Nitrogen doped hollow porous carbon fibers derived from polyacrylonitrile for Li-S batteries
-
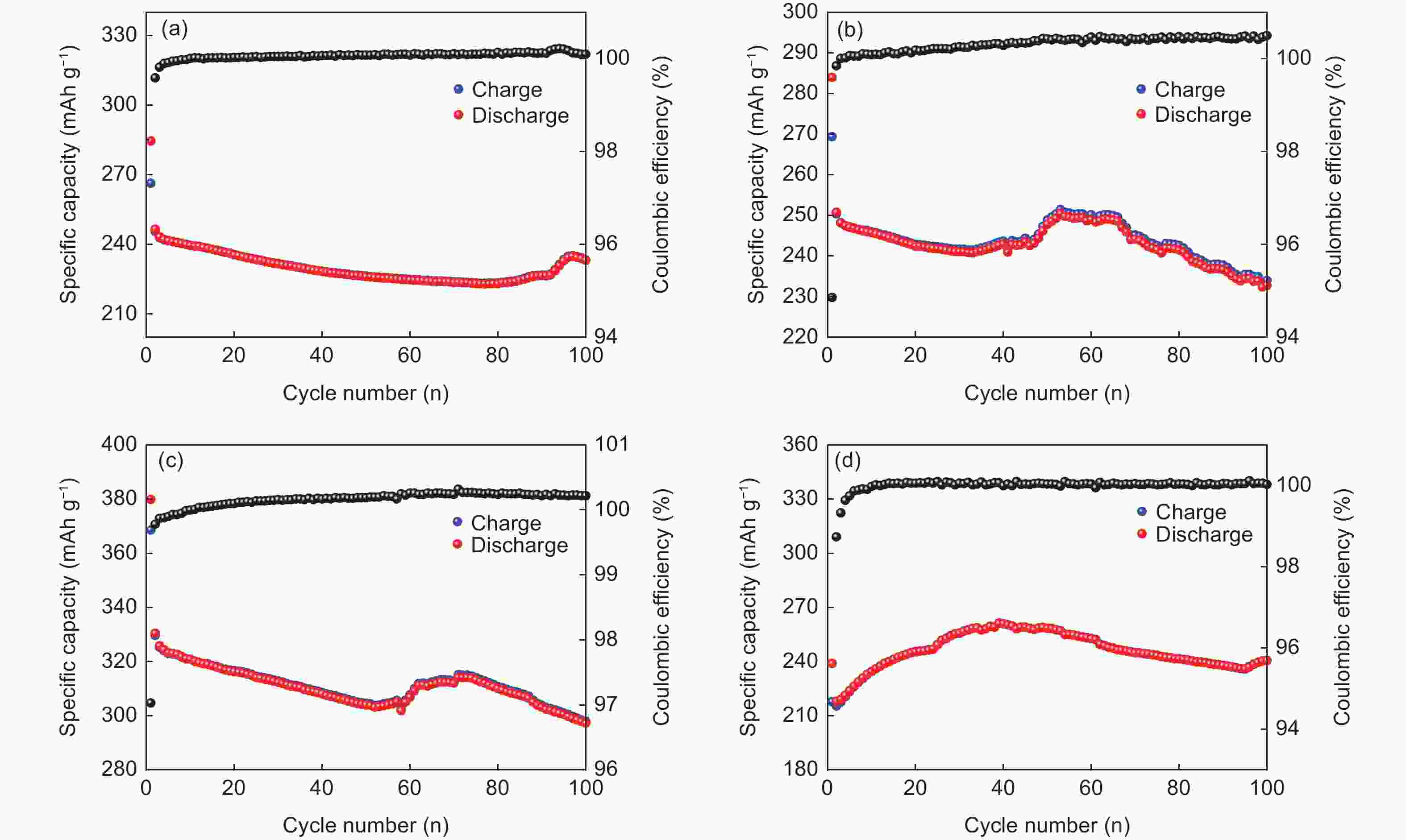
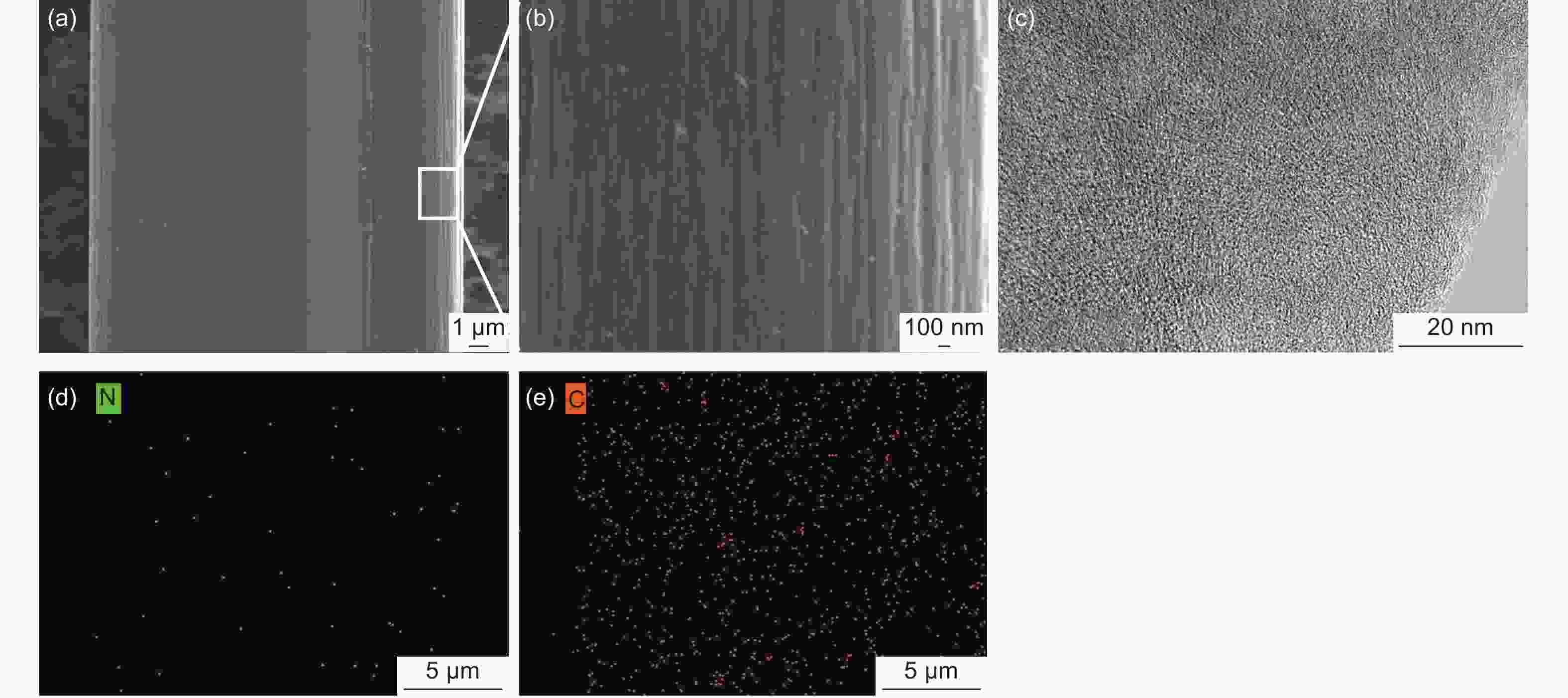
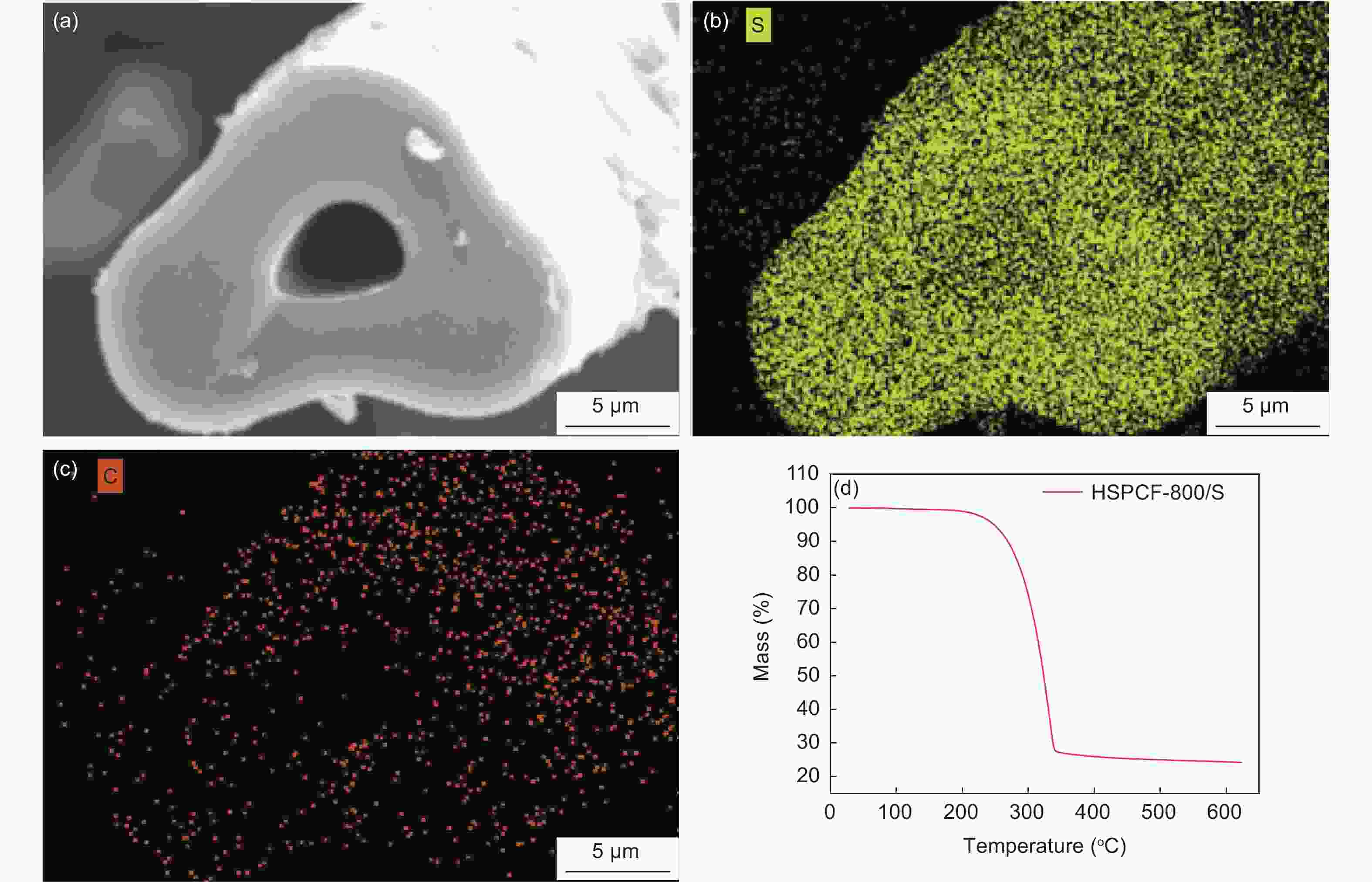
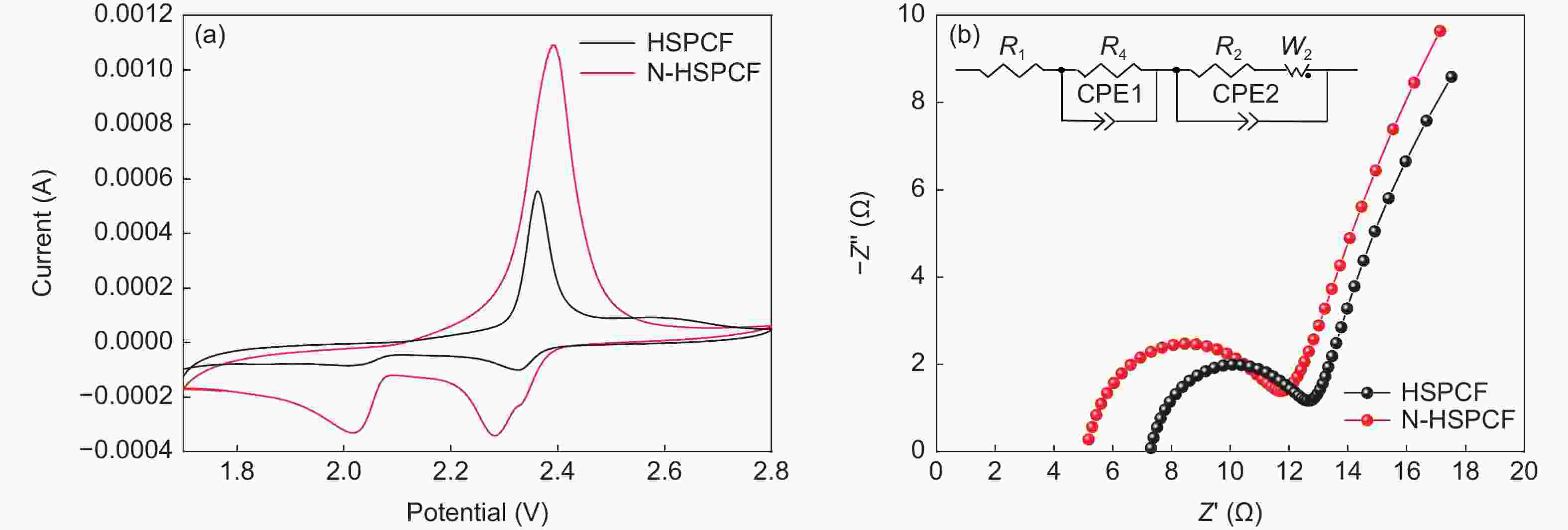
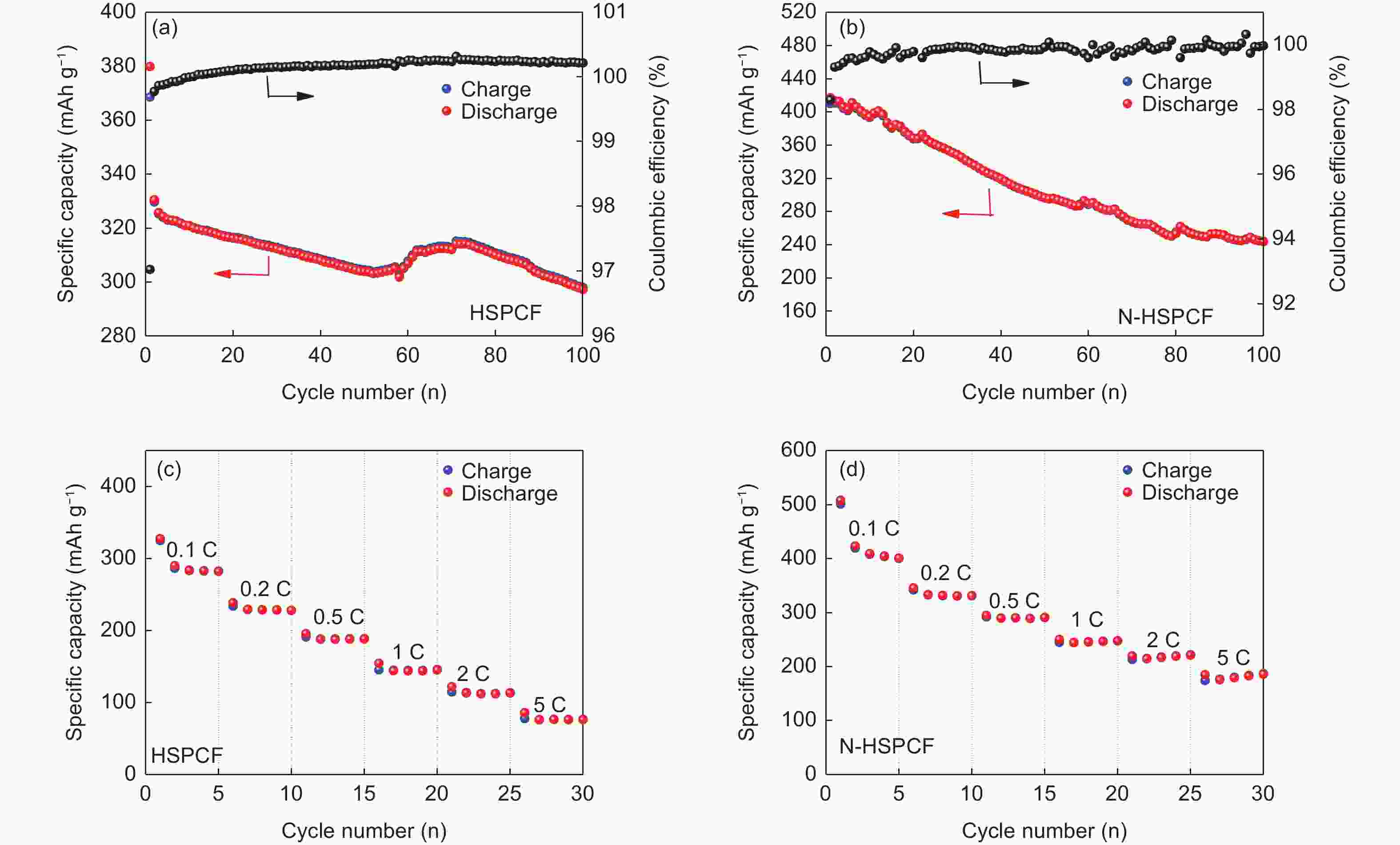
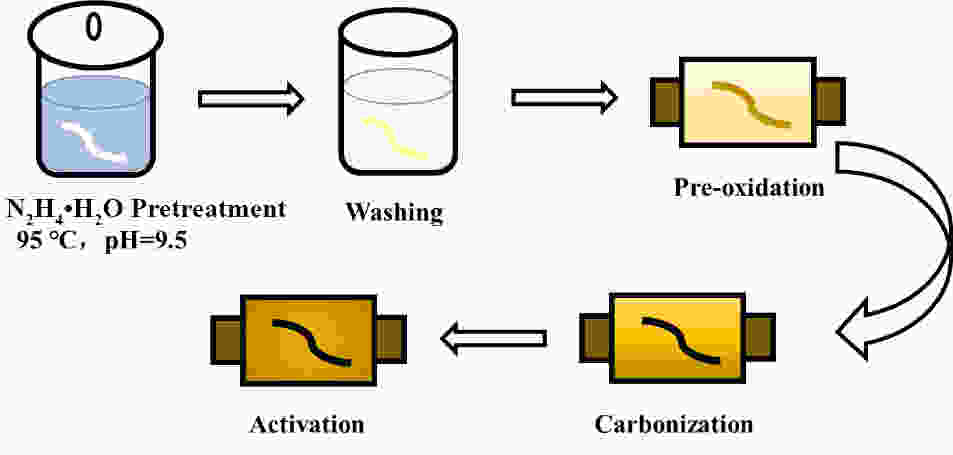
摘要: 以聚丙烯腈(PAN)中空炭纤维为基体,通过KOH活化法制备了PAN中空多孔炭纤维用于锂硫电池正极材料基体。中空炭纤维经活化得到2491 m2·g−1的高比表面积和1.22 cm3·g−1的大孔隙体积。为了进一步提高电化学性能,使用水合肼对纤维前体进行了改性,以制备氮掺杂的中空多孔炭纤维。修饰后的纤维拥有1690 m2·g−1的比表面积,0.84 cm3·g−1的孔隙体积和8.81 at%的高氮含量。由于含氮基团可以增加纤维表面极性和吸附能力,所以在电流密度为1 C时,其起始比容量可以提升至到420 mAh·g−1。Abstract: Hollow porous carbon fibers for Li-S battery electrodes were prepared by the KOH activation of carbon prepared from hollow polyacrylonitrile fibers. The fibers had a high specific surface area of 2 491 m2·g−1, a large pore volume of 1.22 cm3·g−1 and an initial specific capacity of 330 mAh·g−1 at a current density of 1 C. To improve their electrochemical performance, the fibers were modified by treatment with hydrazine hydrate to prepare nitrogen-doped hollow porous carbon fibers with a specific surface area of 1 690 m2·g−1, a pore volume of 0.84 cm3·g−1 and a high nitrogen content of 8.81 at%. Because of the increased polarity and adsorption capacity produced by the nitrogen doping, the initial specific capacity of the fibers was increased to 420 mAh·g−1 at a current density of 1 C.
-
Key words:
- Hollow-shaped carbon fibers /
- Activation /
- Modification /
- Li-S batteries
-
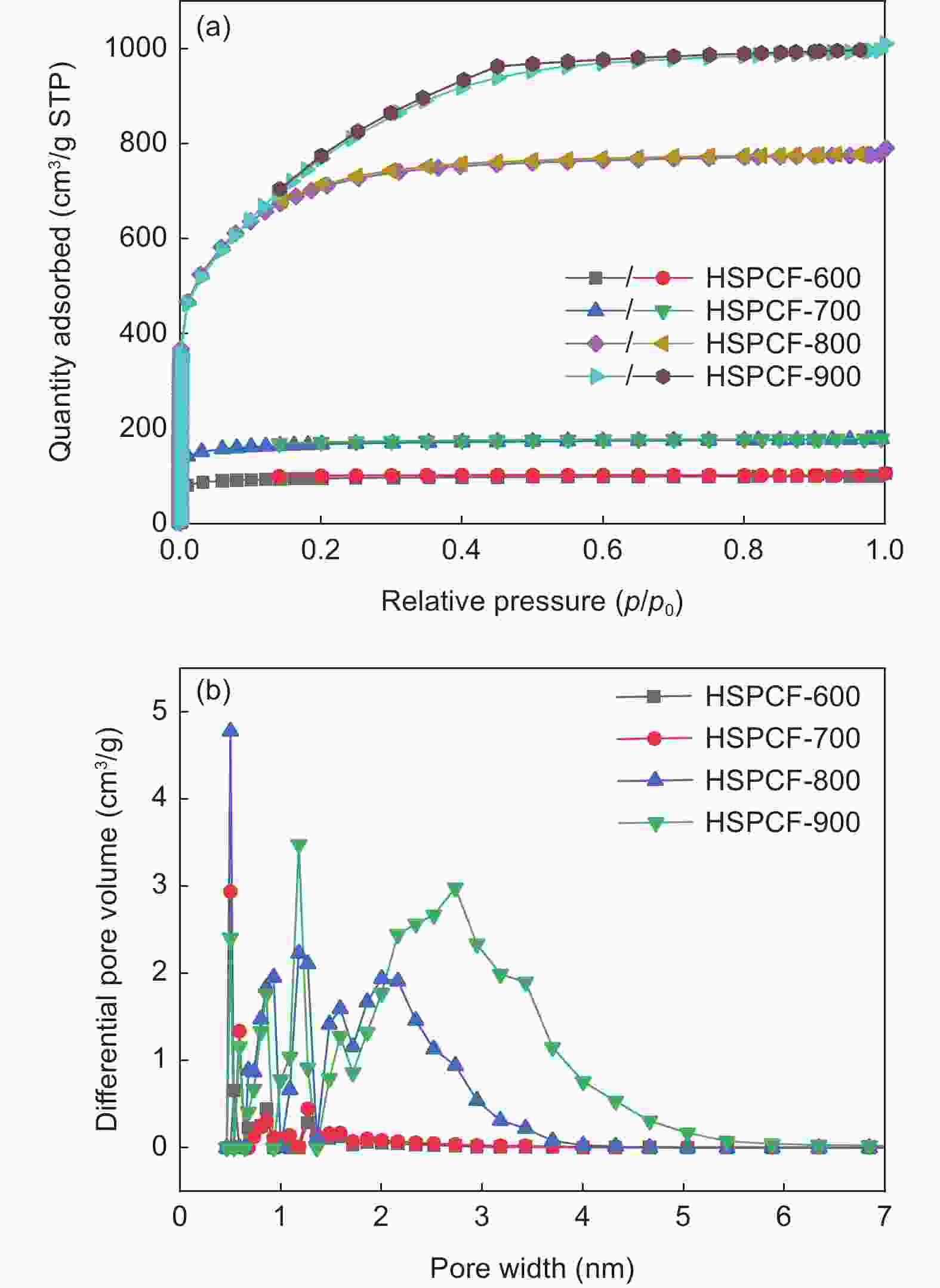
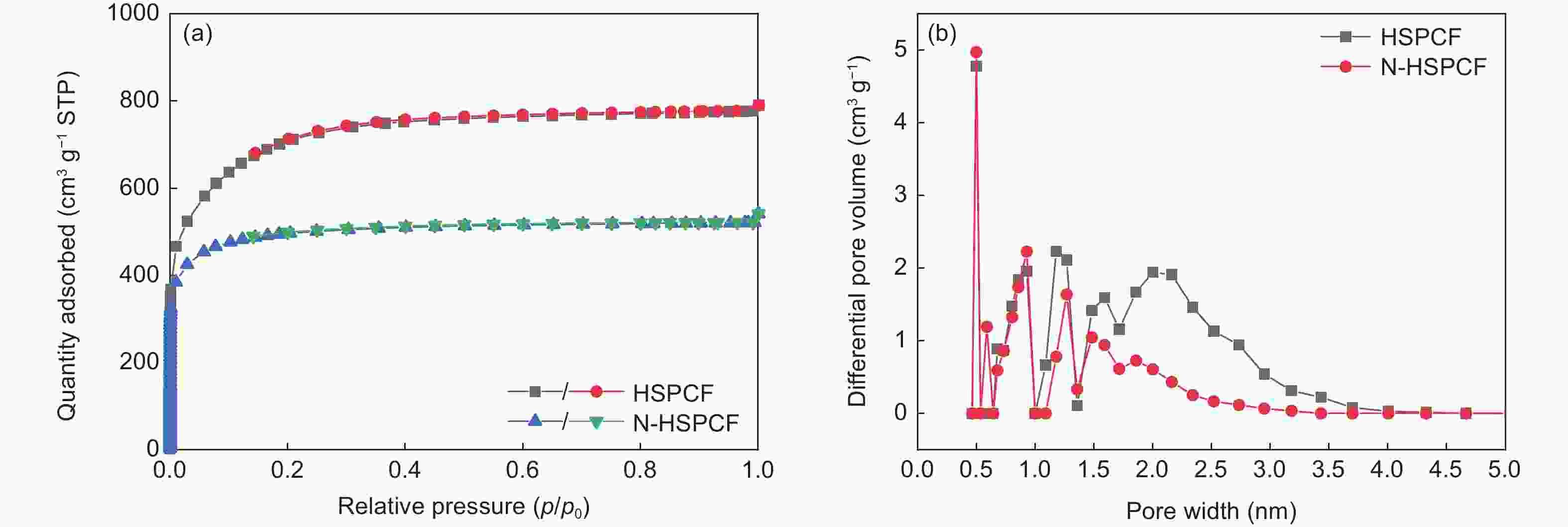
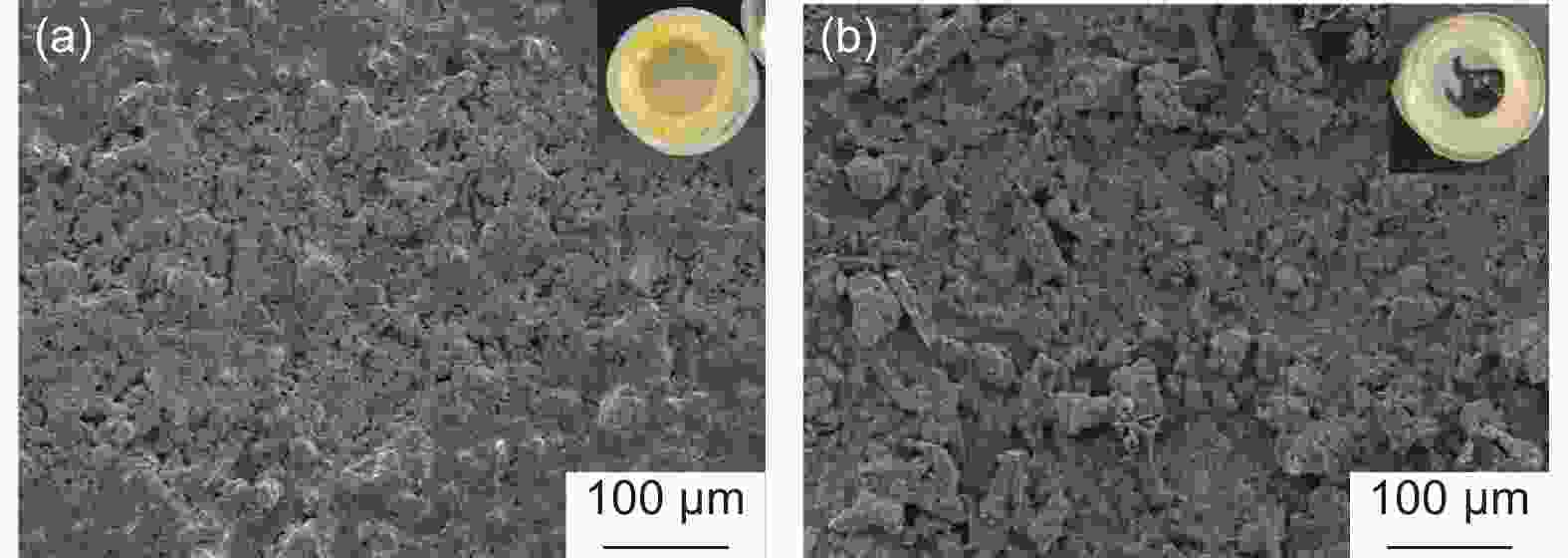
Table 1. Pore structure parameters of samples
Sample SBET(m2 g−1) Smicro(m2 g−1) Vtotal(cm3 g−1) VBJH(cm3 g−1) HSCF 0.19 - - - HSPCF-600 323.31 260.19 0.16 0.021 HSPCF-700 566.05 449.23 0.28 0.045 HSPCF-800 2490.98 992.42 1.22 0.407 HSPCF-900 2813.15 95.90 1.56 1.131 Table 2. Pore structure parameters of HSPCF and N-HSPCF
Samples SBET (m2·g−1) Smicro (m2·g−1) Vtotal (cm3·g−1) VBJH (cm3·g−1) HSPCF 2491 992 1.22 0.41 N-HSPCF 1690 1307 0.84 0.12 Table 3. The content of surface nitrogen-containing functional groups for HSPCF and N-HSPCF
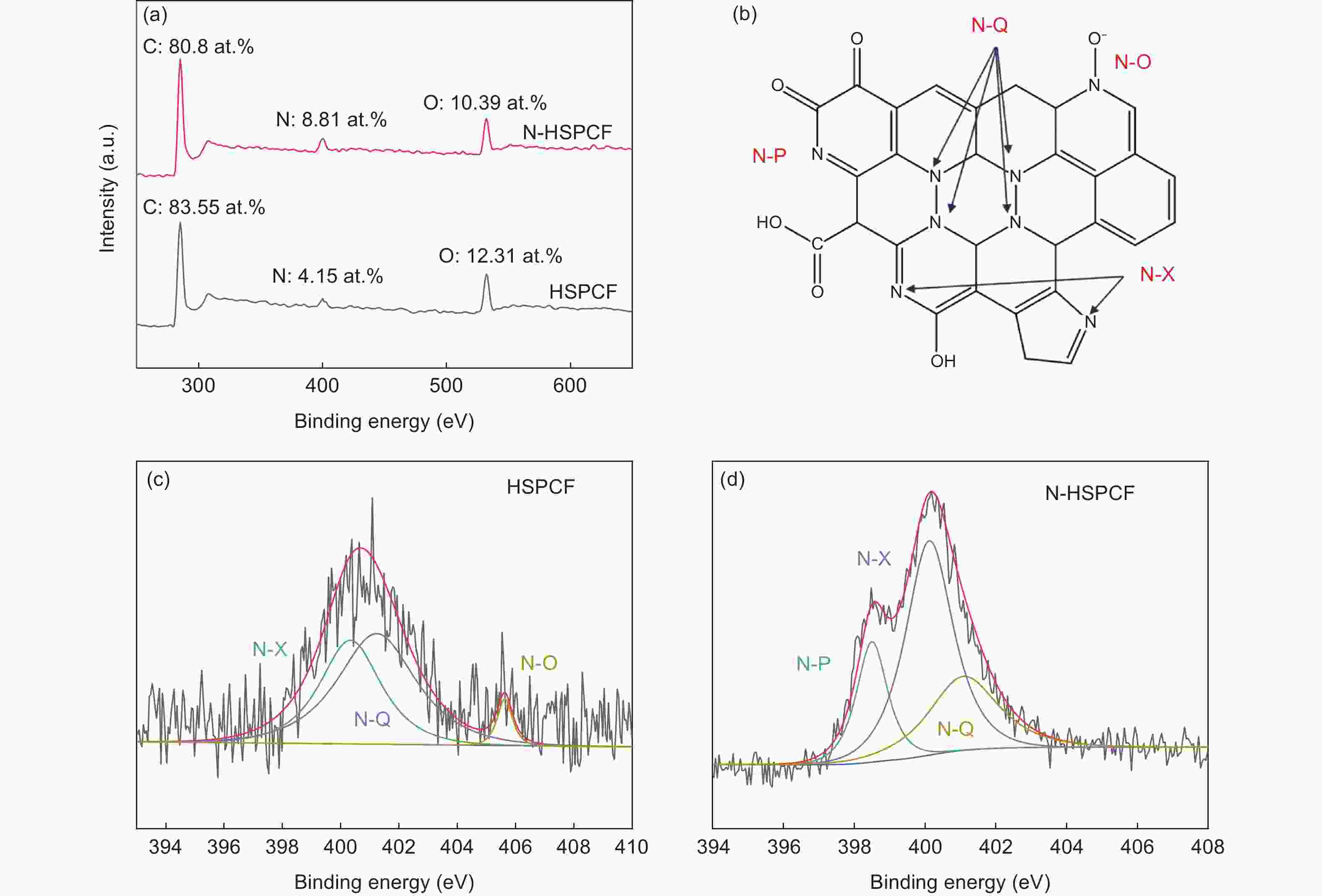
Sample XPS (at. %) Nitrogen functional group (%) C N O N-P N-X N-Q N-O HSPCF 83.55 4.15 12.31 - 40.29 55.16 4.55 N-HSPCF 80.80 8.81 10.39 19.25 53.50 26.75 - -
[1] Zhong Y, Chao D, Deng S, et al. Confining sulfur in integrated composite scaffold with highly porous carbon fibers/vanadium nitride arrays for high‐performance lithium–sulfur batteries[J]. Advanced Functional Materials,2018,28(38):1706391. doi: 10.1002/adfm.201706391 [2] Kim J S, Hwang T H, Kim B G, et al. A lithium-sulfur battery with a high areal energy density[J]. Advanced Functional Materials,2014,24(34):5359-5367. doi: 10.1002/adfm.201400935 [3] Zhou G, Li L, Ma C, et al. A graphene foam electrode with high sulfur loading for flexible and high energy Li-S batteries[J]. Nano Energy,2015,11:356-365. doi: 10.1016/j.nanoen.2014.11.025 [4] You Y, Zeng W, Yin Y X, et al. Hierarchically micro/mesoporous activated graphene with a large surface area for high sulfur loading in Li-S batteries[J]. ournal of Materials Chemistry A,2015,3(9):4799-4802. doi: 10.1039/C4TA06142J [5] Miao L, Wang W, Yuan K, et al. A lithium-sulfur cathode with high sulfur loading and high capacity per area: a binder-free carbon fiber cloth-sulfur material[J]. Chemical communications,2014,50(87):13231-13234. doi: 10.1039/C4CC03410D [6] Fang R, Zhao S, Hou P, et al. 3D interconnected electrode materials with ultrahigh areal sulfur loading for Li-S batteries[J]. Advanced materials,2016,28(17):3374-3382. doi: 10.1002/adma.201506014 [7] Chen K, Fang R, Lian Z, et al. An in-situ solidification strategy to block polysulfides in lithium-sulfur batteries[J]. Energy Storage Materials,2021,37:224-232. doi: 10.1016/j.ensm.2021.02.012 [8] Yang Y, Zheng G, Cui Y. Nanostructured sulfur cathodes[J]. Chemical Society Reviews,2013,42(7):3018-3032. doi: 10.1039/c2cs35256g [9] Pang Q, Liang X, Kwok C Y, et al. Advances in lithium-sulfur batteries based on multifunctional cathodes and electrolytes[J]. Nature Energy,2016,1(9):1-11. [10] Zheng G, Yang Y, Cha J J, et al. Hollow carbon nanofiber-encapsulated sulfur cathodes for high specific capacity rechargeable lithium batteries[J]. Nano Letters,2011,11(10):4462-4467. doi: 10.1021/nl2027684 [11] Zhao M, Chen X, Li X Y, et al. An organodiselenide comediator to facilitate sulfur redox kinetics in lithium-sulfur batteries[J]. Advanced Materials,2021,33(13):2007298. doi: 10.1002/adma.202007298 [12] Feng X, Wang Q, Li R, et al. CoFe2O4 coated carbon fiber paper fabricated via a spray pyrolysis method for trapping lithium polysulfide in Li-S batteries[J]. Applied Surface Science,2019,478:341-346. doi: 10.1016/j.apsusc.2019.01.145 [13] Ye J, He F, Nie J, et al. Sulfur/carbon nanocomposite-filled polyacrylonitrile nanofibers as a long life and high capacity cathode for lithium-sulfur batteries[J]. Journal of Materials Chemistry A,2015,3(14):7406-7412. doi: 10.1039/C4TA06976E [14] Cheng Z, Chen Y, Yang Y, et al. Metallic MoS2 nanoflowers decorated graphene nanosheet catalytically boosts the volumetric capacity and cycle life of lithium–sulfur batteries[J]. Advanced Energy Materials,2021,11(12):2003718. doi: 10.1002/aenm.202003718 [15] Park J H, Choi W Y, Yang J, et al. Nitrogen-rich hierarchical porous carbon paper for a free-standing cathode of lithium sulfur battery[J]. Carbon,2021,172:624-636. doi: 10.1016/j.carbon.2020.10.078 [16] Pang Z, Ma Y, Zhou Y, et al. Tailoring 3D carbon foam using CNTs and MnO2 to fabricate stable lithium/dissolved lithium polysulfide batteries[J]. Langmuir,2021,37(13):4016-4024. doi: 10.1021/acs.langmuir.1c00337 [17] Zeng S, Arumugam G M, Liu X, et al. Encapsulation of Sulfur into N-doped porous carbon cages by a facile, template-free method for stable lithium-sulfur cathode[J]. Small,2020,16(39):2001027. doi: 10.1002/smll.202001027 [18] Singhal R, Chung S H, Manthiram A, et al. A free-standing carbon nanofiber interlayer for high-performance lithium-sulfur batteries[J]. Journal of Materials Chemistry A,2015,3(8):4530-4538. doi: 10.1039/C4TA06511E [19] Elazari R, Salitra G, Garsuch A, et al. Sulfur-impregnated activated carbon fiber cloth as a binder-free cathode for rechargeable Li-S batteries[J]. Advanced materials,2011,23(47):5641-5644. doi: 10.1002/adma.201103274 [20] Zhang J, Li Z, Lou X W. A freestanding selenium disulfide cathode based on cobalt disulfide-decorated multichannel carbon fibers with enhanced lithium storage performance[J]. Angewandte Chemie International Edition,2017,56(45):14107-14112. doi: 10.1002/anie.201708105 [21] Ryu Z, Zheng J, Wang M, et al. Nitrogen adsorption studies of PAN-based activated carbon fibers prepared by different activation methods[J]. Journal of colloid and interface science,2000,230(2):312-319. doi: 10.1006/jcis.2000.7078 [22] Xiong L, Wang X F, Li L, et al. Nitrogen-enriched porous carbon fiber as a CO2 adsorbent with superior CO2 selectivity by air activation[J]. Energy & Fuels,2019,33(12):12558-12567. doi: 10.1021/acs.energyfuels.9b02769 [23] Li L, Wang X F, Zhong J J, et al. Nitrogen-enriched porous polyacrylonitrile-based carbon fibers for CO2 capture[J]. Industrial & Engineering Chemistry Research,2018,57(34):11608-11616. doi: 10.1021/acs.iecr.8b01836 [24] Li Y, Lu C X, Zhang S C, et al. Nitrogen- and oxygen-enriched 3D hierarchical porous carbon fibers: synthesis and superior supercapacity[J]. Journal of Materials Chemistry A,2015,3(28):14817-14825. doi: 10.1039/C5TA02702K [25] Zhou C, Li X, Jiang H, et al. Pulverizing Fe2O3 nanoparticles for developing Fe3C/N‐codoped carbon nanoboxes with multiple polysulfide anchoring and converting activity in Li-S batteries[J]. Advanced Functional Materials,2021,31(18):2011249. doi: 10.1002/adfm.202011249 [26] Chen P, Wu Z, Guo T, et al. Strong chemical interaction between lithium polysulfides and flame-retardant polyphosphazene for lithium-sulfur batteries with enhanced safety and electrochemical performance[J]. Advanced Materials,2021,33(9):2007549. doi: 10.1002/adma.202007549 [27] Yan S, Zhao M, Lei G, et al. Novel tetrazole-functionalized absorbent from polyacrylonitrile fiber for heavy-metal ion adsorption[J]. Journal of Applied Polymer Science,2012,125(1):382-389. doi: 10.1002/app.35641 [28] Pérez-Manríquez L, Aburabi’e J, Neelakanda P, et al. Cross-linked PAN-based thin-film composite membranes for non-aqueous nanofiltration[J]. Reactive and Functional Polymers,2015,86:243-247. doi: 10.1016/j.reactfunctpolym.2014.09.015 [29] Chen D, Zhan W, Fu X, et al. High-conductivity 1T-MoS2 catalysts anchored on a carbon fiber cloth for high-performance lithium-sulfur batteries[J]. Materials Chemistry Frontiers,2021,5(18):6941-6950. doi: 10.1039/D1QM00674F [30] Yang R, Li L, Chen D, et al. The enhancement of polysulfides adsorption for stable lithium-sulfur batteries cathode enabled by N-doped wrinkled graphene using solvothermal method[J]. ChemistrySelect,2017,2(35):11697-11702. doi: 10.1002/slct.201702484 [31] Zhu S, Wang Y, Jiang J, et al. Good low-temperature properties of nitrogen-ienriched porous carbon as sulfur hosts for high-performance Li-S batteries[J]. ACS Applied Materials & Interfaces,2016,8(27):17253-17259. doi: 10.1021/acsami.6b04355 [32] Wang Z, Niu X, Xiao J, et al. First principles prediction of nitrogen-doped carbon nanotubes as a high-performance cathode for Li–S batteries[J]. RSC Advances,2013,3(37):16775-16780. doi: 10.1039/c3ra41333k -





 下载:
下载: