Preparation of carbon dots from carbonized corncobs by electrochemical oxidation and their application in Na-batteries
-
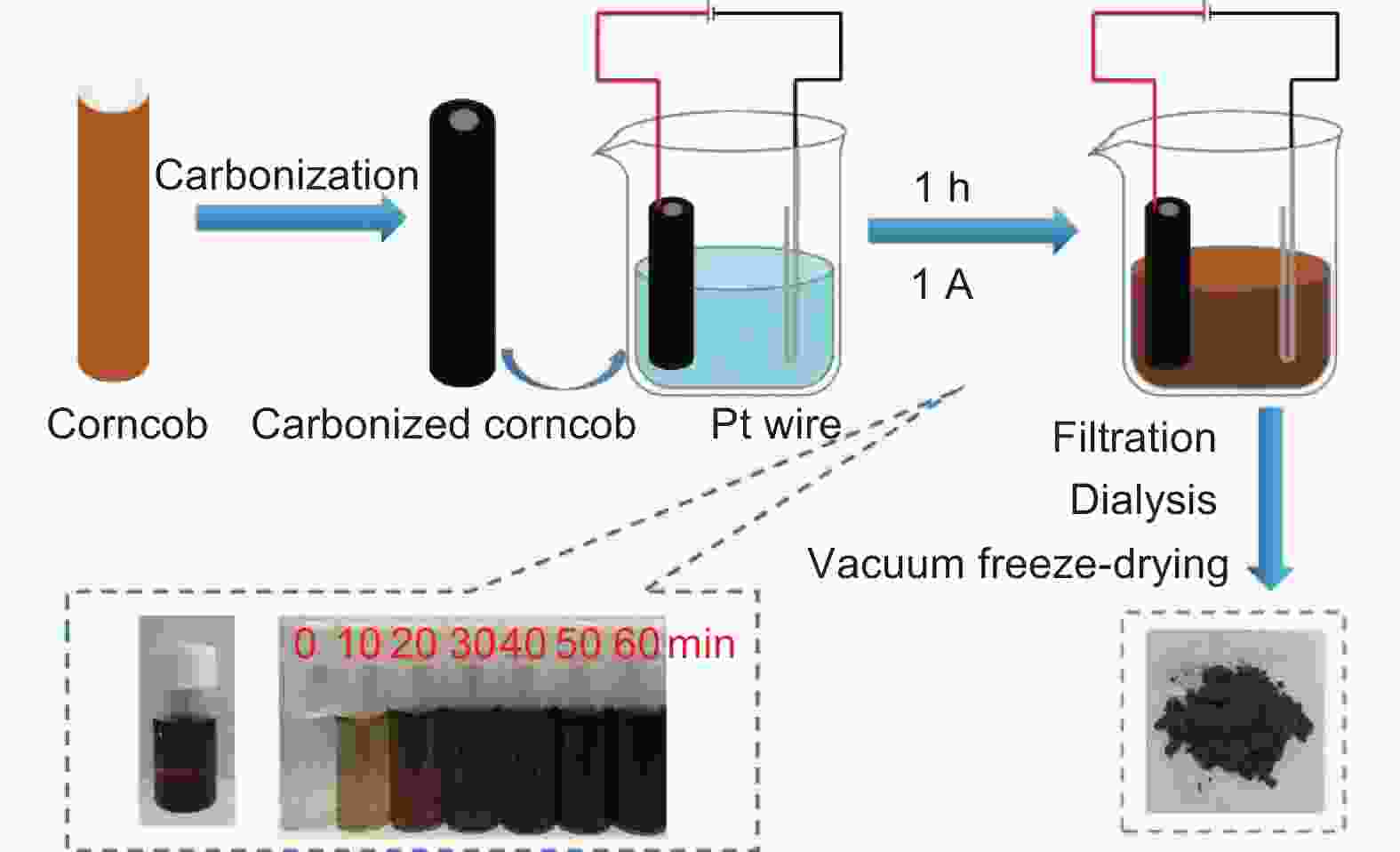
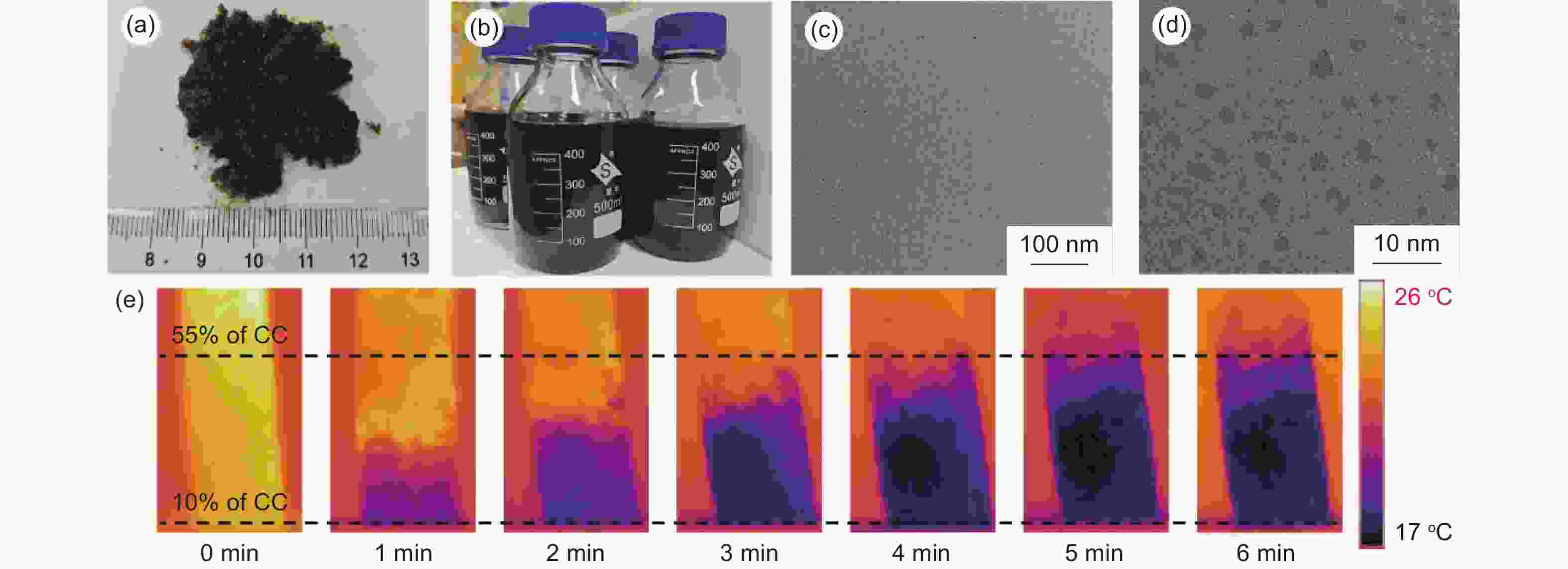
摘要: 碳点(CDs)是一种新兴的碳纳米材料,因其高比表面积、良好的分散性、丰富的表面官能团、低生物毒性和光致发光特性而受到了研究者的广泛关注。然而,低成本、大规模和绿色合成CDs仍面临挑战。本工作基于生物质玉米芯特殊的天然孔隙结构,经直接炭化制备具有定向、贯通微纳米孔道的多孔三维电极材料,在毛细作用下电解液可以充满整个电极材料,内外表面同时发生电化学氧化,实现高效制备CDs。在1 A恒电流下,每克电极材料制备CDs速率达到了79.83 mg h−1。将制备的CDs与氧化石墨烯(GO)水热复合得到复合气凝胶CDs/rGO材料,经热处理后应用于钠离子电池。在1 A g−1下循环1000圈仍保持263.3 mAh g−1的容量。本研究工作采用生物质玉米芯高效制备CDs,为CDs的大规模绿色制备和应用提供了新的途径和思路。Abstract: Carbon dots (CDs) have attracted increasing attention due to their high specific surface area, good dispersion, abundant surface functional groups, low biotoxicity and photoluminescence. However, their preparation on a large-scale is still a great challenge because of the high cost and environmental problems, and this seriously limits their practical applications. Herein, carbonized corncobs were used as the starting material for the preparation of the CDs by electrochemical oxidation. Their natural porous structures with well-developed channels allow the electrode to be filled with electrolyte, and the electrochemical oxidation takes place both on the inside and outside surfaces of the carbonized corncob, achieving a CD output of 79.8 mg h−1 per gram of electrode material at 1 A. The CDs were combined with graphene oxide (GO) to produce CD/rGO composite aerogels by a hydrothermal method. After heat treatment at 600 °C, the materials obtained were used as the anode in a sodium ion battery, which had a capacity of 263.3 mAh g−1 after 1 000 cycles at 1 A g−1. This work suggests a new way to prepare CDs and possibly expand their range of application.
-
Key words:
- Corncob /
- Carbon dots /
- Electrochemical synthesis /
- Sodium ion batteries
-
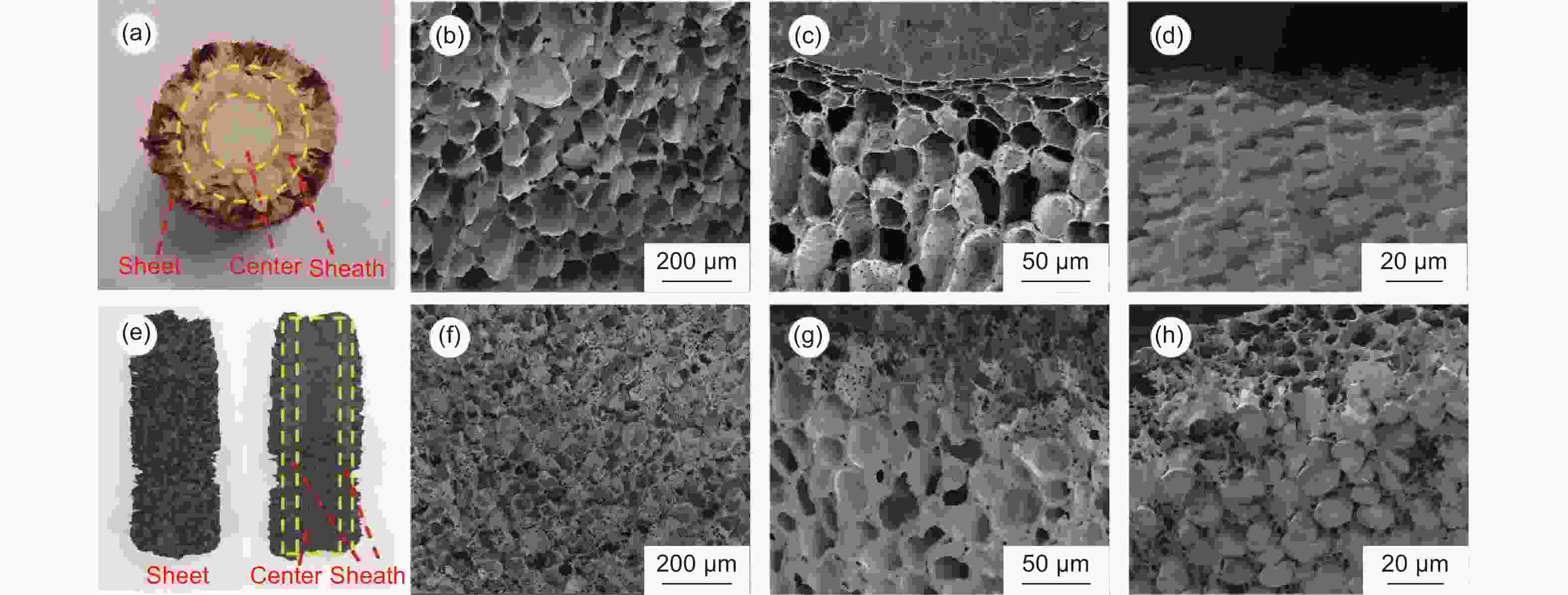
图 2 (a) 玉米芯截面;(b-d) 炭化玉米芯各部分刻蚀前的SEM照片:(b) 中心;(c) 鞘;(d) 外层薄片;(e) 炭化玉米芯刻蚀后轴向剖面;(f-h) 玉米芯各部分刻蚀前的SEM照片:(f) 中心;(g) 鞘;(h) 外层薄片
Figure 2. (a) Cross-section of corncob; (b-d) SEM images of carbonized corncob before etching; (b) center; (c) sheath; (d) sheet; (e) Longitudinal section of carbonized corncob after etching; (f-h) SEM images of carbonized corncob after etching; (f) center; (g) sheath; (h) sheet
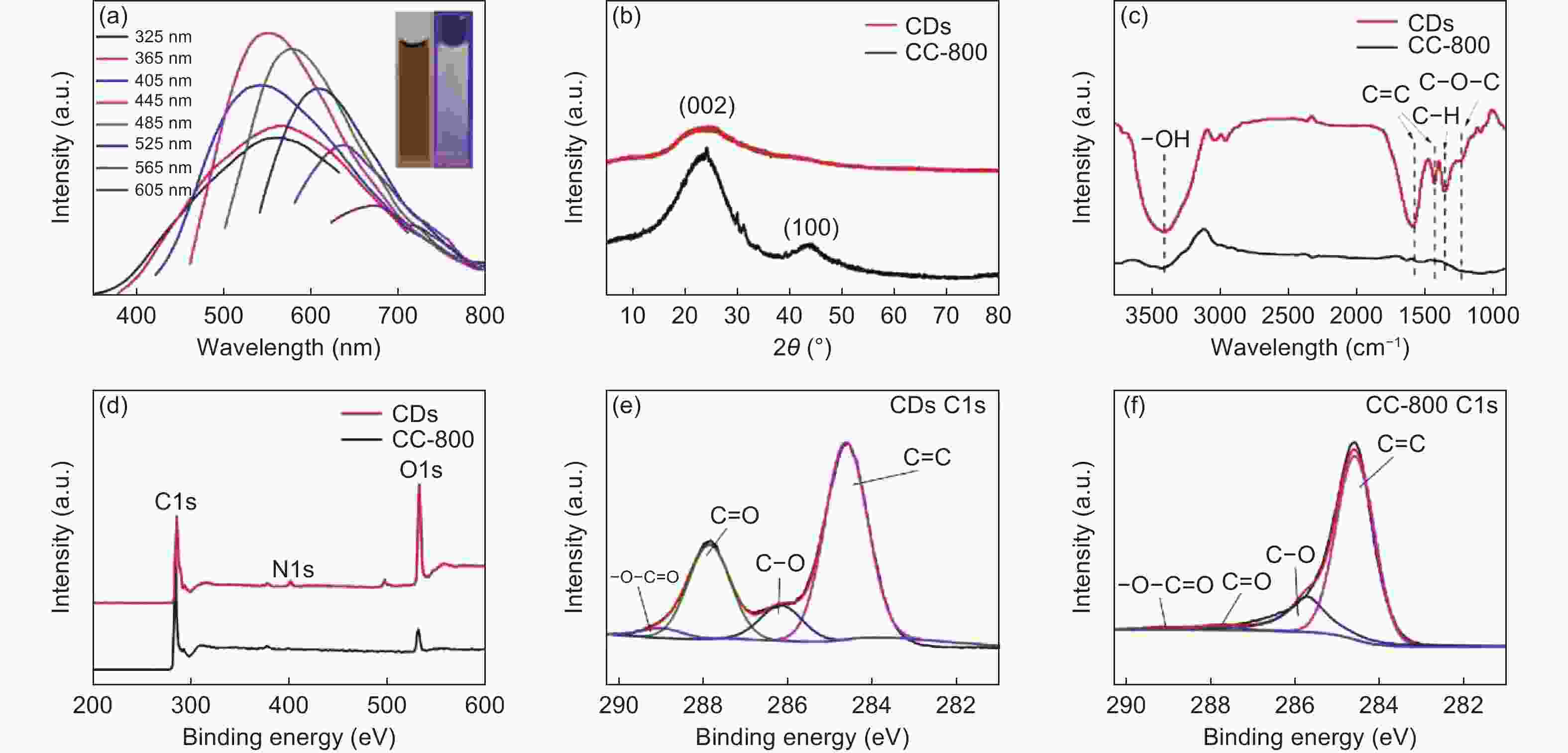
图 4 (a) CDs荧光谱图;(b) CDs和炭化玉米芯(800°C)的XRD谱图;(c) CDs和炭化玉米芯(800 °C)的FTIR光谱图;(d) CDs和炭化玉米芯(800 °C)的XPS谱图;(e) CDs的C1s XPS谱图;(f)炭化玉米芯(800 °C)的C1s XPS谱图
Figure 4. (a) Fluorescence spectra of CDs; (b) XRD patterns of CDs and CC-800; (c) FTIR spectra of CDs and CC-800; (d) XPS survey spectra of CDs and CC-800; (e) XPS survey spectra of C1s for CDs; (f) XPS survey spectra of C1s for CC-800
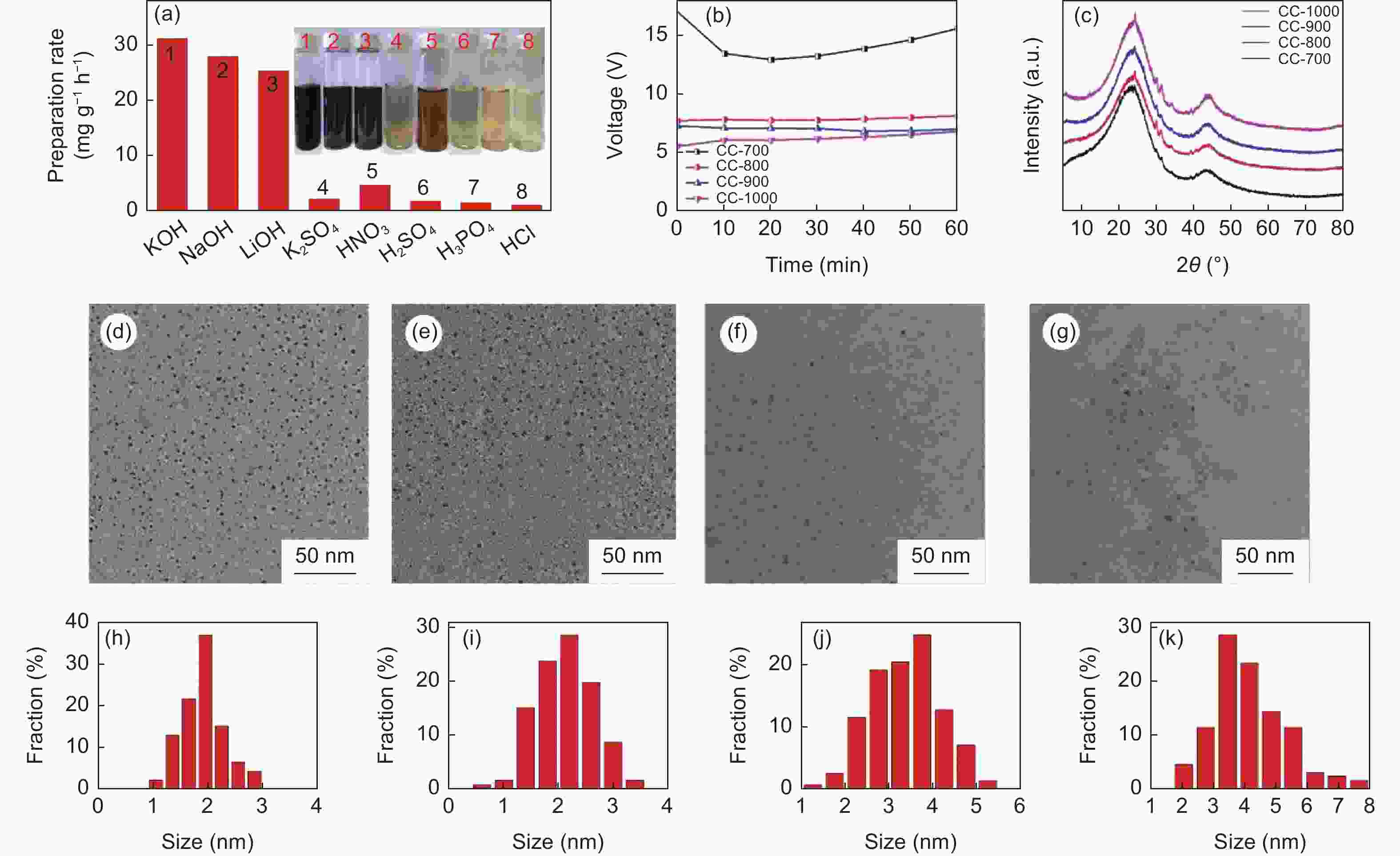
图 5 (a) 不同电解液中CDs制备速率图;(b) 不同炭化温度玉米芯电化学制备CDs电压-时间曲线;(c) 不同炭化温度玉米芯XRD谱图;(d-g) 不同炭化温度玉米芯制备CDs 的TEM图:(d) 700 °C,(e) 800 °C,(f) 900 °C,(g) 1000 °C;(h-k) 不同炭化温度玉米芯制备的CDs尺寸统计图:(h) 700 °C,(i) 800 °C,(j) 900 °C,(k) 1000 °C
Figure 5. (a) Preparation rates of CDs with different electrolytes; (b) Volt-time curve of electrochemical preparation of CDs from CC-T; (c) XRD patterns of CC-T; TEM images of CDs from CC-T: (d) 700 °C, (e) 800 °C, (f) 900 °C, (g) 1000 °C; (h-k) CDs size statistics from CC-T: (h) 700 °C, (i) 800 °C, (j) 900 °C, (k) 1000 °C
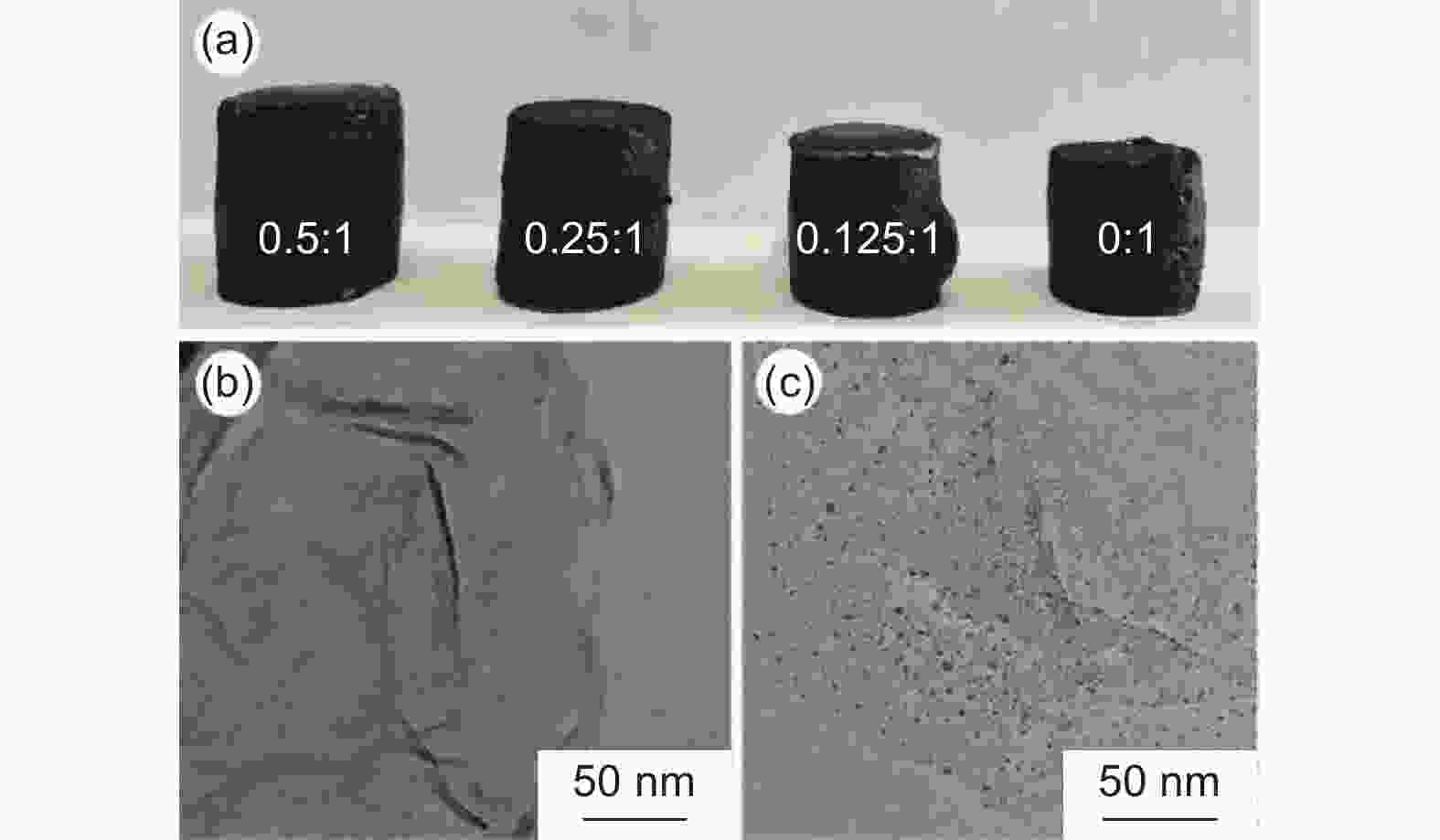
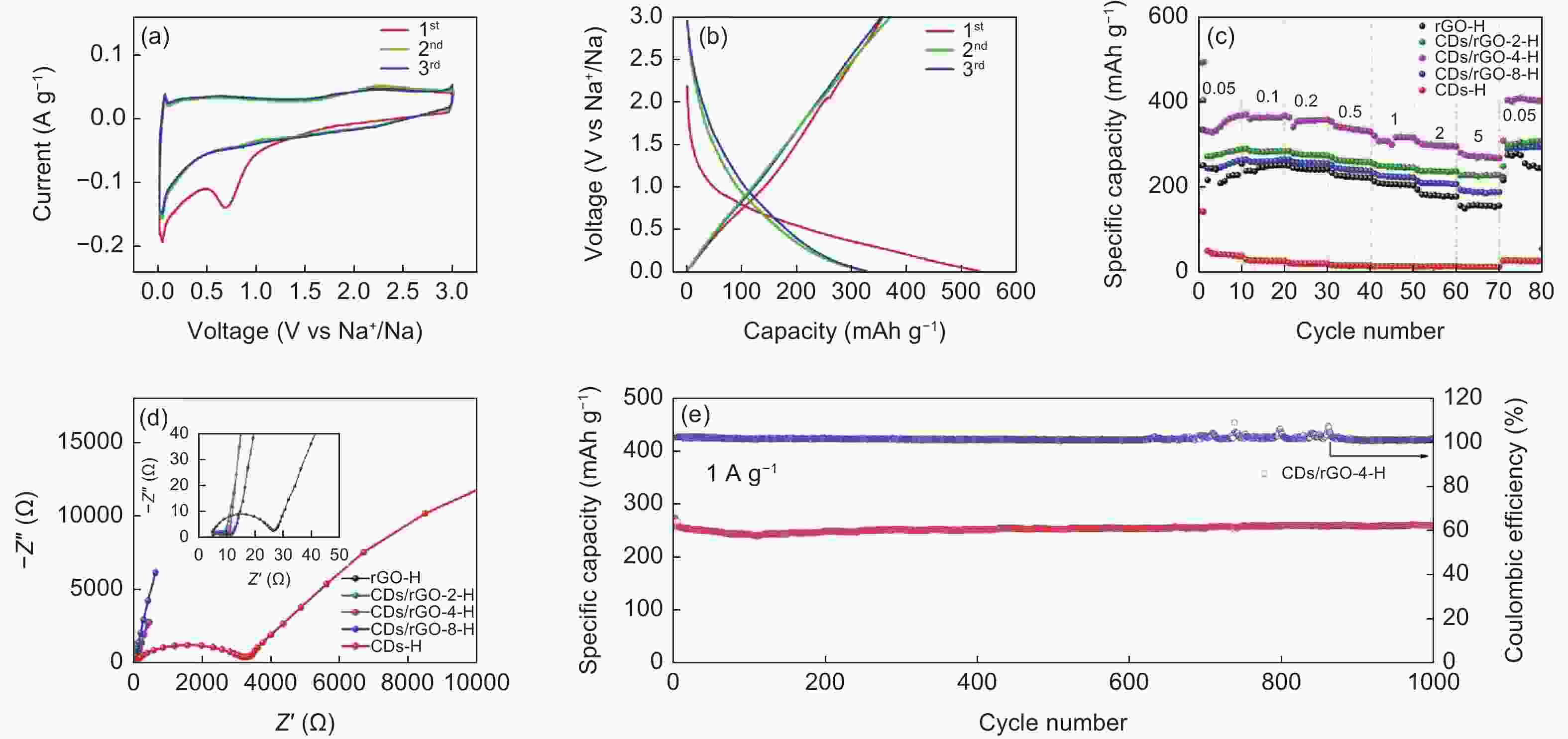
图 7 (a) CDs/rGO-4-H的CV曲线;(b) CDs/rGO-4-H的充放电曲线;(c) 不同CDs与rGO复合比例材料的倍率性能图;(d) 不同CDs与rGO复合比例材料的交流阻抗图;(e) CDs/rGO-4-H在1 A g−1下循环性能图
Figure 7. (a) CV curves of CDs/rGO-4-H; (b) the charge-discharge curves of CDs/rGO-4-H; (c) Ratio performance of materials with different CDs and rGO composite ratio; (d) Nyquist impedance plot of materials with different CDs and rGO composite ratios; (e) Cyclic performance of CDs/rGO-4-H at 1 A g−1
表 1 本研究结果与报道的石墨烯基钠离子电池负极材料性能比较
Table 1. Comparison of the results in this study with reported performance of the graphenebased cathode materials for sodium-ion batteries
Sample Rate capability (mAh g−1)/
current density (A g−1)Cyclic stability (mAh g−1)/
current density (A g−1)Ref. CDs/rGO aerogel 359.0 / 0.1
268.0 / 5263.3 / 1
after 1000 cyclesThis work Expanded graphite 284 / 0.02
91 / 0.2136 / 0.1
after 2000 cycles[31] Graphene oxide nanosheets 220 / 0.03
105 / 5150 / 0.1
after 300 cycles[32] Reduced graphene oxide 509 / 0.1
196 / 5250 / 1
after 1000 cycles[30] Graphene oxide paper 290.5 / 0.05
22.4 / 2210 / 1
after 250 cycles[33] Crumpled graphene paper 183 / 0.1
61 / 8120 / 1
after 500 cycles[34] N-carbon dots pillared
graphene blocks520 / 0.02
118 / 10125 / 10
after 10000 cycles[13] 3D interconnected hollow channel reduced graphene oxide 329.8 / 0.1
163.9 / 2166.8 / 1
after 1000 cycles[35] Holey graphene oxide 365 / 0.1
131 / 10163 / 2
after 3000 cycles[36] Nitrogen-rich graphene hollow microspheres 253.8 / 0.1
66.7 / 2077.8 / 10
after 8000 cycles[37] Nitrogen-doped
graphene foams1057.1 / 0.1
137.7 / 5594 / 0.5
after 500 cycles[38] Graphene nanowires anchored to 3D graphene foam 497 / 0.0375
203 / 7.5291 / 0.375
after 1000 cycles[39] -
[1] Hu C, Li M, Qiu J, et al. Design and fabrication of carbon dots for energy conversion and storage[J]. Chemical Society Reviews,2019,48(8):2315-2337. doi: 10.1039/C8CS00750K [2] GUO Rui-ting, LI lin, JI Xiao-bo, et al. Applications of carbon dots in advanced sodium ion batteries[J]. Chinese Journal of Luminescence,2021,42(08):1182-1195. doi: 10.37188/CJL.20210140 [3] Shi Y, Wei S, Gao Z. Carbon quantum dots and their applications[J]. Chemical Society Reviews,2015,44:362-381. doi: 10.1039/C4CS00269E [4] Yang S, Cao L, Luo P, et al. Carbon dots for optical imaging in vivo[J]. Journal of the American Chemical Society,2009,131(32):11308-11309. doi: 10.1021/ja904843x [5] LV Chun-xiang, LI Li-ping. Progress in research on the preparation of carbon dots and their use in tumor theranostics[J]. New Carbon Materials,2018,33(01):12-18. [6] Ding H, Du F, Liu P, et al. DNA-carbon dots function as fluorescent vehicles for drug delivery[J]. ACS Applied Materials & Interfaces,2015,7(12):6889-6897. [7] Sun C, Zhang Y, Sun K, et al. Combination of carbon dot and polymer dot phosphors for white light-emitting diodes[J]. Nanoscale,2015,7(28):12045-12050. doi: 10.1039/C5NR03014E [8] Zheng J, Liu X, Yang Y, et al. Rapid and green synthesis of fluorescent carbon dots from starch for white light-emitting diodes[J]. New Carbon Materials,2018,33(3):276-288. doi: 10.1016/S1872-5805(18)60339-7 [9] Hu C, Yu C, Li M, et al. Chemically tailoring coal to fluorescent carbon dots with tuned size and their capacity for Cu(II) detection[J]. Small,2015,10(23):4926-4933. [10] Zhuo S, Shao M, Lee S. Upconversion and downconversion fluorescent graphene quantum dots: ultrasonic preparation and photocatalysis[J]. ACS Nano,2012,6(2):1059-1064. doi: 10.1021/nn2040395 [11] Liu H, Liu Z, Zhang J, et al. Boron and nitrogen co-doped carbon dots for boosting electrocatalytic oxygen reduction[J]. New Carbon Materials,2021,36(3):585-593. doi: 10.1016/S1872-5805(21)60043-4 [12] Hou H, Banks C E, Jing M, et al. Sodium-ion batteries: carbon quantum dots and their derivative 3D porous carbon frameworks for sodium-ion batteries with ultralong cycle life[J]. Advanced Materials,2015,27(47):7861-7866. doi: 10.1002/adma.201503816 [13] Liu Z, Zhang L, Sheng L, et al. Edge-nitrogen-rich carbon dots pillared graphene blocks with ultrahigh volumetric/gravimetric capacities and ultralong life for sodium-ion storage[J]. Advanced Energy Materials,2017,8(30):1802042. [14] Hong W, Zhang Y, Yang L, et al. Carbon quantum dot micelles tailored hollow carbon anode for fast potassium and sodium storage[J]. Nano Energy,2019,65:104038-104038. doi: 10.1016/j.nanoen.2019.104038 [15] Li K, Wei L, Yao N, et al. Technical synthesis and biomedical applications of graphene quantum dots[J]. Journal of Materials Chemistry B,2017,5(25):4811-4826. doi: 10.1039/C7TB01073G [16] Zhou J, Booker C, Li R, et al. An electrochemical avenue to blue luminescent nanocrystals from multiwalled carbon nanotubes (MWCNTs)[J]. Journal of the American Chemical Society,2007,129(4):744-745. doi: 10.1021/ja0669070 [17] Li H, He X, Kang Z, et al. Water-soluble fluorescent carbon quantum dots and photocatalyst design[J]. Angewandte Chemie International Edition,2010,49(26):4430-4434. doi: 10.1002/anie.200906154 [18] He M, X Guo, Huang J, et al. Mass production of tunable multicolor graphene quantum dots from an energy resource of coke by a one-step electrochemical exfoliation[J]. Carbon,2018,140:508-520. doi: 10.1016/j.carbon.2018.08.067 [19] NBSC. China Statistical Yearbook-2021[M]. Beijing: China Statistical Publishing House, 2021. [20] Wei Q, Chen Z, Wang X, et al. A two-step method for the preparation of high performance corncob-based activated carbons as supercapacitor electrodes using ammonium chloride as a pore forming additive[J]. New Carbon Materials,2018,33(5):402-408. doi: 10.1016/S1872-5805(18)60348-8 [21] Makishima S, Mizuno M, Sato N, et al. Development of continuous flow type hydrothermal reactor for hemicellulose fraction recovery from corncob[J]. Bioresource Technology,2009,99(11):2842-2848. [22] Sun Y, Zhao Z, Zhao G, et al. High performance carbonized corncob-based 3D solar vapor steam generator enhanced by environmental energy[J]. Carbon,2021,179(11):337-347. [23] Hummers W S, Offeman R E. Preparation of graphitic oxide[J]. Journal of the American Chemical Society,1958,208:1334-1339. [24] Ye R, Xiang C, Lin J, et al. Coal as an abundant source of graphene quantum dots[J]. Nature Communications,2013,4:2943. doi: 10.1038/ncomms3943 [25] Pei S, Wei Q, Huang K, et al. Green synthesis of graphene oxide byseconds timescale water electrolytic oxidation[J]. Nature Communications,2018,9(1):145. doi: 10.1038/s41467-017-02479-z [26] Liu M, Xu Y, Niu F, et al. Carbon quantum dots directly generated from electrochemical oxidation of graphite electrodes in alkaline alcohols and the applications for specific ferric ion detection and cell imaging[J]. Analyst,2016,141:2657-2664. doi: 10.1039/C5AN02231B [27] Yang J X, Wang J, Lim A, et al. One-pot synthesis of fluorescent carbon nanoribbons, nanoparticles, and graphene by the exfoliation of graphite in ionic liquids[J]. ACS Nano,2009,3(8):2367-75. doi: 10.1021/nn900546b [28] Liu F, Jang M, Ha H, et al. Facile synthetic method for pristine graphene quantum dots and graphene oxide quantum dots: origin of blue and green luminescence[J]. Advanced Materials,2013,25(27):3657-3662. doi: 10.1002/adma.201300233 [29] Zhang J, Wang D, Lv W, et al. Achieving superb sodium storage performance on carbon anodes through an ether-derived solid electrolyte interphase[J]. Energy & Environmental Science Ees,2017,10(1):370-376. [30] Zhang S, Lv W, Luo C et al. Commercial carbon molecular sieves as a high performance anode for sodium-ion batteries[J]. Energy Storage Materials,2016,3:18-23. doi: 10.1016/j.ensm.2015.12.004 [31] Wen Y, He K, Zhu Y, et al. Expanded graphite as superior anode for sodium-ion batteries[J]. Nature Communication,2014,5:4033. [32] Luo X, Yang C, Peng Y, et al. Graphene nanosheets, carbon nanotubes, graphite, and activated carbon as anode materials for sodium-ion batteries[J]. Journal of Materials Chemistry A,2015,3:10320-10326. doi: 10.1039/C5TA00727E [33] Mahmood A, Yuan Z, Sui X, et al. Foldable and scrollable graphene paper with tuned interplayer spacing as high areal capacity anodes for sodium-ion batteries[J]. Energy Storage Materials,2021,41:395-403. doi: 10.1016/j.ensm.2021.06.020 [34] Yun Y, Park Y, Chang S, et al. Crumpled graphene paper for high power sodium battery anode[J]. Carbon,2016:658-664. [35] Liang K, Li M, Hao Y, et al. Reduced graphene oxide with 3D interconnected hollow channel architecture as high-performance anode for Li/Na/K-ion batteries[J]. Chemical Engineering Journal,2020,394:124956. doi: 10.1016/j.cej.2020.124956 [36] Zhao J, Zhang Y, Zhang F, et al. Partially reduced holey graphene oxide as high performance anode for sodium-ion batteries[J]. Advanced Energy Materials,2019,9(7):1803215-1803211. doi: 10.1002/aenm.201803215 [37] Yue X, Huang N, Jiang Z, et al. Nitrogen-rich graphene hollow microspheres as anode materials for sodium-ion batteries with super-high cycling and rate performance[J]. Carbon,2018,130:574-583. doi: 10.1016/j.carbon.2018.01.053 [38] Xu J, Wang M, Dai L, et al. High-performance sodium ion batteries based on a 3D anode from nitrogen-doped graphene foams[J]. Advanced Materials,2015,27:2042-2048. doi: 10.1002/adma.201405370 [39] Liu X, Chao D, Su D, et al. Graphene nanowires anchored to 3D graphene foam via self-assembly for high performance Li and Na ion storage[J]. Nano Energy,2017,37:108-117. doi: 10.1016/j.nanoen.2017.04.051 -






 下载:
下载: