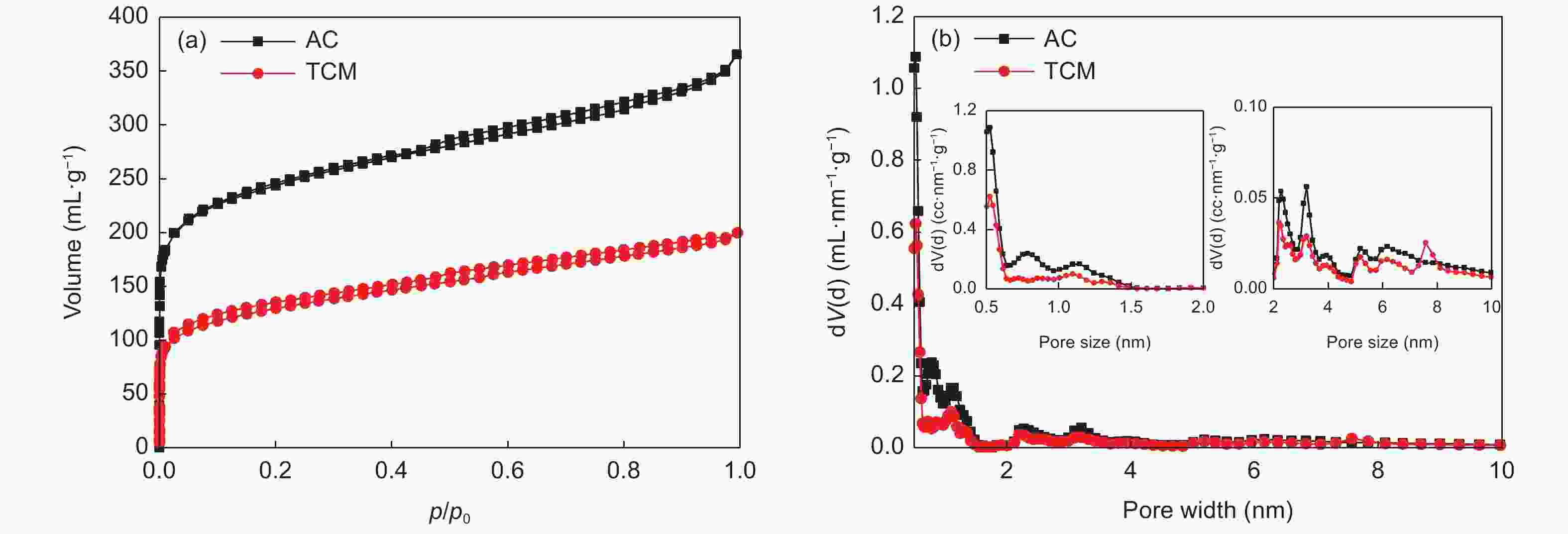
Sample SBET (m2·g−1) Smicro (m2·g−1) Vtotal (mL·g−1) Vmicro (mL·g−1) Vmeso (mL·g−1) AC 860.8 574.3 0.677 0.388 0.295 TCM 517.7 312.4 0.309 0.223 0.120 -
[1] 刘春花. 微污染水源水处理技术研究进展和对策分析[J]. 环境与发展,2018,30(11):83-84.Liu Chun-hua. Research progress and countermeasure analysis of micro polluted source water treatment technology[J]. Environment and Development,2018,30(11):83-84. [2] 张玲玲, 顾平. 微滤和超滤膜技术处理微污染水源水的研究进展[J]. 膜科学与技术,2008,28(5):103-109. doi: 10.3969/j.issn.1007-8924.2008.05.021Zhang Ling-ling, Gu Ping. Research progress of microfiltration and ultrafiltration membrane technology for the treatment of slightly polluted water source[J]. Membrane Science and Technology,2008,28(5):103-109. doi: 10.3969/j.issn.1007-8924.2008.05.021 [3] Agenson K. Retention of a wide variety of organic pollutants by different nanofiltration/reverse osmosis membranes: Controlling parameters of process[J]. Journal of Membrane Science,2003,225(1-2):91-103. doi: 10.1016/j.memsci.2003.08.006 [4] Xue W C, Xiao K, Liang P, et al. Roles of membrane and organic fouling layers on the removal of endocrine disrupting chemicals in microfiltration[J]. Jourmal of Environment Sciences,2018,72:176-184. doi: 10.1016/j.jes.2018.01.004 [5] Ren Y, Ma Y L, Min G Y, et al. A mini review of multifunctional ultrafiltration membranes for wastewater decontamination: Additional functions of adsorption and catalytic oxidation[J]. Science of the Total Environment,2021,762:143083. doi: 10.1016/j.scitotenv.2020.143083 [6] Feng Z X, Xu Y N, Yue W X, et al. Recent progress in the use of graphene/polymer composites to remove oil contaminants from water[J]. New Carbon Materials,2021,36(2):235-252. doi: 10.1016/S1872-5805(21)60018-5 [7] Dong L J, Wu S Y, Li S B, et al. Sorption behaviors and mechanismsof Eu(III) on rice straw-derived biochar[J]. Journal of Inorganic Materials,2020,35(03):390-398. [8] 杜旭东, 唐城元, 杨小丽, 等. 生物源碳酸钙对污水中Pb(Ⅱ)和甲基橙吸附行为的研究[J]. 无机材料学报,2020,35(03):315-323.Du Xu-dong, Tang Cheng-yuan, Yang Xiao-li, et al. High-efficiency biogenic calcium carbonate for adsorption of Pb(II) and methyl orange from wastewater[J]. Journal of Inorganic Materials,2020,35(03):315-323. [9] Zhou L, Xiao G, He Y, et al. Multifunctional filtration membrane with anti-viscous-oils-fouling capacity and selective dyes adsorption ability for complex wastewater remediation[J]. Journal of Hazardous Materials,2021,413:125379. doi: 10.1016/j.jhazmat.2021.125379 [10] 肖鹏飞, 安璐, 吴德东. 炭材料在过硫酸盐高级氧化技术中的应用进展[J]. 新型炭材料,2020,35(6):667-683. doi: 10.1016/S1872-5805(20)60521-2Xiao Peng-fei, An Lu, Wu De-dong. The use of carbon materials in persulfate-based advanced oxidation processes: A review[J]. New Carbon Materials,2020,35(6):667-683. doi: 10.1016/S1872-5805(20)60521-2 [11] Fan X, Zhao H, Liu Y, et al. Enhanced permeability, selectivity, and antifouling ability of CNTs/Al2O3 membrane under electrochemical assistance[J]. Environmental Science & Technology,2015,49(4):2293-2300. doi: 10.1021/es5039479 [12] Pan Z L Yu F P, Li L, et al. Electrochemical filtration carbon membrane derived from coal for wastewater treatment: Insights into the evolution of electrical conductivity and electrochemical performance during carbonization[J]. Separation and Purification Technology,2020:247. [13] Pan Z L, Yu F P, Li L, et al. Electrochemical microfiltration treatment of bisphenol A wastewater using coal-based carbon membrane[J]. Separation and Purification Technology,2019,227:115695. doi: 10.1016/j.seppur.2019.115695 [14] Pan Z L Yu F P, Li L, et al. Low-cost electrochemical filtration carbon membrane prepared from coal via self-bonding[J]. Chemical Engineering Journal,2020:385. [15] Shi B Y, Fang L P, Li Z J, et al. Adsorption behavior of DOM by PACs with different particle sizes[J]. Clean Soil, Air, Water,2014,42(10):1363-1369. doi: 10.1002/clen.201300518 [16] Jiang Y, Liu Y, Shi D T, et al. Membrane fouling in a powdered activated carbon – membrane bioreactor (PAC-MBR) for micro-polluted water purification: Fouling characteristics and the roles of PAC[J]. Journal of Cleaner Production,2020,277:122341. doi: 10.1016/j.jclepro.2020.122341 [17] Venkataraman K, Choate W T, Torre E R, et al. Characterization studies of ceramic membranes - a novel technique using a coulter porometer[J]. Journal of Membrane Science,1988,39(3):259-271. doi: 10.1016/S0376-7388(00)80933-3 [18] 代洁, 李鹏程, 朱蓉琪, 等. 一种高残炭新型苯并噁嗪树脂的固化及热解动力学[J]. 功能高分子学报,2018,31(2):114-120.Dai Jie, Li Peng-cheng, Zhu Rong-qi, et al. Curing and pyrolysis kinetics of a new benzoxazine resin with high char yield[J]. Journal of Functional Polymers,2018,31(2):114-120. [19] Kercher A K, Nagle D C. Microstructural evolution during charcoal carbonization by X-ray diffraction analysis[J]. Carbon,2003,41(1):15-27. doi: 10.1016/S0008-6223(02)00261-0 [20] 江泽慧, 张东升, 费本华, 等. 炭化温度对竹炭微观结构及电性能的影响[J]. 新型炭材料,2004,19(4):249-253. doi: 10.3321/j.issn:1007-8827.2004.04.002Jiang Ze-hui, Zhang Dong-sheng, Fei Ben-hua, et al. Effects of carbonization temperature on the microstructure and electrical conductivity of bamboo charcoal[J]. New Carbon Materials,2004,19(4):249-253. doi: 10.3321/j.issn:1007-8827.2004.04.002 [21] Schnoor M H, Vecitis C D. Quantitative examination of aqueous ferrocyanide oxidation in a carbon nanotube electrochemical filter: Effects of flow rate, ionic strength, and cathode material[J]. The Journal of Physical Chemistry C,2013,117(6):2855-2867. doi: 10.1021/jp3112099 [22] Zaky A M, Chaplin B P. Porous substoichiometric TiO2 anodes as reactive electrochemical membranes for water treatment[J]. Environmental Science Technology,2013,47(12):6554-6563. doi: 10.1021/es401287e [23] Liu H, Vecitis C D. Reactive Transport mechanism for organic oxidation during electrochemical filtration: Mass-transfer, physical adsorption, and electron-transfer[J]. The Journal of Physical Chemistry C,2011,116(1):374-383. [24] Fang X F, Li J S, Li X, et al. Internal pore decoration with polydopamine nanoparticle on polymeric ultrafiltration membrane for enhanced heavy metal removal[J]. Chemical Engineering Journal,2017,314:38-49. doi: 10.1016/j.cej.2016.12.125 [25] Vecitis C D, Schnoor M H, Rahaman M S, et al. Electrochemical multiwalled carbon nanotube filter for viral and bacterial removal and inactivation[J]. Environmental Science Technology,2011,45(8):3672-3679. doi: 10.1021/es2000062 [26] 沈存花, 李丹, 曹美玲, 等. 含重金属离子有机废水处理研究进展[J]. 应用化工,2018,47(7):1488-1492. doi: 10.3969/j.issn.1671-3206.2018.07.041Shen Cun-hua, Li Dan, Cao Mei-ling, et al. Progress in the treatment of organic wastewater containing heavy metal ions[J]. Applied Chemical Industry,2018,47(7):1488-1492. doi: 10.3969/j.issn.1671-3206.2018.07.041 -





 下载:
下载: