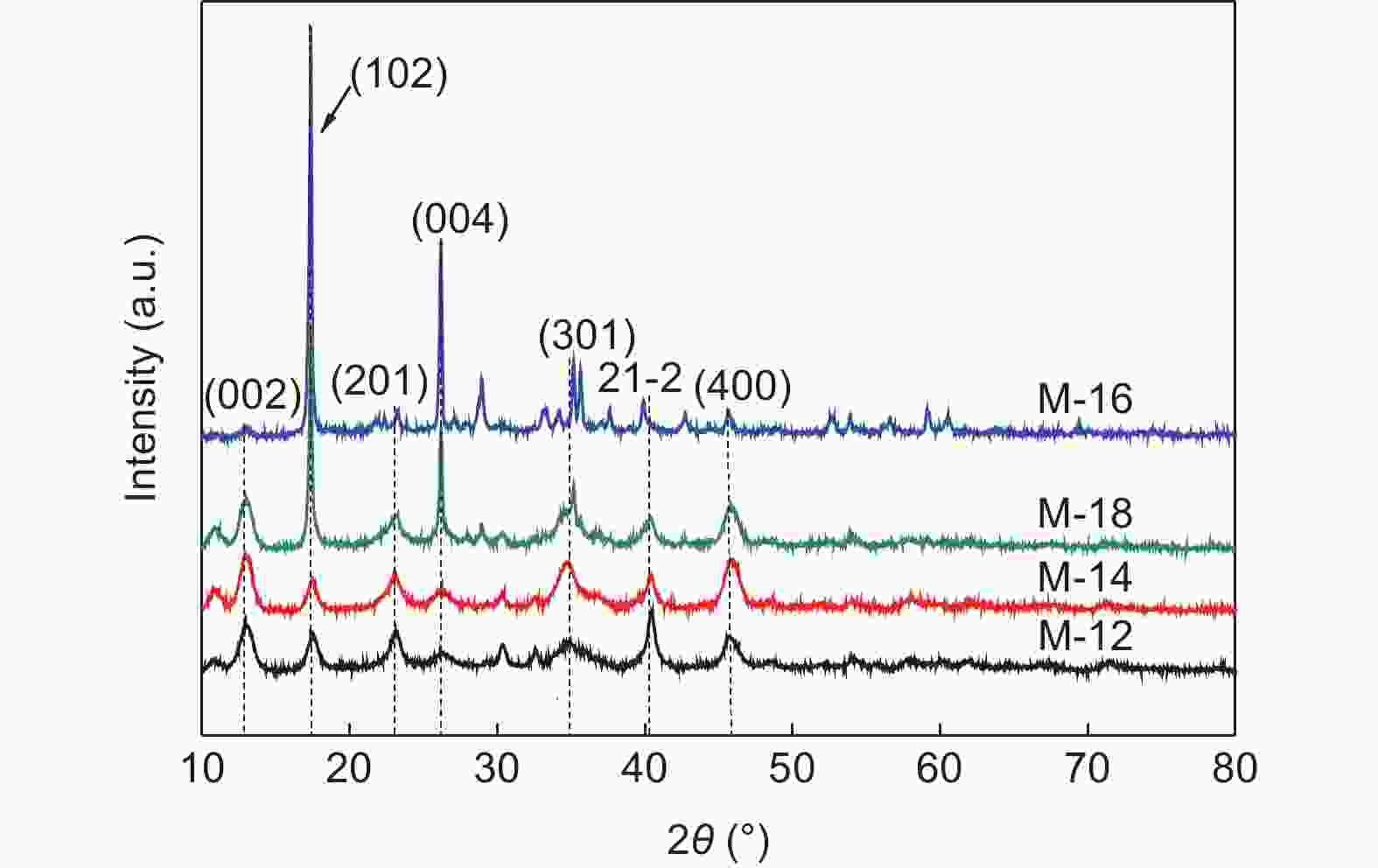
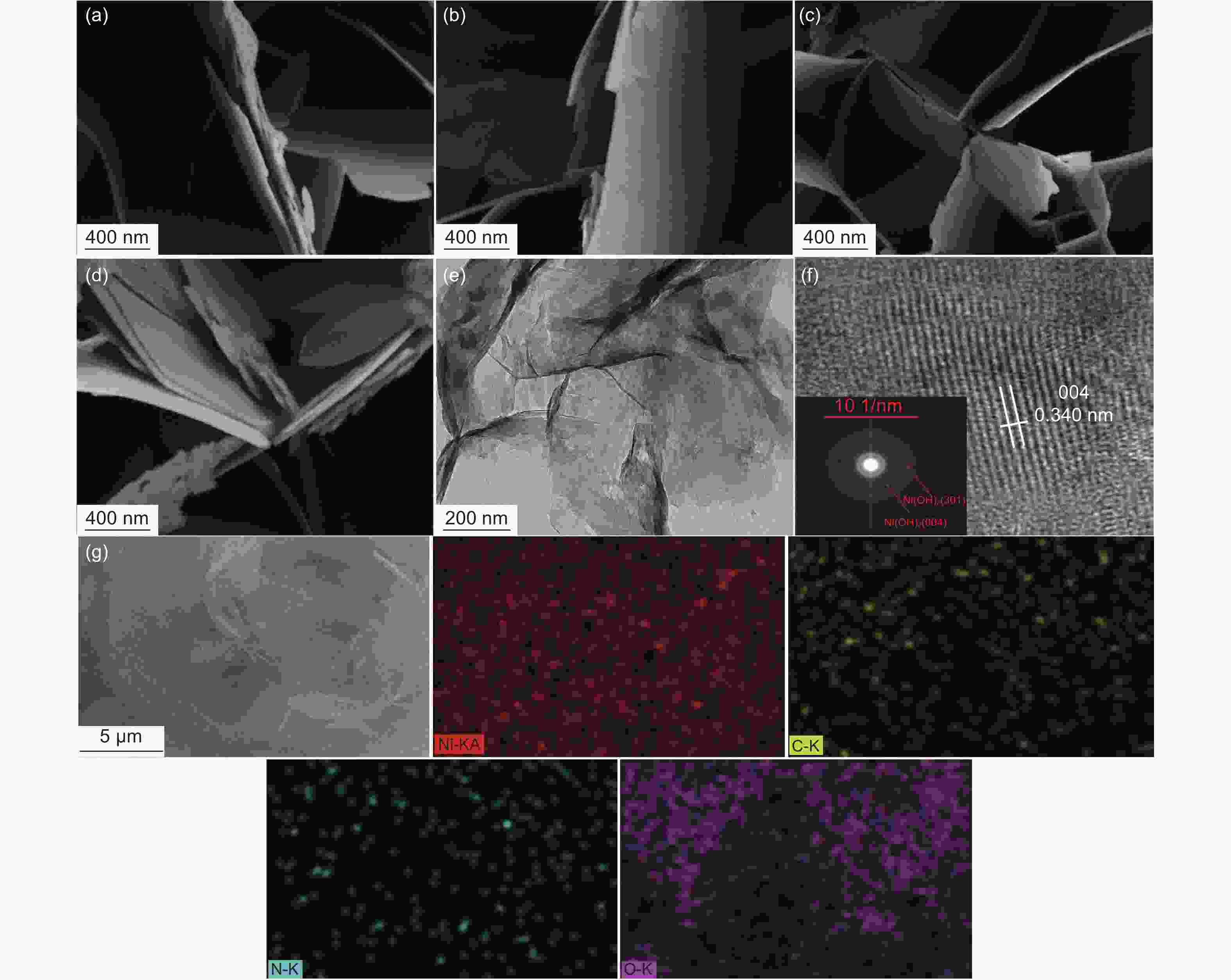
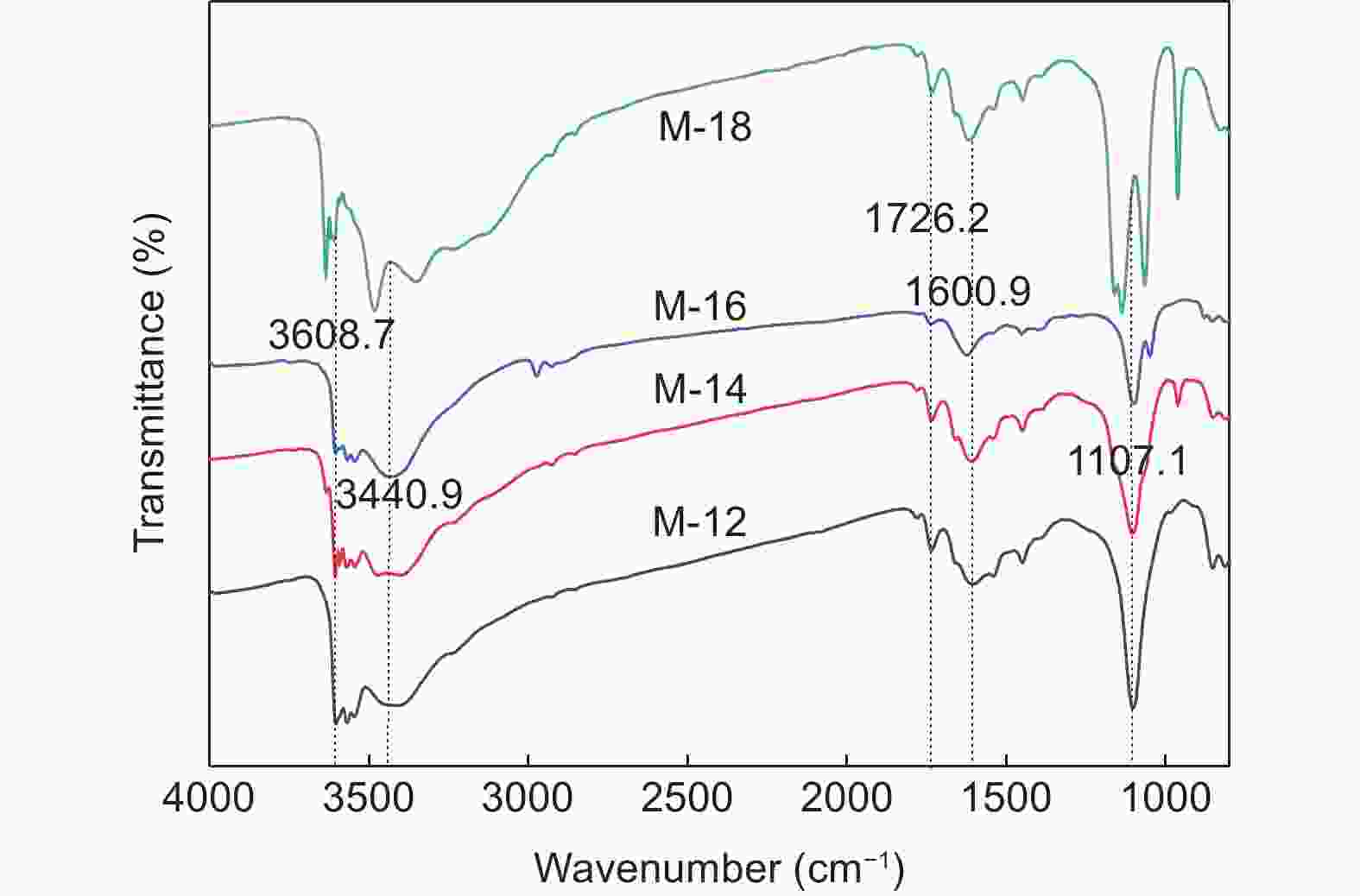
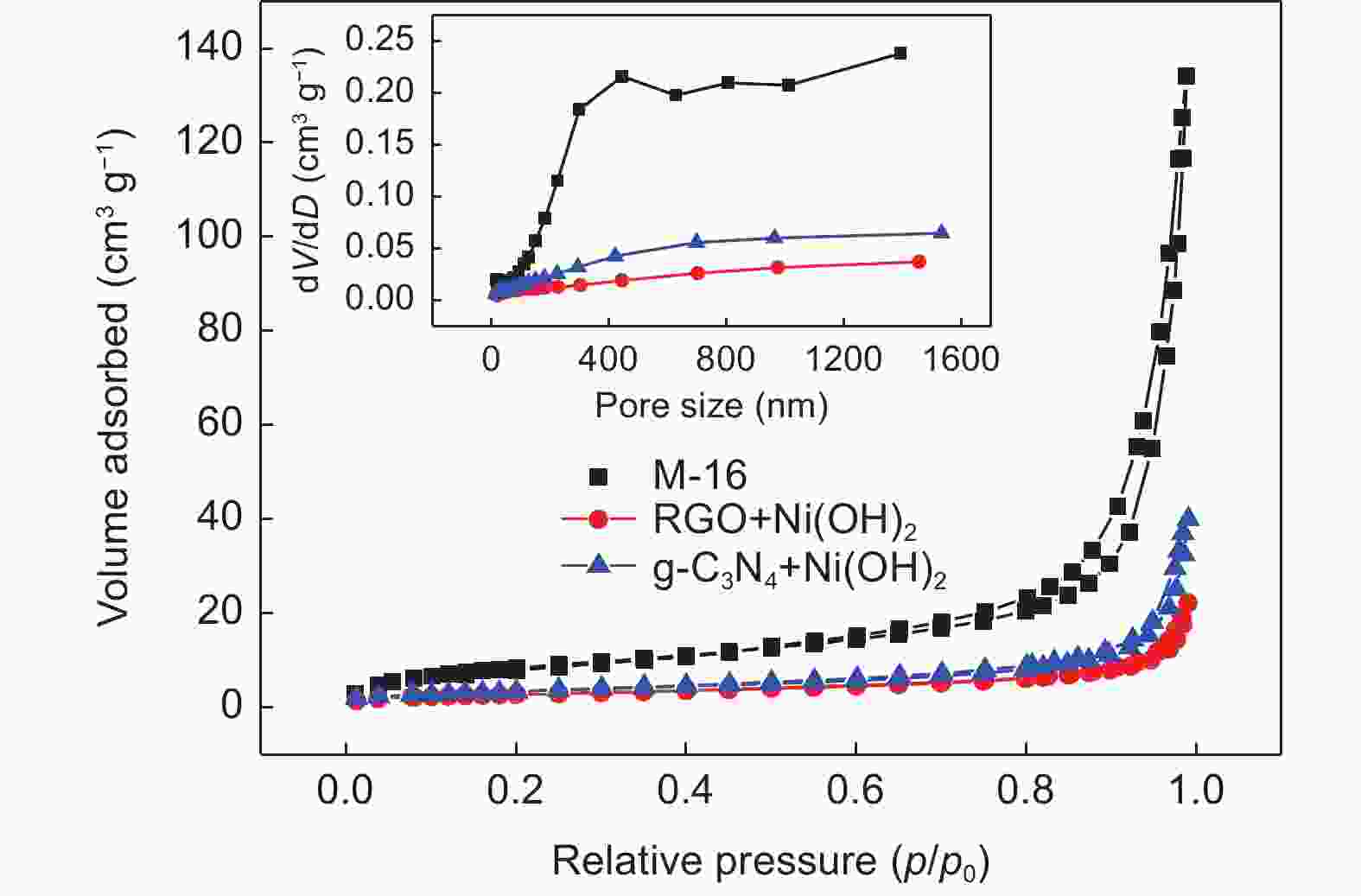
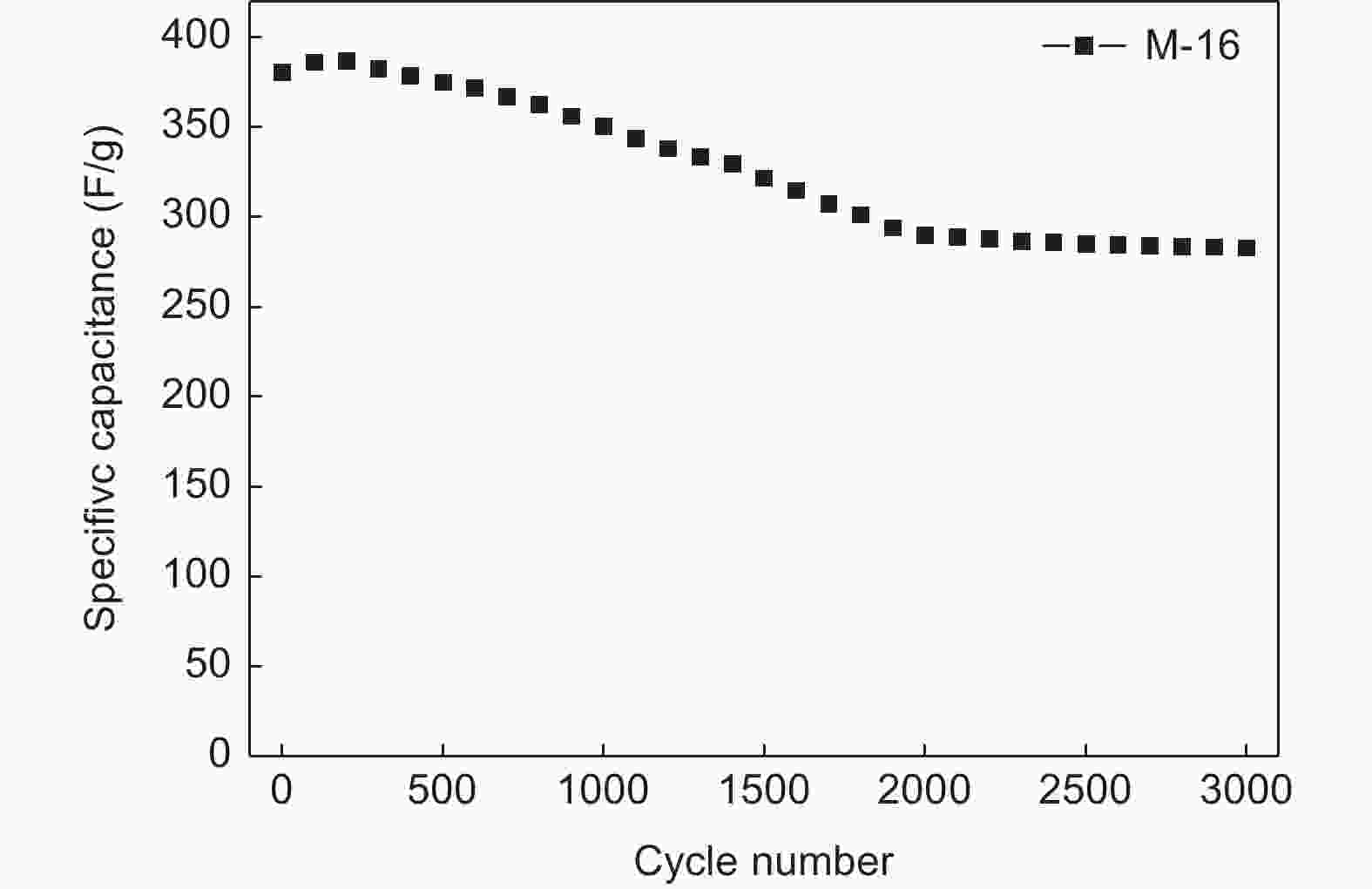
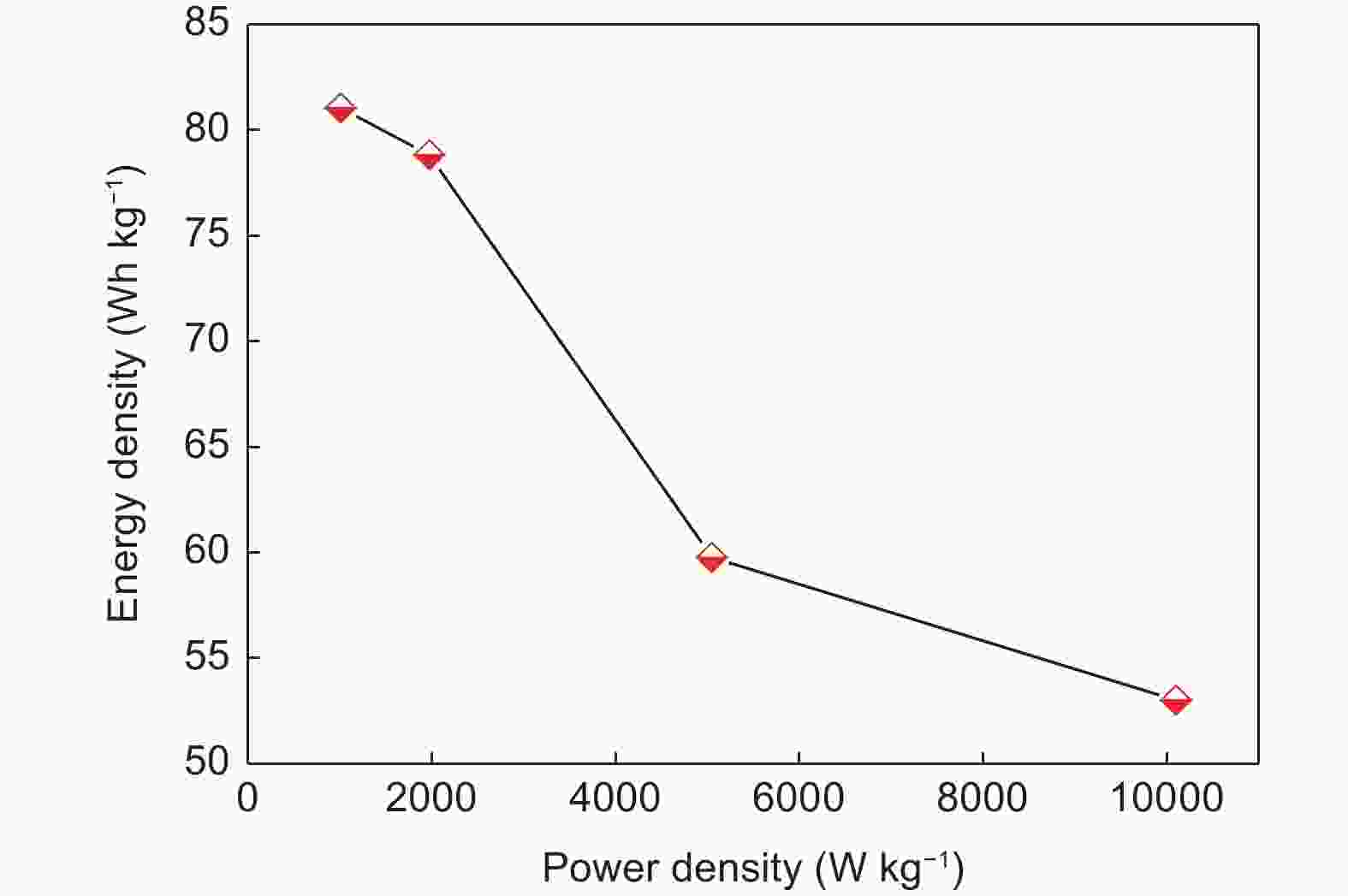
| [1] |
Hu H J, Liu J Y, Xu Z, et al. Hierarchical porous Ni/Co-LDH hollow dodecahedron with excellent adsorption property for Congo red and Cr(VI) ions[J]. Appl Surf Sci,2019,478:981-990. doi: 10.1016/j.apsusc.2019.02.008
|
| [2] |
Huang B, Wang W S, Pu T, et al. Two-dimensional porous (Co, Ni)-based monometallic hydroxides and bimetallic layered double hydroxides thin sheets with honeycomb-like nanostructure as positive electrode for high-performance hybrid supercapacitors[J]. J Colloid Interf Sci,2018,532:630-640. doi: 10.1016/j.jcis.2018.08.019
|
| [3] |
Jia H N, Wang Z Y, Zheng X H, et al. Interlaced Ni-Co LDH nanosheets wrapped Co9S8 nanotube with hierarchical structure toward high performance supercapacitors[J]. Chem Eng J,2018,351:348-355. doi: 10.1016/j.cej.2018.06.113
|
| [4] |
Liu J L, Wang Y H, Hu R J, et al. High-performance supercapacitor electrode based on 3D rose-like β-Ni(OH)2/rGO nanohybrid[J]. J Phys Chem Solids,2020,138:109297. doi: 10.1016/j.jpcs.2019.109297
|
| [5] |
Zhang X Y, Liao H W, Liu X, et al. Graphitic carbon nitride nanosheets made by different methods as electrode material for supercapacitors[J]. Ionics,2020,26(7):3599-3607. doi: 10.1007/s11581-020-03458-z
|
| [6] |
Bandyopadhyay P, Li X Y, Hoon N K, et al. Graphitic carbon nitride modified graphene/Ni Al layered double hydroxide and 3D functionalized graphene for solid-state asymmetric supercapacitors[J]. Chem Eng J,2018,353:824-838. doi: 10.1016/j.cej.2018.07.172
|
| [7] |
Kumar Y A, Kulurumotlakatla D K, Sangaraju S, et al. Facile synthesis of novel and highly efficient CoNi2S4-Ni(OH)2 nanosheet arrays as pseudocapacitive-type electrode material for high-performance electrochemical supercapacitors[J]. J Energy Storage,2020,31:101623. doi: 10.1016/j.est.2020.101623
|
| [8] |
Lai L Q, Clark M, Su S Y, et al. Dip-coating synthesis of rGO/α-Ni(OH)2@nickel foam with layer-by-layer structure for high performance binder-free supercapacitors[J]. Electrochim Acta,2021,368:137589. doi: 10.1016/j.electacta.2020.137589
|
| [9] |
Han C, Cao W Y, Si H Z, et al. One-step electrodeposition synthesis of high performance carbon nanotubes/graphene-doped Ni(OH)2 thin film electrode for high-performance supercapacitor[J]. Electrochim Acta,2019,322:134747. doi: 10.1016/j.electacta.2019.134747
|
| [10] |
Hong Y W, Yang J X, Choi W M, et al. B-doped g-C3N4 quantum dots-modified Ni(OH)2 nanoflowers as an efficient and stable electrode for supercapacitors[J]. Acs Appl Energ Mater,2021,4(2):1496-1504. doi: 10.1021/acsaem.0c02680
|
| [11] |
Aranganathan V, Adka N S. The high energy supercapacitor from rGO/Ni(OH)2/PANI nanocomposite with methane sulfonic acid as dopant[J]. J Colloid Interf Sci,2019,557:367-380. doi: 10.1016/j.jcis.2019.09.036
|
| [12] |
Zeng W, Feng Q N, Yuan J L. Ni(OH)2/3D-rGO supercapacitor material with high specific capacitance[J]. Mater Today Commun,2021,27:102292. doi: 10.1016/j.mtcomm.2021.102292
|
| [13] |
Sheng K X, Xu Y X, Li C, et al. High-performance self-assembled graphene hydrogels prepared by chemical reduction of graphene oxide[J]. New Carbon Mater,2011,26(1):9-15. doi: 10.1016/j.carbon.2011.02.032
|
| [14] |
Gao T, Jelle B P. Paraotwayite-Type α-Ni(OH)2 Nanowires: Structural, Optical and Electrochemical Properties[J]. J PHYS CHEM C,2013,117(33):17294-17302. doi: 10.1021/jp405149d
|
| [15] |
Liu F Y, Chu X, Zhang H T, et al. Synthesis of self-assembly 3D porous Ni(OH)2 with high capacitance for hybrid supercapacitors[J]. Electrochim Acta,2018,269:102-110. doi: 10.1016/j.electacta.2018.02.130
|
| [16] |
Li B J, Cao H Q, Shao J, et al. Improved performances of β-Ni(OH)2@reduced-graphene-oxide in Ni-MH and Li-ion batteries[J]. Chem Comun,2011,47(11):3159. doi: 10.1039/c0cc04507a
|
| [17] |
Wu, S C, Xu B J, Long Y F, et al. Oxygen-functionalized g-C3N4 layers anchored with Ni(OH)2 nanoparticles assembled onto Ni foam as binder-free outstanding electrode for supercapacitors[J]. Synthetic Met,2020,270:116601. doi: 10.1016/j.synthmet.2020.116601
|
| [18] |
Ding M, Qu Y D, Zhang X Y, et al. Reduced graphene oxide/g-C3N4 modified carbon fibers for high performance fiber supercapacitors[J]. New J Chem,2021,45(2):923-929. doi: 10.1039/d0nj05072e
|
| [19] |
Qiu H R, An S L, Sun X J, et al. MWCNTs-GONRs/Co3O4@Ni(OH)2 core-shell array structure with a high performance electrode for supercapacitor[J]. Chem Eng J,2020,380:122490. doi: 10.1016/j.cej.2019.122490
|
| [20] |
Zhang Q, Zang Q, Shi Q Q, et al. Formation of V6O11@Ni(OH)2/NiOOH hollow double-shell nanoflowers for the excellent cycle stability of supercapacitors[J]. Dalton T,2021,50(10):3693-3700. doi: 10.1039/D0DT04134C
|
| [21] |
Tahir M U, Arshad H, Xie W Y, et al. Synthesis of morphology controlled NiCo-LDH microflowers derived from ZIF-67 using binary additives and their excellent asymmetric supercapacitor properties[J]. Appl Surf Sci,2020,529:147073. doi: 10.1016/j.apsusc.2020.147073
|
| [22] |
Li X Y, Chen R R, Zhao Y H, et al. Layer-by-layer inkjet printing GO film anchored Ni(OH)2 nanoflakes for high-performance supercapacitors[J]. Chem Eng J,2019,375:121988. doi: 10.1016/j.cej.2019.121988
|
| [23] |
Tang Y F, Liu Y Y, Yu S X, et al. Hydrothermal synthesis of a flower-like nano-nickel hydroxide for high performance supercapacitors[J]. Electrochim Acta,2014,123:158-166. doi: 10.1016/j.electacta.2013.12.187
|
| [24] |
Huang J C, Xu P P, Cao D X, et al. Asymmetric supercapacitors based on β-Ni(OH)2 nanosheets and activated carbon with high energy density[J]. J Power Sources,2014,246:371-376. doi: 10.1016/j.jpowsour.2013.07.105
|





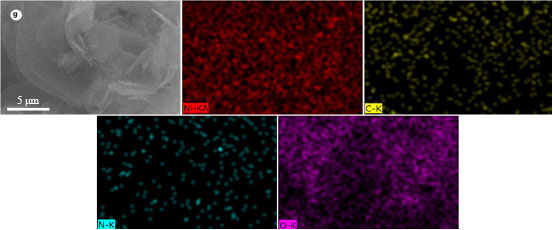
 下载:
下载: