Preparation and performance of a graphene-(Ni-NiO)-C hybrid as the anode of a lithium-ion battery
-
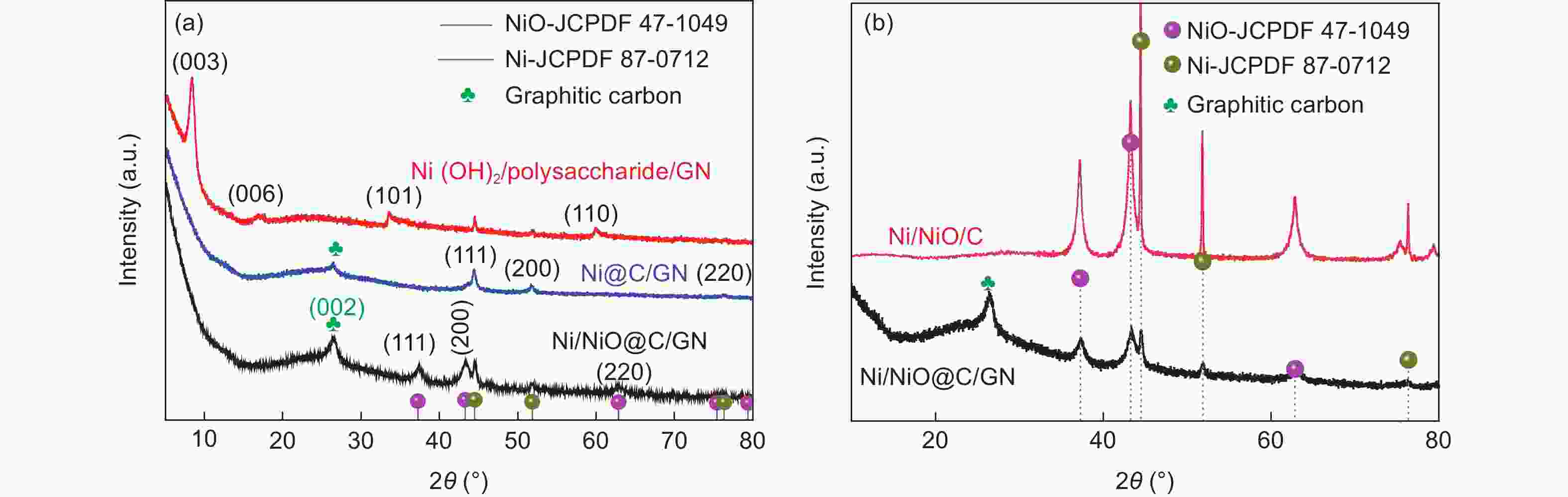
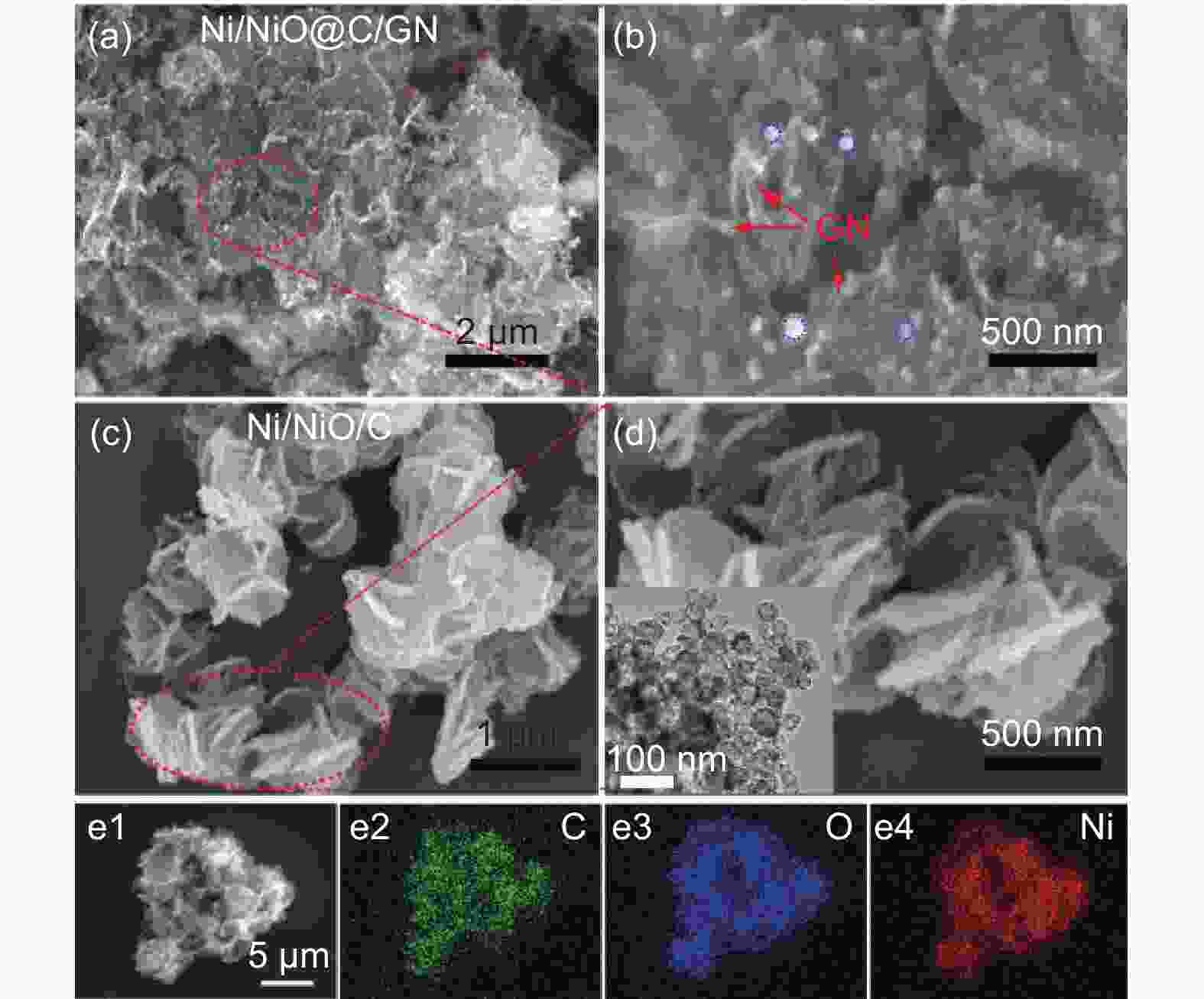
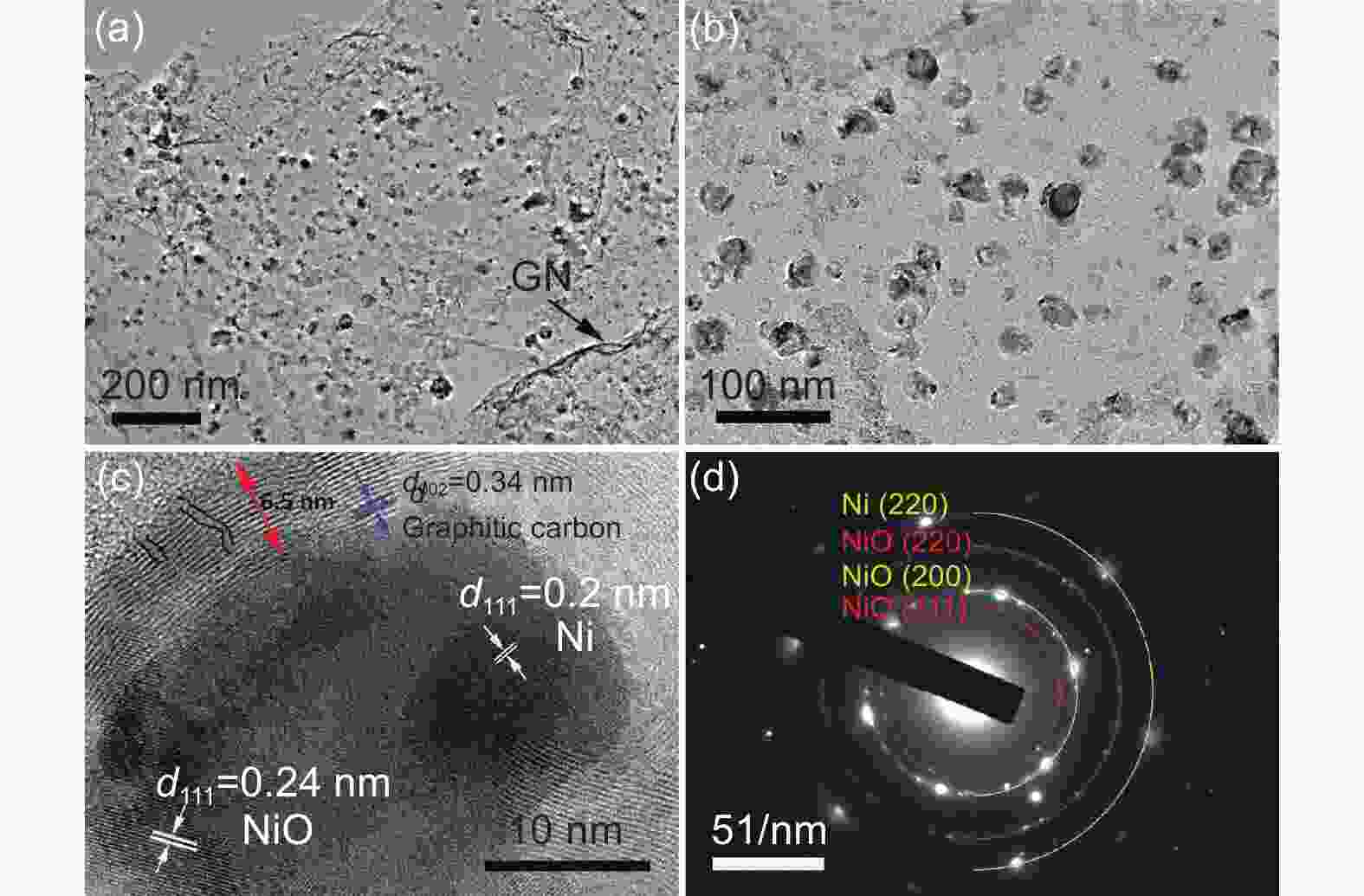
摘要: 将醋酸镍和葡萄糖溶于水中,与氧化石墨烯(GO)水悬浮液均匀混合,在180 °C下水热处理24 h,再在Ar中700 °C下炭化3 h,然后在空气中300 °C下煅烧3 h得到三维Ni/NiO@C/GN。结果表明,水热处理过程中葡萄糖衍生的炭层将Ni(OH)2完全包裹,并在炭化过程中转化为金属Ni,部分金属Ni在空气中煅烧中被氧化为NiO。当作为锂离子电池的负极材料时,其初始容量为711.6 mA h g−1,300次循环后增加到772.1 mA h g−1。作为对比,没有添加GO的材料的初始容量较低,仅为584.7 mA h g−1,300次循环后下降到148.8 mA h g−1。这些结果表明炭层可以抑制Ni/NiO纳米颗粒的团聚,有效缓解锂化过程中的体积膨胀,抑制循环过程中的电极开裂。GO的加入可形成丰富的导电网络,提高导电性。较大的比表面积可增加活性位点,有利于电解液快速浸润电极材料。这些因素显著改善了Ni/NiO@C/GN负极的电化学性能。
-
关键词:
- Ni/NiO@C/GN /
- 锂离子电池 /
- 石墨烯 /
- 电化学性能
Abstract: A graphene-(Ni-NiO)-C hybrid was prepared by dissolving nickel acetate and glucose in water to form a solution that was mixed with a graphene oxide (GO) aqueous suspension, hydrothermally treated at 180 °C for 24 h, carbonized at 700 °C for 3 h in Ar and calcined at 300 °C for 3 h in air. Results indicated that Ni(OH)2 formed during the hydrothermal treatment was converted to metallic Ni during carbonization, which was partly oxidized to NiO during calcination. When used as the anode material of a lithium-ion battery, it had a high initial capacity of 711.6 mA h g−1, which increased to 772.1 mA h g−1 after 300 cycles. For comparison, the sample without added GO had a much lower initial capacity of 584.7 mA h g−1, which decreased to 148.8 mA h g−1 after 300 cycles. Hybridization of the Ni-NiO nanoparticles with carbon inhibited their aggregation. The GO addition led to the formation of a conducting network, which alleviated the large volume expansion during lithiation, prevented the electrode from cracking during cycling and increased the surface area for easy access of the electrolyte. These factors jointly contributed to the obvious improvement in the electrochemical performance of the graphene-(Ni-NiO)-C anode.-
Key words:
- Ni/NiO@C/GN /
- Lithium-ion batteries /
- Graphene /
- Electrochemical performance
-
Figure 7. (a) Rate capability in the current density range from 50 to 2000 mA g−1. (b) Nyquist plots of AC impedance spectra of Ni/NiO@C/GN and Ni/NiO/C, and the corresponding equivalent circuits (inset). (c) Cyclic performance and Coulombic efficiencies of Ni/NiO@C/GN and Ni/NiO/C electrodes at 300 mA g−1
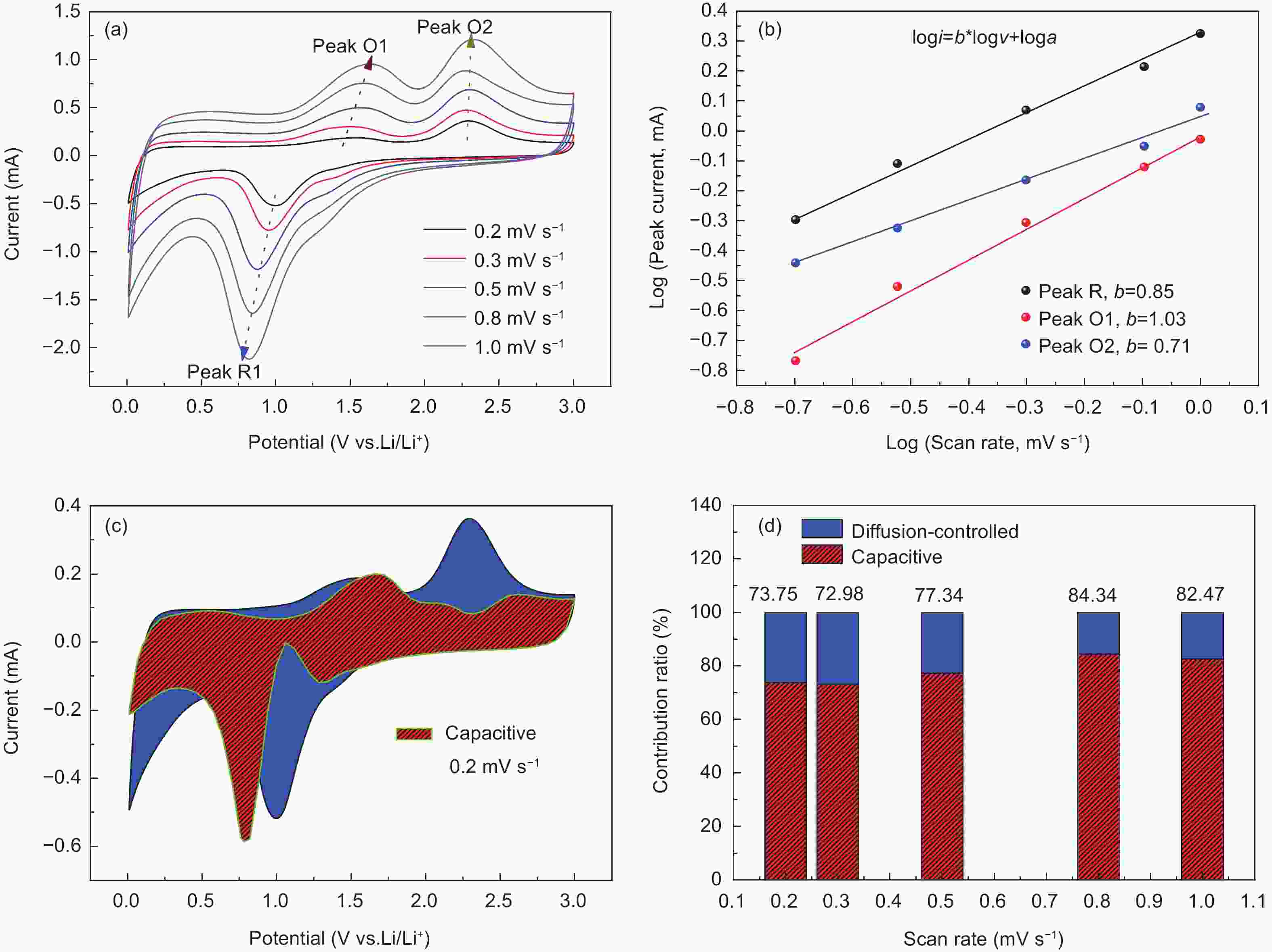
Figure 9. (a) CV profiles of Ni/NiO@C/GN at various scan rates from 0.2 to 1.0 mV s−1. (b) Fitted linear relation of log (i) vs log (v), where slope of slash line is the value b. (c) The separation of capacity contribution at a scan rate of 0.2 mV s−1. (d) The contribution of capacitance and diffusion controlled at different scan rates
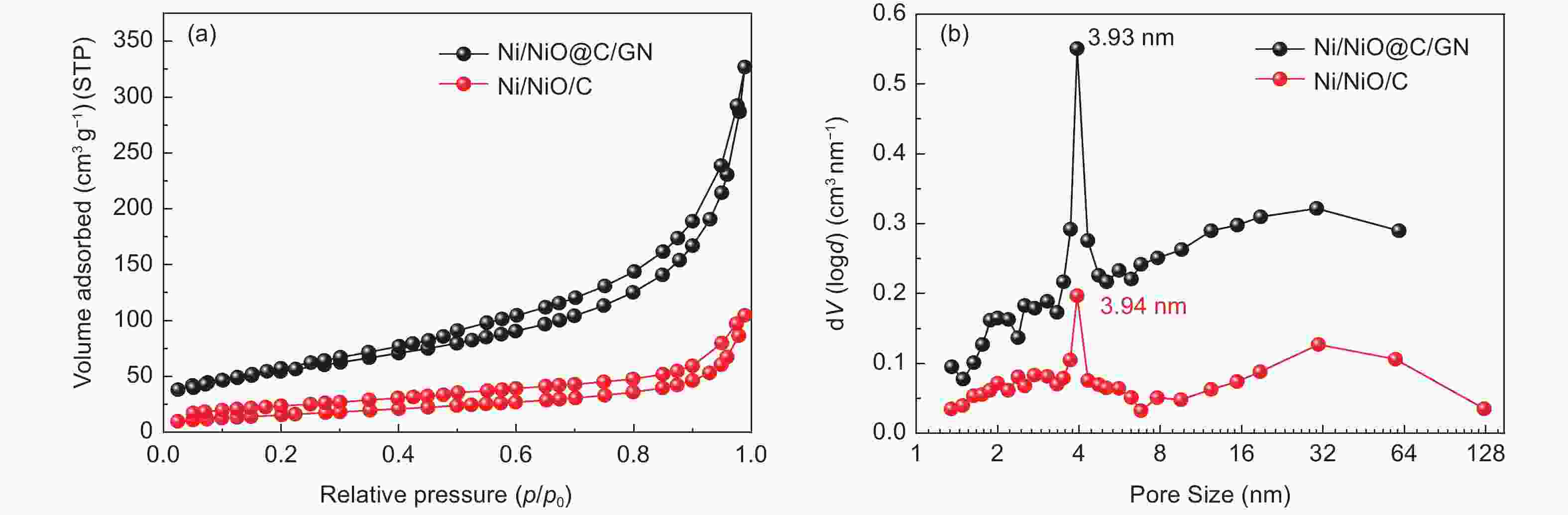
Table 1. Surface area and pore parameters of Ni/NiO@C/GN and Ni/NiO/C
BET (m2 g−1) t-method external
superficial area
(m2 g−1)t-method micropore
superficial area
(m2 g−1)BJH method
desorption pore
diameter (nm)BJH method
adsorption pore
diameter (nm)DFT pore
diameter (nm)Ni/NiO@C/GN 193.9 186.4 7.466 3.934 2.517 2.897 Ni/NiO/C 57.98 57.98 / 3.938 1.937 1.688 Table 2. Fitting results of Ni/NiO@C/GN before and after cycling test
Sample Cycles Rs RSEI Rct Ni/NiO/C 1 7.1 23.78 130 Ni/NiO@C/GN 1 4.31 32 45 Ni/NiO@C/GN 300 4.1 4.8 23 -
[1] Song R, Zhang N, Dong H, et al. Self-standing three-dimensional porous NiO/Ni anode materials for high-areal capacity lithium storage[J]. Materials & Design,2022,215:110448. [2] Pal N, Jo J W, Narsimulu D, et al. Hierarchical multi-metal-doped mesoporous NiO-silica nanoparticles towards a viable platform for Li-ion battery electrode application[J]. Korean Journal of Chemical Engineering,2022,39:1959-1967. doi: 10.1007/s11814-021-1003-1 [3] Peng J, Zhang W, Zheng M, et al. Propelling electrochemical kinetics of transition metal oxide for high-rate lithium-ion battery through in situ deoxidation[J]. Journal of Colloid and Interface Science,2021,587:590-596. doi: 10.1016/j.jcis.2020.11.016 [4] Yang G, Han T, Lu X, et al. "Powder electrodeposition" synthesis of NiO-Ni/CNTs composites with high performances of lithium storage battery[J]. Journal of Alloys and Compounds,2022,898:163005. [5] Ding K, Chen J, Liu Y, et al. Peony-shaped micron-sized NiO particles: their excellent electrochemical performances as anode materials of lithium ion batteries (LIBs)[J]. Journal of Solid State Electrochemistry,2022,26:985-996. [6] Zhu Y, Guo H, Wu Y, et al. Surface-enabled superior lithium storage of high-quality ultrathin NiO nanosheets[J]. Journal of Materials Chemistry A,2014,2:7904-7911. doi: 10.1039/c4ta00257a [7] Jo M S, Ghosh S, Jeong S M, et al. Coral-like yolk-shell-structured nickel oxide/carbon composite microspheres for high-performance li-ion storage anodes[J]. Nano-Micro Letters,2019,11(1):42-59. doi: 10.1007/s40820-019-0274-0 [8] Pan Y, Zeng W, Hu R, et al. Investigation of Cu doped flake-NiO as an anode material for lithium ion batteries[J]. RSC Advances,2019,9(62):35948-35956. doi: 10.1039/C9RA05618A [9] Zou Y, Guo Z, Ye L, et al. Co/La-doped NiO hollow nanocubes wrapped with reduced graphene oxide for lithium storage[J]. ACS Applied Nano Materials,2021,4(3):2910-2920. doi: 10.1021/acsanm.1c00070 [10] Yin X, Zhi C, Sun W, et al. Multilayer NiO@Co3O4@graphene quantum dots hollow spheres for high-performance lithium-ion batteries and supercapacitors[J]. Journal of Materials Chemistry A,2019,7(13):7800-7814. doi: 10.1039/C8TA11982A [11] Archana S, Athika M, Elumalai P. Supercapattery and full-cell lithium-ion battery performances of a Ni(Schiff base)-derived Ni/NiO/nitrogen-doped carbon heterostructure[J]. New Journal of Chemistry,2020,44(29):12452-12464. doi: 10.1039/D0NJ01602K [12] Zhong Y, Wang L, Yu Z, et al. Hierarchical stratiform of a fluorine-doped NiO prism as an enhanced anode for lithium-ion storage[J]. Journal of Physical Chemistry Letters,2021,12(46):11460-11469. doi: 10.1021/acs.jpclett.1c02843 [13] Kawade U V V, Kadam S R R, Kulkarni M V V, et al. Synergic effects of the decoration of nickel oxide nanoparticles on silicon for enhanced electrochemical performance in LIBs[J]. Nanoscale Advances,2020,2(2):823-832. doi: 10.1039/C9NA00727J [14] Duraisamy E, Sujithkrishnan E, Kannadasan K, et al. Facile metal complex-derived Ni/NiO/Carbon composite as anode material for Lithium-ion battery[J]. Journal of Electroanalytical Chemistry,2021,887:115168. [15] Du M, Li Q, Pang H. Oxalate-derived porous prismatic nickel/nickel oxide nanocomposites toward lithium-ion battery[J]. Journal of Colloid and Interface Science,2020,580:614-622. doi: 10.1016/j.jcis.2020.07.009 [16] Ma L, Pei X Y, Mo D C, et al. Facile fabrication of NiO flakes and reduced graphene oxide (NiO/RGO) composite as anode material for lithium-ion batteries[J]. Journal of Materials Science-Materials in Electronics,2019,30(6):5874-5880. doi: 10.1007/s10854-019-00885-1 [17] Ou J, Wu S, Yang L, et al. Facile preparation of NiO@graphene nanocomposite with superior performances as anode for li-ion batteries[J]. Acta Metallurgica Sinica-English Letters,2022,35(2):212-222. doi: 10.1007/s40195-021-01283-5 [18] Yang C C, Zhang D M, Du L, et al. Hollow Ni–NiO nanoparticles embedded in porous carbon nanosheets as a hybrid anode for sodium-ion batteries with an ultra-long cycle life[J]. Journal of Materials Chemistry A,2018,6(26):12663-12671. doi: 10.1039/C8TA03692F [19] Wang Y, Wang Y, Lu L, et al. Hierarchically hollow and porous NiO/NiCo2O4 nanoprisms encapsulated in graphene oxide for lithium storage[J]. Langmuir,2020,36(33):9668-9674. doi: 10.1021/acs.langmuir.0c00801 [20] Fu J, Kang W, Guo X, et al. 3D hierarchically porous NiO/Graphene hybrid paper anode for long -life and high rate cycling flexible Li-ion batteries[J]. Journal of Energy Chemistry,2020,47:172-179. doi: 10.1016/j.jechem.2019.11.022 [21] Zhang X, Huang Q, Zhang M, et al. Pine wood-derived hollow carbon fibers@NiO@rGO hybrids as sustainable anodes for lithium-ion batteries[J]. Journal of Alloys and Compounds,2020,822:153718. doi: 10.1016/j.jallcom.2020.153718 [22] Wang Z, Zhang X, Liu X, et al. Bimodal nanoporous NiO@Ni-Si network prepared by dealloying method for stable Li-ion storage[J]. Journal of Power Sources,2020,449:227550. doi: 10.1016/j.jpowsour.2019.227550 [23] Zhang X, Gao X, Li D, et al. Flower-like NiO/ZnO hybrid coated with N-doped carbon layer derived from metal-organic hybrid frameworks as novel anode material for high performance sodium-ion batteries[J]. Journal of Colloid and Interface Science,2020,563:354-362. doi: 10.1016/j.jcis.2019.12.090 [24] Zhang Z, Mei T, Yang K, et al. Heterojunction-structured MnCO3@NiO composites and their enhanced electrochemical performance[J]. Dalton Transactions,2020,49(41):14483-14489. doi: 10.1039/D0DT02780D [25] Wang X, Liu J, Hu Y, et al. Oxygen vacancy-expedited ion diffusivity in transition-metal oxides for high-performance lithium-ion batteries[J]. Science China-Materials,2022,65(6):1421-1430. doi: 10.1007/s40843-021-1909-5 [26] Ranjbar-Azad M, Behpour M. Facile in situ co-precipitation synthesis of CuO-NiO/rGO nanocomposite for lithium-ion battery anodes[J]. Journal of Materials Science-Materials in Electronics,2021,32(13):18043-18056. doi: 10.1007/s10854-021-06346-y [27] Kim C, Cho H J, Yoon K R, et al. Synergistic interactions of different electroactive components for superior lithium storage performance[J]. ACS Applied Materials & Interfaces,2021,13(1):587-596. [28] Sun P P, Zhang Y H, Pan G X, et al. Application of NiO-modified NiCo2O4 hollow spheres for high performance lithium ion batteries and supercapacitors[J]. Journal of Alloys and Compounds,2020,832:154954. doi: 10.1016/j.jallcom.2020.154954 [29] Wu D, Zhao W, Wu H, et al. Holey graphene confined hollow nickel oxide nanocrystals for lithium ion storage[J]. Scripta Materialia,2020,178:187-192. doi: 10.1016/j.scriptamat.2019.11.015 [30] Dai H Y, Zhang R, Zhong M. Effects of the inherent tubular structure and graphene coating on lithium ion storage performances of electrospun NiO/Co3O4 nanotubes[J]. Journal of Physical Chemistry C,2020,124:143-151. [31] Zhao Y, Dong W, Nong S, et al. Assembling iron oxide nanoparticles into aggregates by Li3PO4: A universal strategy inspired by frogspawn for robust Li-storage[J]. ACS Nano,2022,16:2968-2977. doi: 10.1021/acsnano.1c10235 [32] Guo H, Zhou J, Li Q, et al. Emerging dual-channel transition-metal-oxide quasiaerogels by self-embedded templating[J]. Advanced Functional Materials,2020,30(15):2000024. doi: 10.1002/adfm.202000024 -





 下载:
下载: