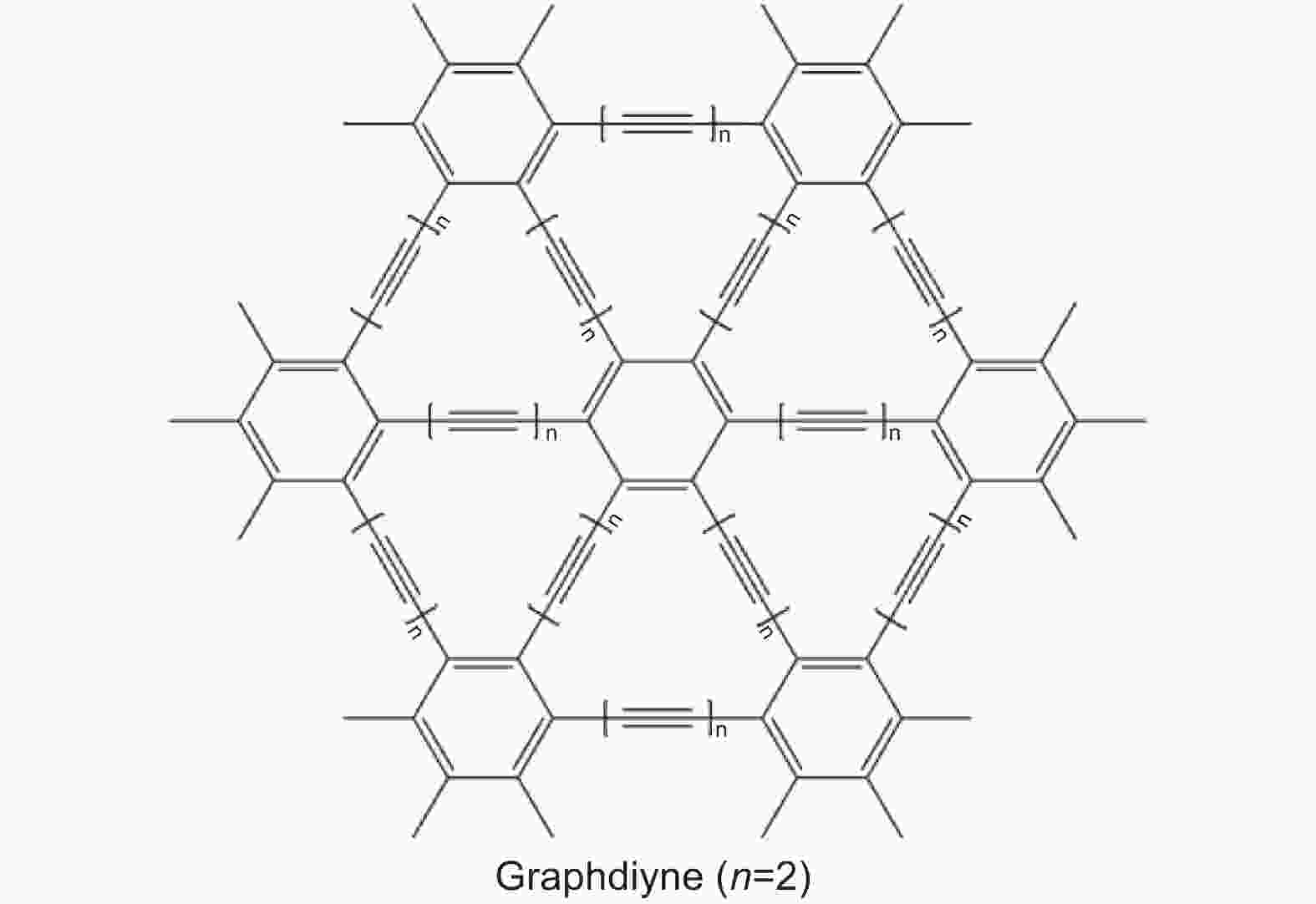
Graphdiyne: Synthesis, modification and application of a two-dimensional carbonaceous material
-
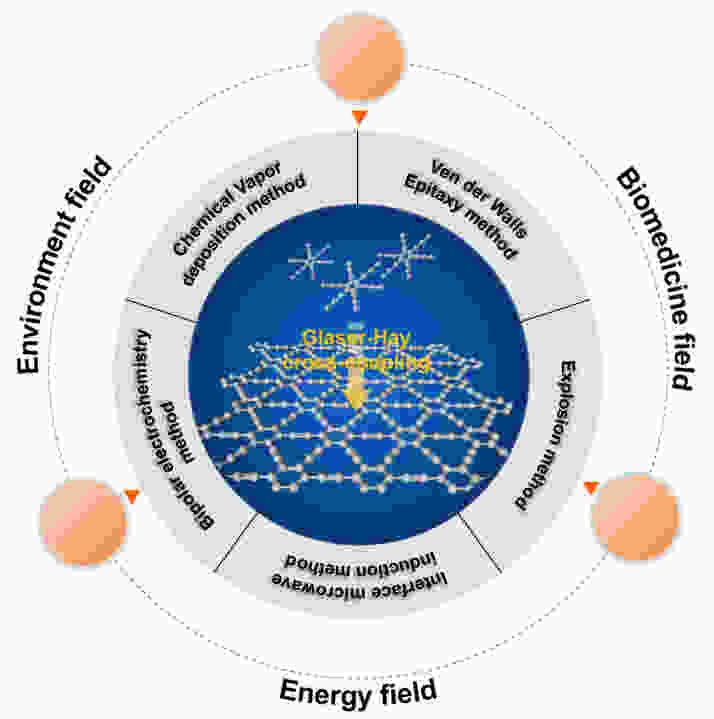
摘要: 石墨炔是一类由sp和sp2杂化碳原子共同组成的新型二维材料。高度共轭及碳环大小可调的分子结构赋予石墨炔特异的物理化学性能,也为其功能化改性及应用提供了便利。近十年来,关于石墨炔的理论及实验研究正在广泛开展,在多个领域取得了一系列重要进展。本文首先对石墨炔性质进行了简要介绍,总结了不同形貌石墨炔的主要合成方法,包括Glaser-Hay交叉偶联、化学气相沉积法、范德华外延生长法、爆炸法、界面限域合成法及双极电化学法等。然后,对金属、非金属原子掺杂、修饰改性及其对石墨炔性能影响的理论计算和实验研究进行了综述;并就石墨炔基材料在环境、能源、生物医学等主要领域的研究进展进行了阐述和总结。最后,探讨了石墨炔发展亟待解决的问题和面临挑战。该综述能够为开展石墨炔相关研究提供有价值的前沿信息和方法参考。Abstract: Graphdiyne is a new kind of two-dimensional carbonaceous material that is composed of sp and sp2 hybridized carbon atoms. The highly conjugated and adjustable carbocyclic molecular structure gives it special physicochemical properties, which also facilitate its functional modification and wide application. In the past ten years, there has been extensive theoretical and experimental research on graphdiyne, and a series of important advances has been made in many fields. The properties of graphdiyne are briefly introduced, and its main synthesis methods with different morphologies are summarized, including Glaser-Hay cross-coupling, chemical vapor deposition, van der Waals epitaxial growth, thermal explosion, interface- confined synthesis and a bipolar electrochemical method. Theoretical calculations and experimental studies on non-metal and metal atom doping and chemical group modification are summarized, and their corresponding effects on the graphdiyne properties are reviewed. Finally, urgent problems and challenges in the development of graphdiyne are discussed. This review provides fundamental information on graphdiyne and guidance for the design of its functionalized forms.
-
Key words:
- Two-dimensional materials /
- Graphdiyne /
- Ion batteries /
- Electrocatalysis /
- Water splitting
-
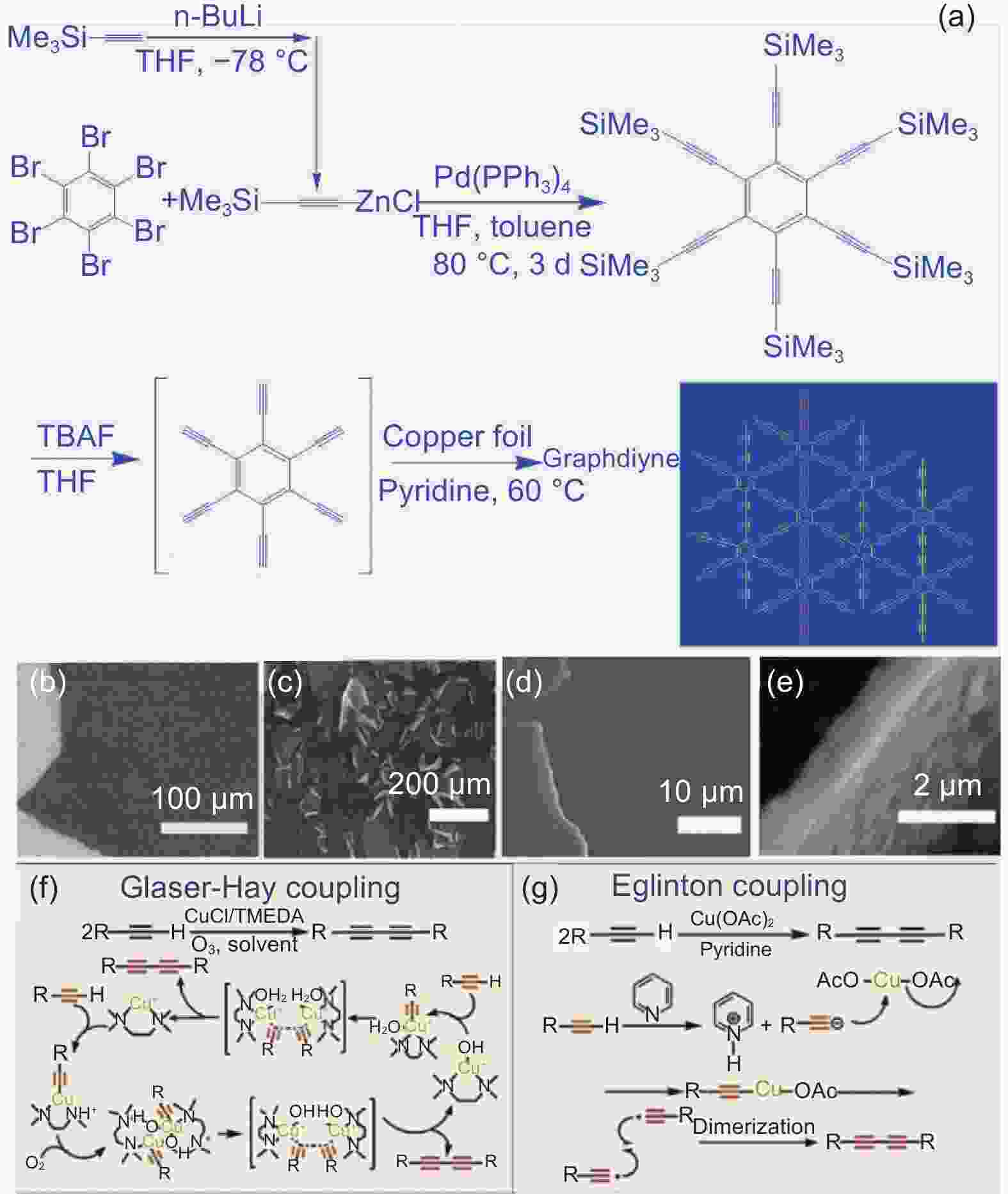
图 2 (a) Glaser-Hay交叉偶联法合成路线[23];(b-e) 石墨炔的SEM图像[23];(f) Glaser-Hay偶联化学反应机理;(g) Eglinton偶联化学反应机理[28]
Figure 2. (a) Synthesis route by Glaser-Hay cross coupling method [23]; (b-e) SEM images of graphdiyne[23]; Proposed mechanism for Glaser-Hay coupling reaction (f) and Eglinton coupling reaction (g)[28]. Reprinted with permission
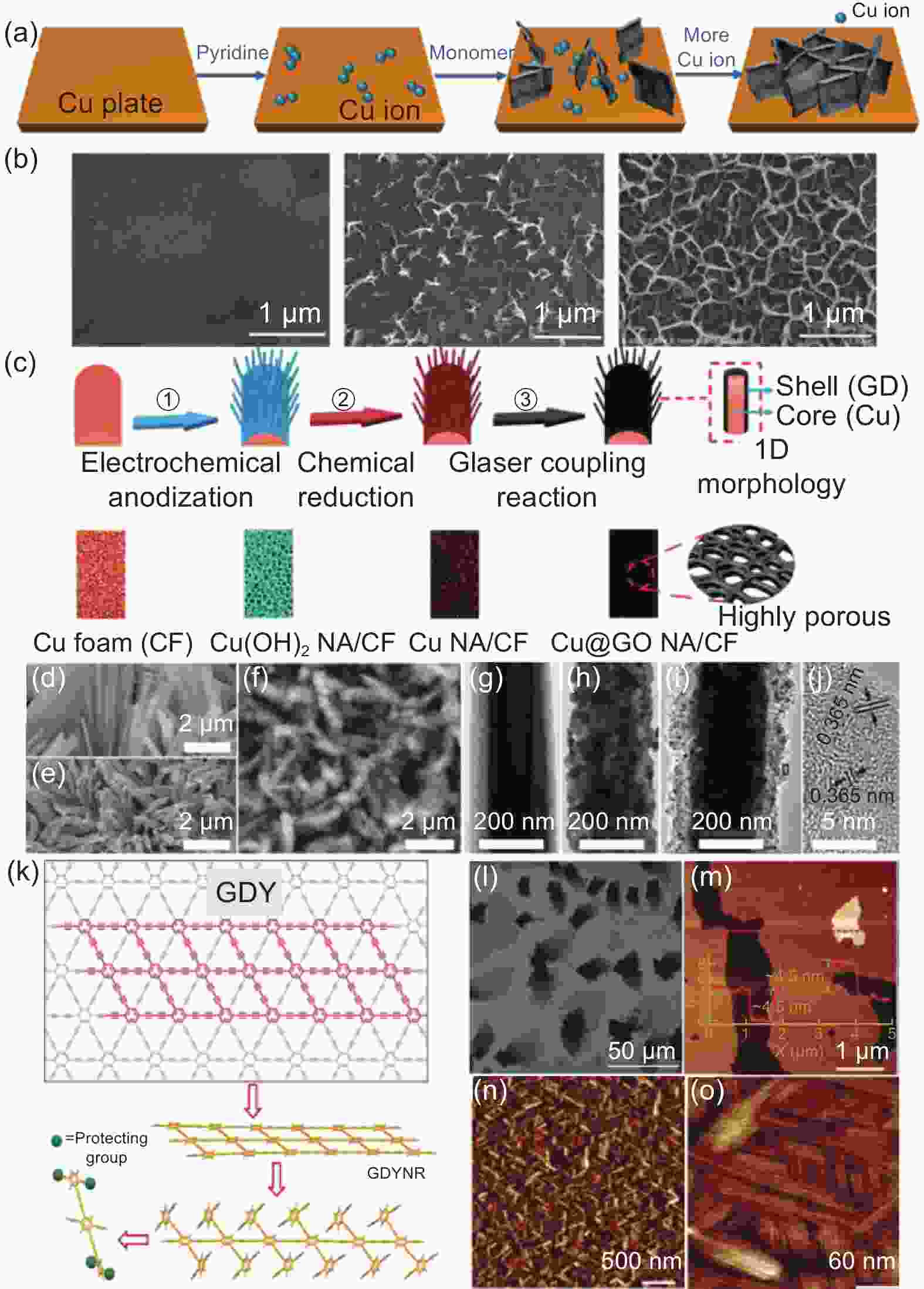
图 3 (a) 石墨炔纳米壁的形成过程;(b)反应前后8、10 h的SEM图[38];(c) Cu@GD NA/CF的制备示意图;(d-f) Cu(OH)2 NA/CF、Cu NA/CF、Cu@GDY NA/CF的SEM图像;(g-i) Cu(OH)2、Cu,、Cu@GDY纳米线的TEM图像;(j) Cu@GDY纳米线的HRTEM图像[39];(k) GDYNR的合成策略;(l) GDYNR的SEM图像;(m-o) GDYNR的AFM图像[43]
Figure 3. (a) Formation process of graphdiyne nanowall; (b) SEM images before and 8 and 10 h after the reaction[38]; (c) Schematic diagram of preparation of Cu@GD NA/CF; (d-f) SEM images of Cu(OH)2 NA/CF, Cu NA/CF and Cu@GD NA/CF; (g-h) TEM images of Cu(OH)2, Cu, and Cu@GD nanowires; (j) HRTEM image of Cu@GD nanowires[39]; (k) Synthesis strategy of GDYNR; (l) SEM image of GDYNR; (m-o) AFM image of GDYNR[43]. Reprinted with permission
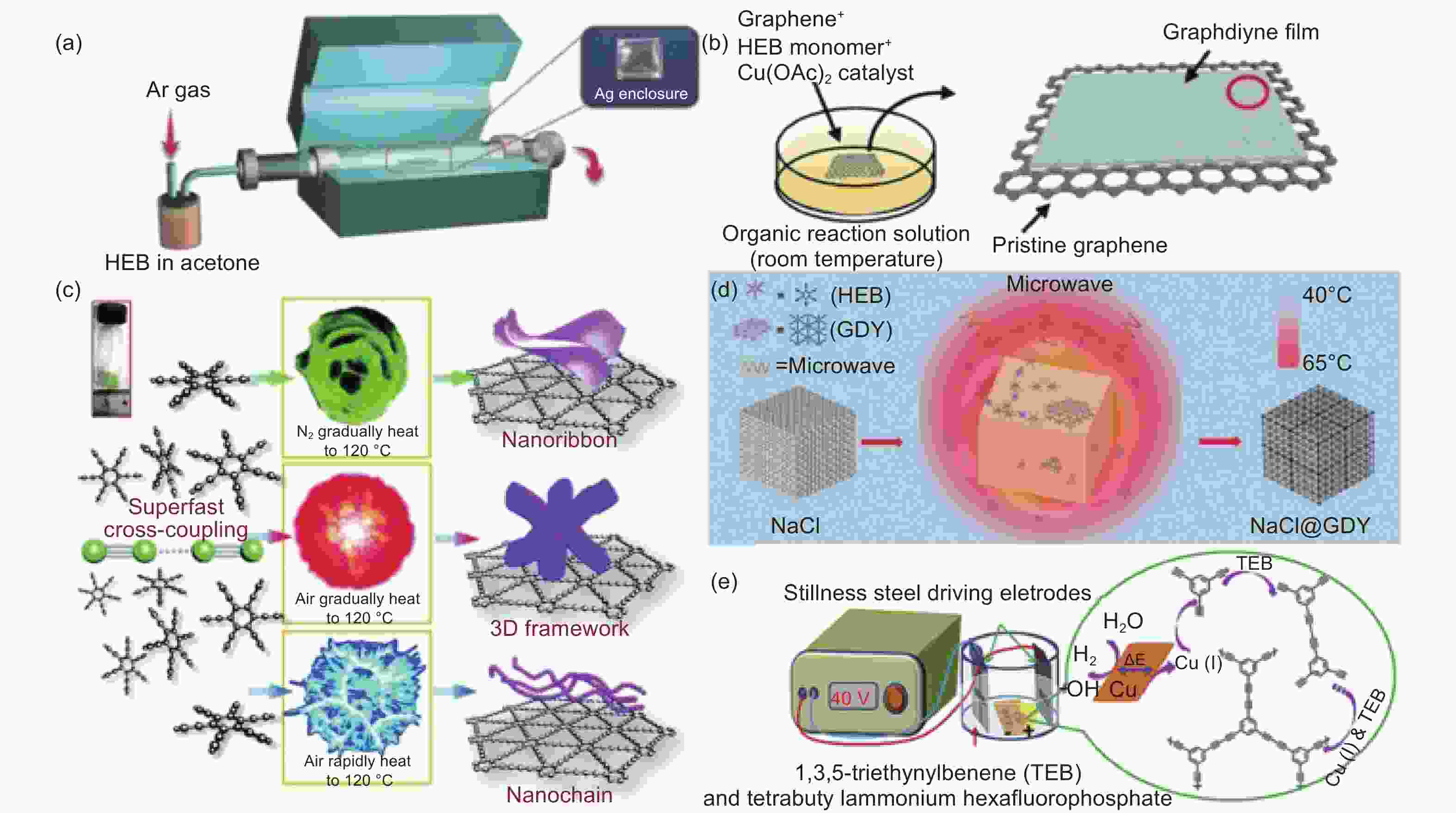
图 4 (a) 化学气相沉积法合成示意图[44];(b) 液相范德华外延法合成示意图[45];(c) 爆炸法合成示意图[46];(d)界面微波诱导法合成示意图[47];(e) 双极电化学法合成示意图[51]
Figure 4. Synthetic schematic diagram of (a) chemical vapor deposition method[44]; (b) liquid phase van der Waals epitaxy method[45]; (c) explosion method[46]; (d) interface microwave induction method[47]; (e) bipolar electrochemical method[51]. Reprinted with permission
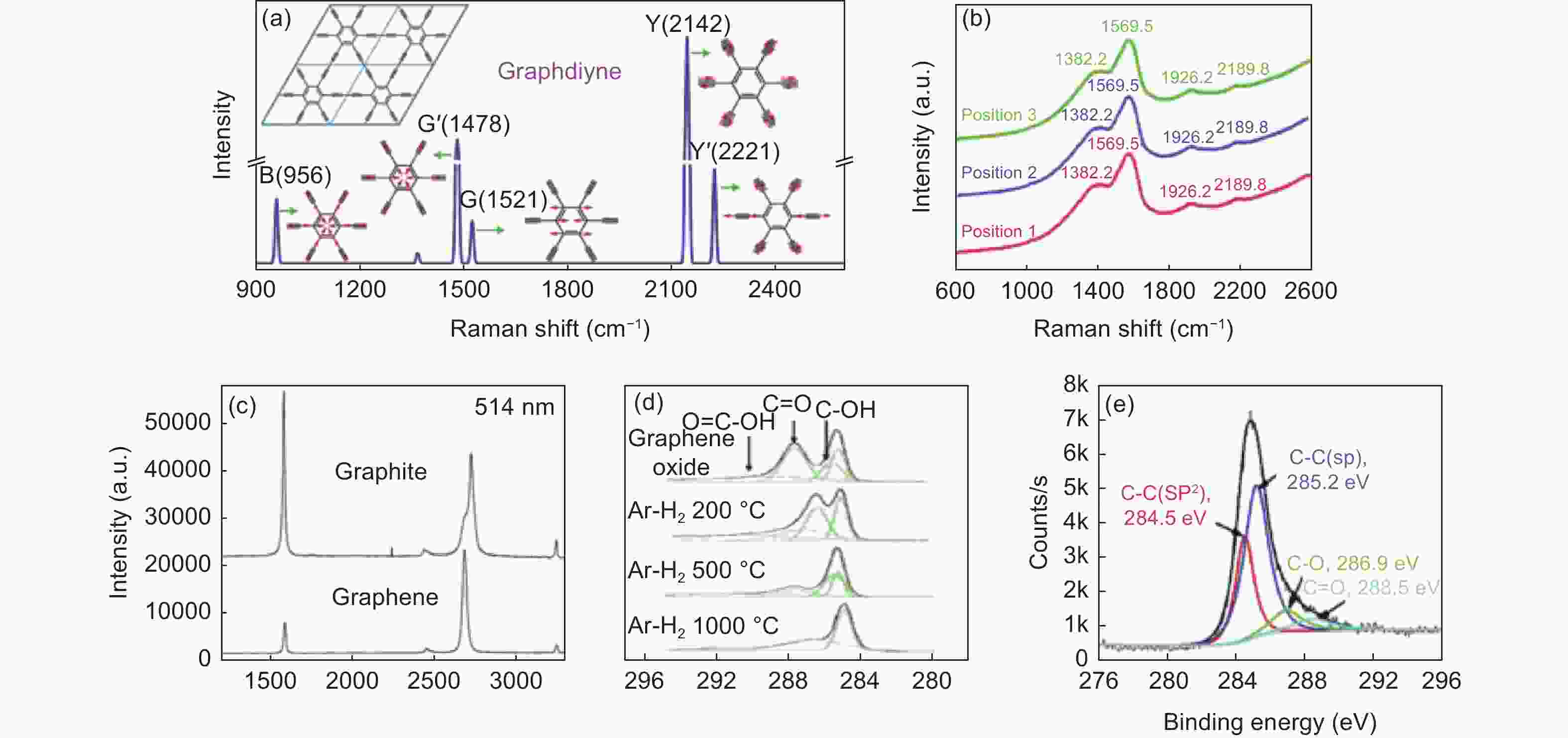
图 5 (a)GDY理论计算[53]和(b)实际样品Raman光谱图[23];(c)石墨和石墨烯的Raman光谱图[55];(d)氧化石墨烯[54]和(e)GDY的XPS谱图[23]
Figure 5. (a) Calculate[53] and (b) experimental Raman spectrum of GDY[23]; (c)Experimental Raman spectra of graphite and graphene[55]; XPS C1s spectra of (d) graphene oxide[54] and (e) graphdiyne[23]. Reprinted with permission
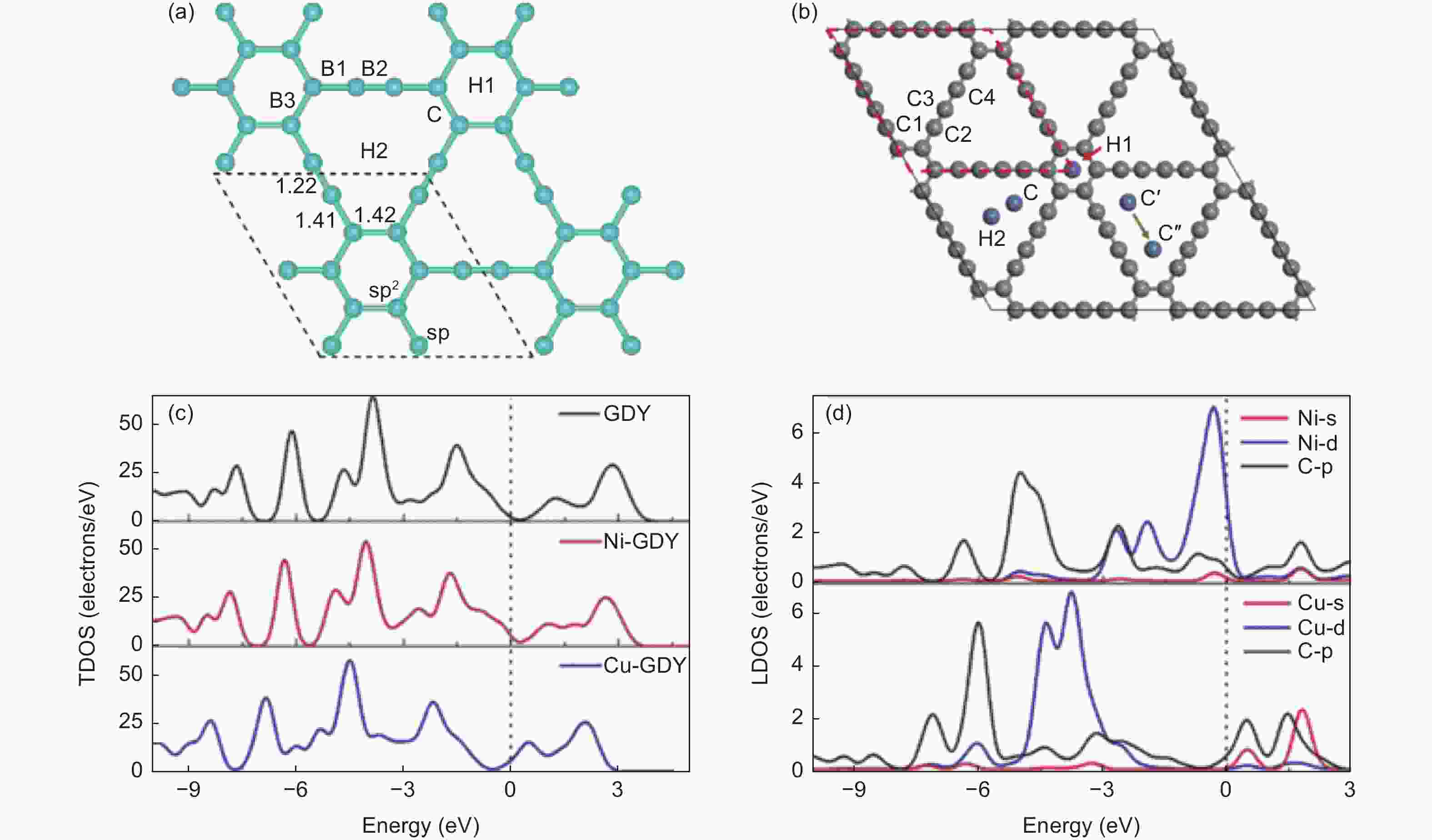
图 7 (a) 单原子贵金属(Au、Pt、Ir、Pd、Rh、Ru)吸附位点[62];(b) Ni、Cu在GDY上可能嵌入的三个位点;(c) 本征GDY和在C位点上嵌有Ni和Cu原子时的TDOS;(d) 掺杂金属原子s和d轨道相邻四个碳原子p轨道的LDOS投影[63]
Figure 7. (a) Adsorption sites of monatomic precious metals (Au, Pt, Ir, Pd, Rh, Ru)[62]; (b) Three possible sites of Ni and Cu in GDY; (c) Intrinsic GDY and TDOS with Ni and Cu atoms embedded at the c site; (d) LDOS projections of the p orbitals of four carbon atoms adjacent to the S and d orbitals of the doped metal atoms[63]. Reprinted with permission
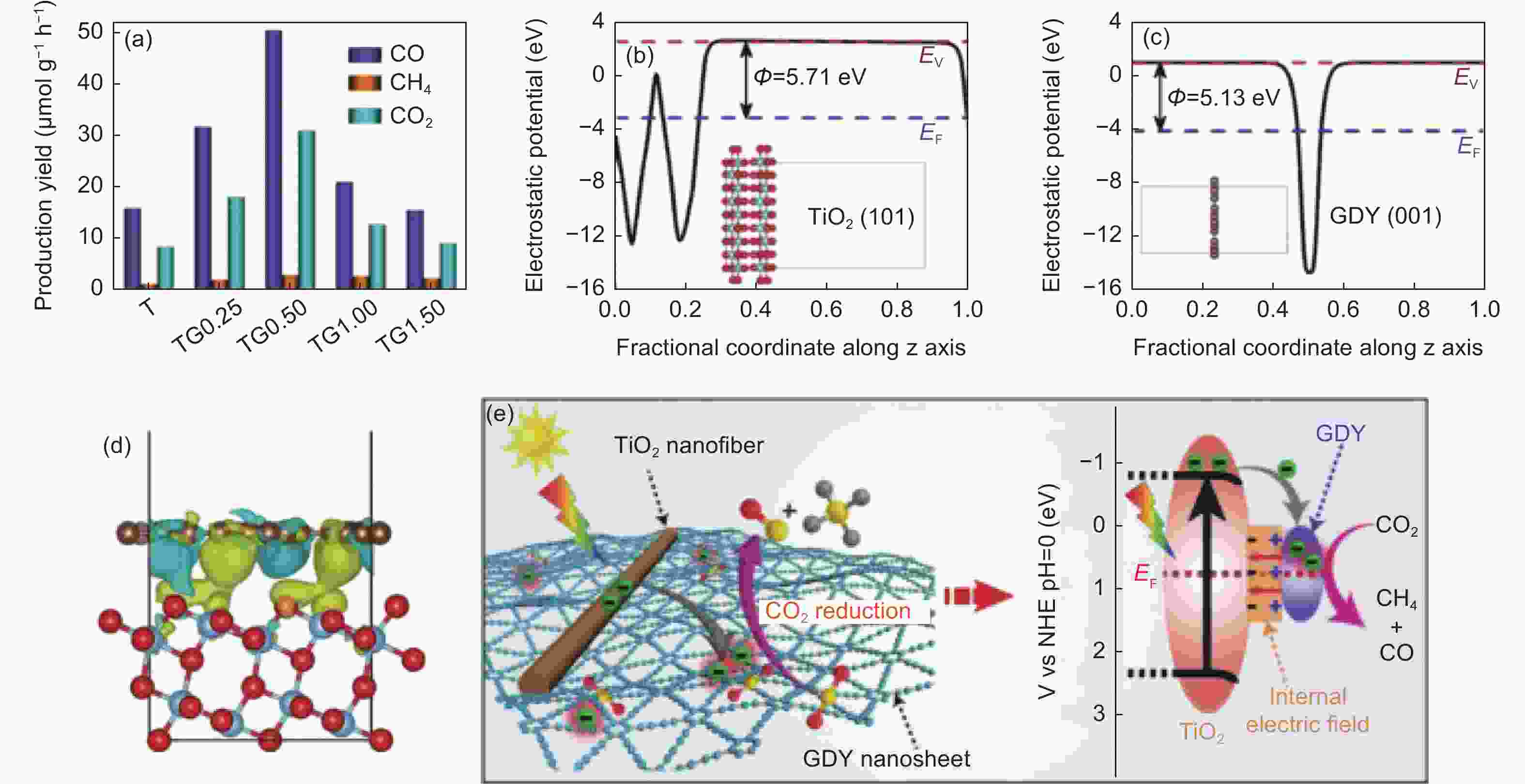
图 8 (a) 不同负载量时TiO2/GDY的CO2还原光催化活性;(b) TiO2的(101)面和(c) GDY的静电势;(d) TiO2/GDY电荷密度差的侧视图;(e) CO2光还原中TiO2/GDY在紫外光照射下内部电场诱导电荷转移和分离示意图[77]
Figure 8. (a) Photocatalytic activity of TiO2/GDY for CO2 reduction with different loading capacity; (b) TiO2(101) surface and (c)GDY electrostatic potential; (d) Side view of TiO2/GDY charge density difference; (e) Schematic illustration of TiO2/GDY heterojunction: internal electric field-induced charge transfer and separation under UV–visible light irradiation for CO2 photoreduction[77]. Reprinted with permission
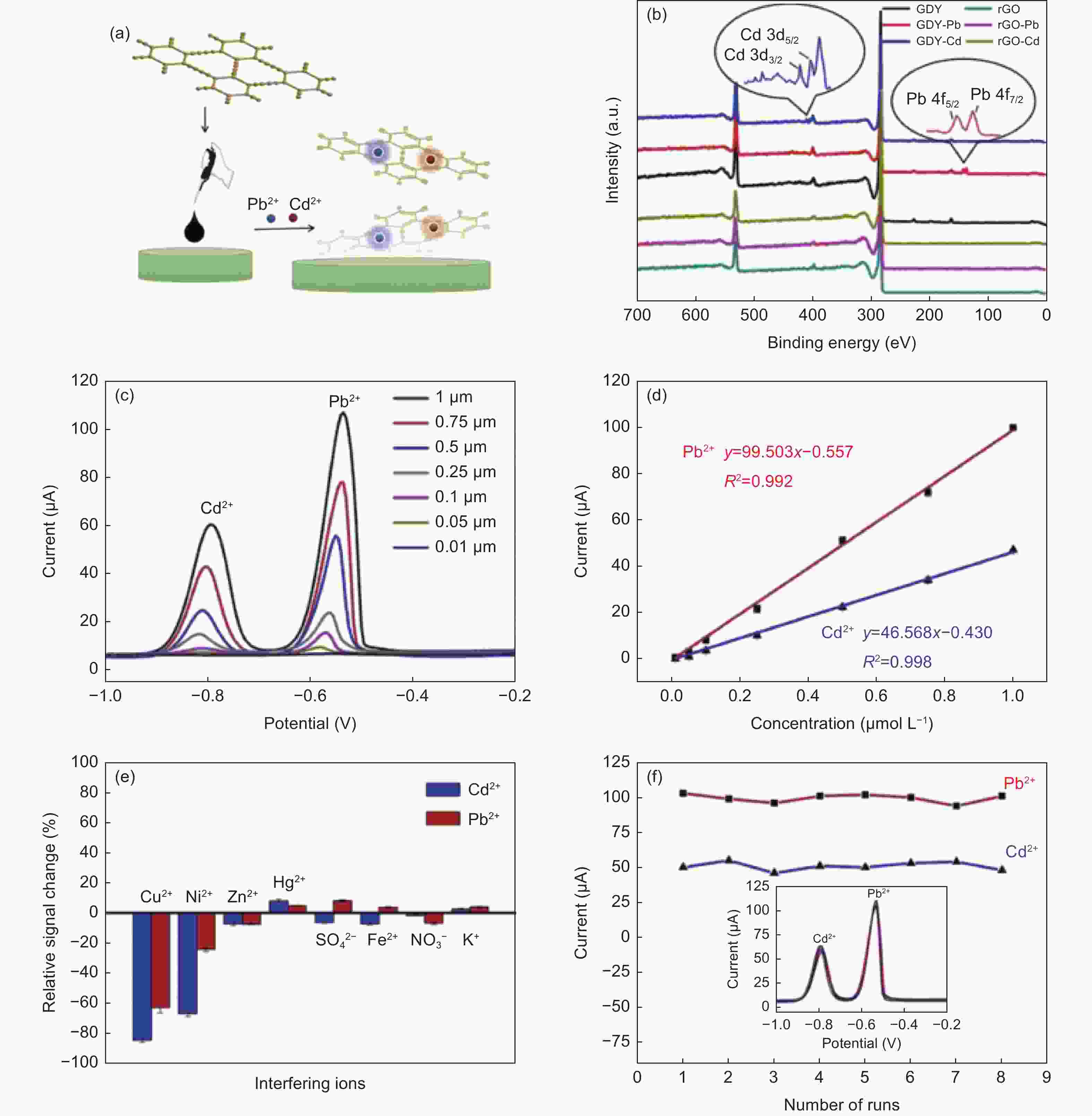
图 9 (a) GDY/GCE实现重金属离子检测的示意图;(b) GDY和rGO吸附Cd2+或Pb2+前后的XPS光谱;(c) Cd2+和Pb2+的电化学响应及(d)相应的线性关系;(e) GDY/GCE在5 μM干扰离子存在下的选择性;(f) GDY/GCE的重现性[85]
Figure 9. (a) Schematic diagram of detection of heavy metal ions by GDY/GCE; (b) XPS spectra before and after adsorption of Cd2+ or Pb2+ by GDY and rGO; (c) Electrochemical responses of Cd2+ and Pb2+ and (d) corresponding calibration curves; (e) Selectivity of GDY/GCE in the presence of 5 μM interfering ions; (f) Reproducibility of GDY/GCE[85]. Reprinted with permission
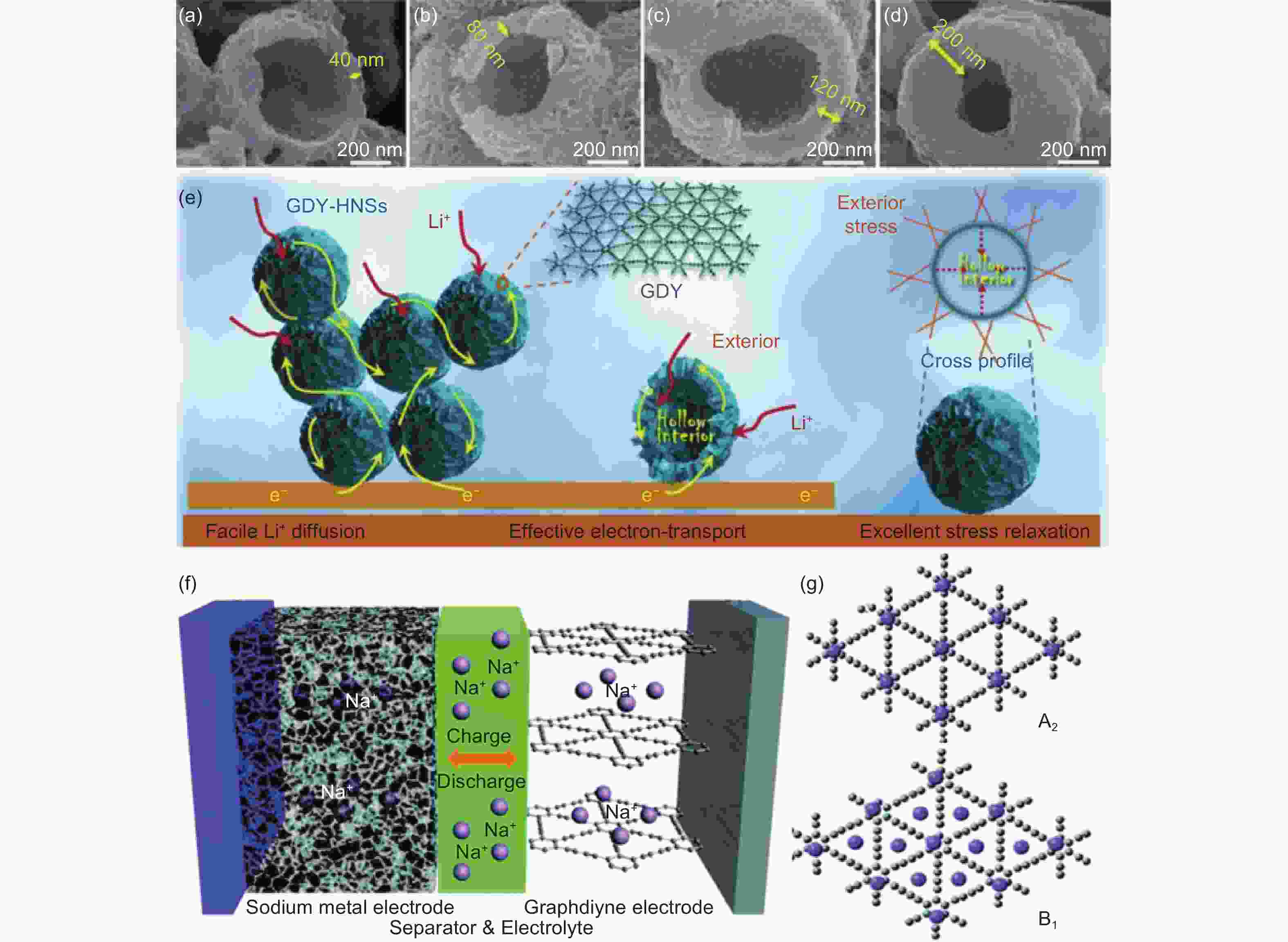
图 10 (a-d) 不同壳层厚度GDY-HNSs的SEM图像;(e) GDY-HNSs阳极的锂离子电池原理图;(f) GDY-HNSs电子传输、锂离子扩散和应力松弛的示意图[101];(g) Na+-GDY的可能构型[104]
Figure 10. (a-d) SEM images of GDY-HNSS with different shell thickness;(e) Schematic diagram of lithium-ion battery with GDY-HNSS anode; (f) Schematic diagram of electron transport, lithium ion diffusion and stress relaxation in GDY-HNSs[101]; (g) Possible configurations of the Na+-GDY[104]. Reprinted with permission
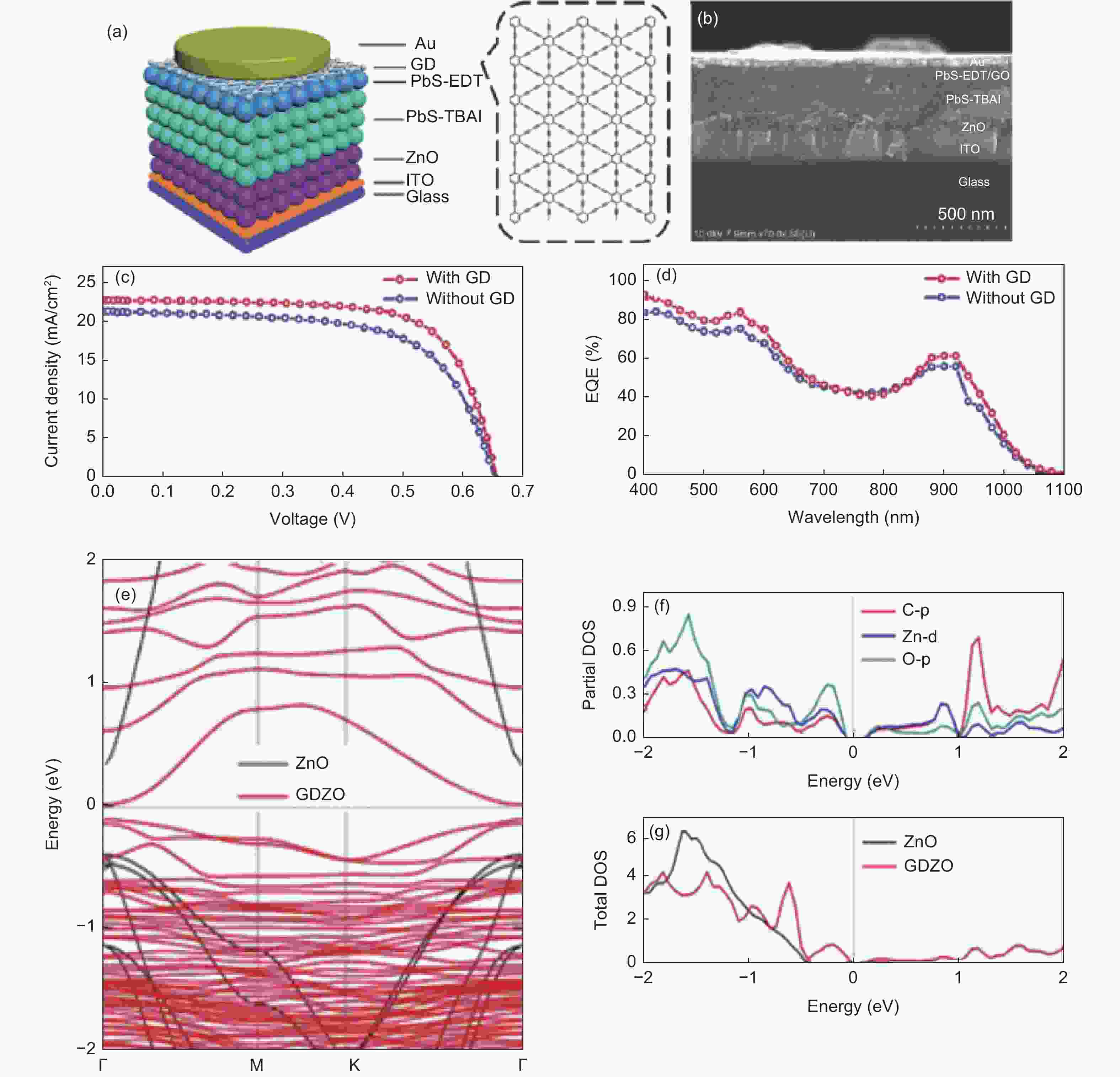
图 11 (a) GD阳极缓冲层的PbS CQD太阳能电池示意图;(b)该电池的SEM截面图;(c) 模拟AM 1.5 G辐照的J-V特性曲线和(d) EQE光谱[111];(e)ZnO和GDZO的能带结构;ZnO和GDZO的(f)总态密度和(g)部分态密度[112]
Figure 11. (a) Schematic diagram of PbS CQD solar cell with GD anode buffer layer; (b) SEM cross-section of the battery;(c) Simulated J-V characteristic curve and (d) EQE spectrum under irradiation[111]; (e) Band structure of ZnO and GDZO ;(f) Total state density and (g)partial state density of ZnO and GDZO[112]. Reprinted with permission
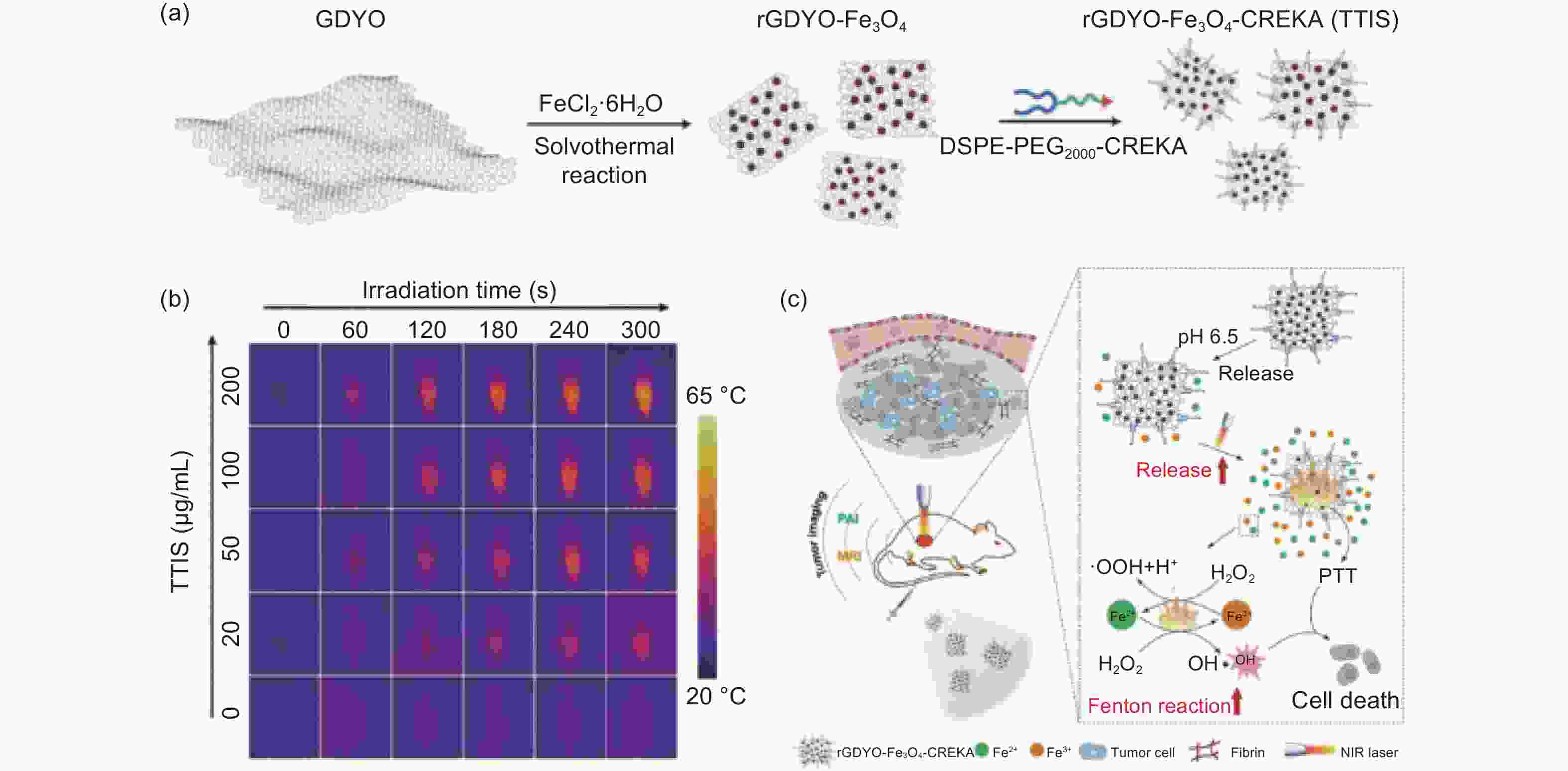
图 12 (a) TTIS制备示意图;(b) TTIS在激光照射下的近红外照片;(c) TTIS介导光热增强芬顿反应的肿瘤治疗示意图[126]
Figure 12. (a) Schematic illustration of the fabrication of TTIS; (b) corresponding near-infrared photographs of TTIS under laser irradiation; (c) Schematic illustration of TTIS mediated tumor therapy via a photothermally enhanced Fenton reaction[126]. Reprinted with permission
-
[1] Shomali Z, Asgari R. Effects of low-dimensional material channels on energy consumption of nano-devices[J]. International Communications in Heat and Mass Transfer,2018,94:77-84. doi: 10.1016/j.icheatmasstransfer.2018.03.014 [2] Chen Z, Molina-Jirón C, Klyatskaya S, et al. 1D and 2D Graphdiynes: Recent Advances on the Synthesis at Interfaces and Potential Nanotechnological Applications[J]. Annalen der Physik,2017,529(11):1700056. doi: 10.1002/andp.201700056 [3] Lv Y, Wu X, Lin H, et al. A novel carbon support: Few-layered graphdiyne-decorated carbon nanotubes capture metal clusters as effective metal-supported catalysts[J]. Small,2021,17(12):e2006442. doi: 10.1002/smll.202006442 [4] Qin X, Liu Y, Chi B, et al. Origins of Dirac cones and parity dependent electronic structures of alpha-graphyne derivatives and silagraphynes[J]. Nanoscale,2016,8(33):15223-15232. doi: 10.1039/C6NR03603A [5] Jia Z, Li Y, Zuo Z, et al. Synthesis and properties of 2D carbon-graphdiyne[J]. Accounts of chemical research,2017,50(10):2470-2478. doi: 10.1021/acs.accounts.7b00205 [6] Yang Y, Yang Y, Xiao Y, et al. Tunable electronic structure of graphdiyne/MoS2 van der Waals heterostructure[J]. Materials Letters,2018,228:289-292. doi: 10.1016/j.matlet.2018.06.038 [7] Liu D, Kim E, Weck P F, et al. Strain-controlled magnetic ordering in 2D carbon metamaterials[J]. Carbon,2020,161:219-223. doi: 10.1016/j.carbon.2020.01.053 [8] Yu X, Jiajun L, Xianglin Y, et al. Preparation of graphdiyne-doped TiO2/SiO2 composite for enhanced photocatalytic activity[J]. Journal of Nanoparticle Research,2020,22(12):365. doi: 10.1007/s11051-020-05097-x [9] Sun C, Liu Y, Wang Z, et al. Self-assembled g-C3N4 nanotubes/graphdiyne composite with enhanced photocatalytic CO2 reduction[J]. Journal of Alloys and Compounds,2021,868:159045. doi: 10.1016/j.jallcom.2021.159045 [10] Zhang J, Feng X. Graphdiyne electrocatalyst[J]. Joule,2018,2(8):1396-1398. doi: 10.1016/j.joule.2018.07.031 [11] Cui M, Hu T, Chen L, et al. Recent progress in graphdiyne for electrocatalytic reactions[J]. ChemElectroChem,2020,7(24):4843-4852. doi: 10.1002/celc.202001313 [12] Zuo Z, Wang D, Zhang J, et al. Synthesis and applications of graphdiyne-based metal-free catalysts[J]. Advanced Materials,2019,31(13):e1803762. doi: 10.1002/adma.201803762 [13] Wang N, He J, Wang K, et al. Graphdiyne-based materials: Preparation and application for electrochemical energy storage[J]. Advanced Materials,2019,31(42):e1803202. doi: 10.1002/adma.201803202 [14] Huang C, Li Y, Wang N, et al. Progress in research into 2D graphdiyne-based materials[J]. Chemical Reviews,2018,118(16):7744-7803. doi: 10.1021/acs.chemrev.8b00288 [15] Matthew J, Allen, Vincent C. Tung, Kaner R B. Honeycomb carbon: A review of graphene[J]. Chemical Reviews,2010,110:132-145. doi: 10.1021/cr900070d [16] Baughman R H, Zakhidov A A, de Heer W A. Carbon nanotubes-the route toward applications[J]. Science,2002,297(5582):787-792. doi: 10.1126/science.1060928 [17] KROTO. H W, ALLAF. A W, BALM S P. C60: Buckminsterfullerene[J]. Chemical Reviews,1991,91:1213-1235. doi: 10.1021/cr00006a005 [18] Bunz U H F, Rubin Y, Tobe Y. Polyethynylated cyclic π-systems: scaffoldings for novel two and three-dimensional carbon networks[J]. Chemical Society Reviews,1999,28(2):107-119. doi: 10.1039/a708900g [19] Li Y, He J, Shen H. Journey from small-molecule diyne structures to 2D graphdiyne: synthetic strategies[J]. Chemistry-A European Journal,2020,26(54):12310-12321. doi: 10.1002/chem.202001898 [20] Chen J, Xi J, Wang D, et al. Carrier mobility in graphyne should be even larger than that in graphene: A theoretical prediction[J]. The Journal of Physical Chemistry Letters,2013,4(9):1443-1448. doi: 10.1021/jz4005587 [21] Torres-Pinto A, Silva C G, Faria J L, et al. Advances on Graphyne-Family Members for Superior Photocatalytic Behavior[J]. Advanced Science,2021,8(10):2003900. doi: 10.1002/advs.202003900 [22] Baughman R H, Eckhardt H, Kertesz M. Structure‐property predictions for new planar forms of carbon: Layered phases containing sp2 and sp atoms[J]. The Journal of Chemical Phyics.,1987,87(11):6687-6699. doi: 10.1063/1.453405 [23] Li G, Li Y, Liu H, et al. Architecture of graphdiyne nanoscale films[J]. Chemical Communications,2010,46(19):3256-3258. doi: 10.1039/b922733d [24] Xie C, Hu X, Guan Z, et al. Tuning the properties of graphdiyne by introducing electron-withdrawing/donating groups[J]. Angewandte Chemie International Edition,2020,59(32):13542-13546. doi: 10.1002/anie.202004454 [25] Huang C, Zhao Y, Li Y. Graphdiyne: The Fundamentals and Application of an Emerging Carbon Material[J]. Advanced Materials,2019,31(42):e1904885. doi: 10.1002/adma.201904885 [26] Sakamoto R, Fukui N, Maeda H, et al. The accelerating world of graphdiynes[J]. Advanced Materials,2019,31(42):e1804211. doi: 10.1002/adma.201804211 [27] Han Y-Y, Lu X-L, Tang S-F, et al. Metal-free 2D/2D heterojunction of graphitic carbon nitride/graphdiyne for improving the hole mobility of graphitic carbon nitride[J]. Advanced Energy Materials,2018,8(16):1703992. [28] KONG Y, LI J, ZENG S, et al. Bridging the gap between reality and ideality of graphdiyne: The advances of synthetic methodology[J]. Chemical Review,2020,6(8):1933-1951. [29] Huang C, Zhang S, Liu H, et al. Graphdiyne for high capacity and long-life lithium storage[J]. Nano Energy,2015,11:481-489. doi: 10.1016/j.nanoen.2014.11.036 [30] He J, Bao K, Cui W, et al. Construction of large-area uniform graphdiyne film for high-performance lithium-ion batteries[J]. Chemistry-A European Journal,2018,24(5):1187-1192. doi: 10.1002/chem.201704581 [31] Kong Y, Li X, Wang L, et al. Rapid synthesis of graphdiyne films on hydrogel at the superspreading interface for antibacteria[J]. ACS Nano,2022,16(7):11338-11345. doi: 10.1021/acsnano.2c04984 [32] Zhou J, Zhang J, Liu Z. Advanced progress in the synthesis of graphdiyne[J]. Acta Physico-Chimica Sinica,2018,34(9):977-991. doi: 10.3866/PKU.WHXB201801243 [33] Zhou J, Li J, Liu Z, et al. Exploring approaches for the synthesis of few-layered graphdiyne[J]. Advanced Materials,2019,31(42):e1803758. doi: 10.1002/adma.201803758 [34] Li G, Li Y, Qian X, et al. Construction of tubular molecule aggregations of graphdiyne for highly efficient field emission[J]. The Journal of Physical Chemistry C,2011,115(6):2611-2615. doi: 10.1021/jp107996f [35] Qian X, Ning Z, Li Y, et al. Construction of graphdiyne nanowires with high-conductivity and mobility[J]. Dalton Transactions,2012,41(3):730-733. doi: 10.1039/C1DT11641J [36] Qian X, Liu H, Huang C, et al. Self-catalyzed growth of large-area nanofilms of two-dimensional carbon[J]. Scientific Reports,2015,5:7756. doi: 10.1038/srep07756 [37] Xue Z, Yang H, Gao J, et al. Controlling the Interface Areas of Organic/Inorganic Semiconductor Heterojunction Nanowires for High-Performance Diodes[J]. ACS Applied Materials & Interfaces,2016,8(33):21563-21569. [38] Zhou J, Gao X, Liu R, et al. Synthesis of graphdiyne nanowalls using acetylenic coupling reaction[J]. Journal of the American Chemical Society,2015,137(24):7596-7599. doi: 10.1021/jacs.5b04057 [39] Xue Y, Guo Y, Yi Y, et al. Self-catalyzed growth of Cu@graphdiyne core–shell nanowires array for high efficient hydrogen evolution cathode[J]. Nano Energy,2016,30:858-866. doi: 10.1016/j.nanoen.2016.09.005 [40] Wang H, Gao Y, Li Q, et al. Electronic structures and charge carrier mobilities of boron-graphdiyne sheet and nanoribbons[J]. Physica E:Low-dimensional Systems and Nanostructures,2020,124:114354. doi: 10.1016/j.physe.2020.114354 [41] Kang J, Wu F, Li J. Modulating the bandgaps of graphdiyne nanoribbons by transverse electric fields[J]. Journal of Physics:Condensed Matter,2012,24(16):165301. doi: 10.1088/0953-8984/24/16/165301 [42] Bai H, Zhu Y, Qiao W, et al. Structures, stabilities and electronic properties of graphdiyne nanoribbons[J]. RSC Advances,2011,1(5):768. doi: 10.1039/c1ra00481f [43] Zhou W, Shen H, Zeng Y, et al. Controllable synthesis of graphdiyne nanoribbons[J]. Angewandte Chemie International Edition,2020,59(12):4908-4913. doi: 10.1002/anie.201916518 [44] Liu R, Gao X, Zhou J, et al. Chemical vapor deposition growth of linked carbon monolayers with acetylenic scaffoldings on silver foil[J]. Advanced Materials,2017,29(18):1604665. doi: 10.1002/adma.201604665 [45] Gao X, Zhu Y, Yi D, et al. Ultrathin graphdiyne film on graphene through solution-phase van der Waals epitaxy[J]. Science Advances,2018,4:eaat6378. doi: 10.1126/sciadv.aat6378 [46] Zuo Z, Shang H, Chen Y, et al. A facile approach for graphdiyne preparation under atmosphere for an advanced battery anode[J]. Chemical Communications,2017,53(57):8074-8077. doi: 10.1039/C7CC03200E [47] Yin C, Li J, Li T, et al. Catalyst‐free synthesis of few‐layer graphdiyne using a microwave‐induced temperature gradient at a solid/liquid interface[J]. Advanced Functional Materials,2020,30(23):1001396. [48] Matsuoka R, Sakamoto R, Hoshiko K, et al. Crystalline graphdiyne nanosheets produced at a gas/liquid or liquid/liquid interface[J]. Journal of The American Chemical Society,2017,139(8):3145-3152. doi: 10.1021/jacs.6b12776 [49] Sopha H, Hromadko L, Motola M, et al. Fabrication of TiO2 nanotubes on Ti spheres using bipolar electrochemistry[J]. Electrochemistry Communications,2020:111. [50] Asoh H, Miura S, Hashimoto H. One-pot synthesis of Pt/alumina composites via AC-bipolar electrochemistry[J]. ACS Applied Nano Materials,2019,2(4):1791-1795. doi: 10.1021/acsanm.9b00268 [51] Navaee A, Salimi A, Sham T-K. Bipolar electrochemistry as a powerful technique for rapid synthesis of ultrathin graphdiyne nanosheets: Improvement of photoelectrocatalytic activity toward both hydrogen and oxygen evolution[J]. International Journal of Hydrogen Energy,2021,46(24):12906-12914. doi: 10.1016/j.ijhydene.2021.01.117 [52] Wang K, Wang N, He J, et al. Preparation of 3D architecture graphdiyne nanosheets for high-performance sodium-ion batteries and capacitors[J]. ACS Applied Materials & Interfaces,2017,9(46):40604-40613. [53] Zhang S, Wang J, Li Z, et al. Raman spectra and corresponding strain effects in graphyne and graphdiyne[J]. The Journal of Physical Chemistry C,2016,120(19):10605-10613. doi: 10.1021/acs.jpcc.5b12388 [54] YANG D, Velamakanni A, Bozoklu G, et al. Chemical analysis of graphene oxide films after heat and chemical treatments by X-ray photoelectron and Micro-Raman spectroscopy[J]. Carbon,2009,47(1):145-152. doi: 10.1016/j.carbon.2008.09.045 [55] Ferrari A C, Meyer J C, Scardaci V, et al. Raman spectrum of graphene and graphene layers[J]. Physical Review Letters,2006,97(18):187401. doi: 10.1103/PhysRevLett.97.187401 [56] Gu J, Magagula S, Zhao J, et al. Boosting ORR/OER Activity of Graphdiyne by Simple Heteroatom Doping[J]. Small Methods,2019,3(9):1800550. doi: 10.1002/smtd.201800550 [57] Kong X, Peng Z, Jiang R, et al. Nanolayered heterostructures of N-doped TiO2 and N-doped carbon for hydrogen evolution[J]. ACS Applied Nano Materials,2020,3(2):1373-1381. doi: 10.1021/acsanm.9b02217 [58] Yang Z, Shen X, Wang N, et al. Graphdiyne containing atomically precise n atoms for efficient anchoring of lithium ion[J]. ACS Applied Materials & Interfaces,2019,11(3):2608-2617. [59] Chen Y, Li J, Liu H. Preparation of Graphdiyne-organic conjugated molecular composite materials for lithium ion batteries[J]. Acta Physico-Chimica Sinica,2018,34(9):1074-1079. doi: 10.3866/PKU.WHXB201801231 [60] Zhao Y, Wan J, Yao H, et al. Few-layer graphdiyne doped with sp-hybridized nitrogen atoms at acetylenic sites for oxygen reduction electrocatalysis[J]. Nature Chemistry,2018,10(9):924-931. doi: 10.1038/s41557-018-0100-1 [61] Zhao Y, Yang N, Yao H, et al. Stereodefined codoping of sp-N and S atoms in few-layer graphdiyne for oxygen evolution reaction[J]. Journal of the American Chemical Society,2019,141(18):7240-7244. doi: 10.1021/jacs.8b13695 [62] Ma D W, Li T, Wang Q, et al. Graphyne as a promising substrate for the noble-metal single-atom catalysts[J]. Carbon,2015,95:756-765. doi: 10.1016/j.carbon.2015.09.008 [63] Liu X, Tang W, Liu S, et al. CO oxidation on Ni and Cu embedded graphdiyne as efficient noble metal-free catalysts: A first-principles density-functional theory investigation[J]. Applied Surface Science,2021,539:148287. doi: 10.1016/j.apsusc.2020.148287 [64] Li X. Design of novel graphdiyne-based materials with large second-order nonlinear optical properties[J]. Journal of Materials Chemistry C,2018,6(28):7576-7583. doi: 10.1039/C8TC02146E [65] Shehzadi K, Ayub K, Mahmood T. Theoretical study on design of novel superalkalis doped graphdiyne: A new donor–acceptor (D-π-A) strategy for enhancing NLO response[J]. Applied Surface Science,2019,492:255-263. doi: 10.1016/j.apsusc.2019.06.221 [66] Yin X P, Wang H J, Tang S F, et al. Engineering the coordination environment of single-atom platinum anchored on graphdiyne for optimizing electrocatalytic hydrogen evolution[J]. Angewandte Chemie International Edition,2018,57(30):9382-9386. doi: 10.1002/anie.201804817 [67] Xue Y, Huang B, Yi Y, et al. Anchoring zero valence single atoms of nickel and iron on graphdiyne for hydrogen evolution[J]. Nature Communications,2018,9(1):1460. doi: 10.1038/s41467-018-03896-4 [68] Wang X, Yang Z, Si W, et al. Cobalt-nitrogen-doped graphdiyne as an efficient bifunctional catalyst for oxygen reduction and hydrogen evolution reactions[J]. Carbon,2019,147:9-18. doi: 10.1016/j.carbon.2019.02.033 [69] Chen Y, Li J, Wang F, et al. Chemical modification: Toward solubility and processability of graphdiyne[J]. Nano Energy,2019,64:103932. doi: 10.1016/j.nanoen.2019.103932 [70] Guo J, Guo M, Wang F, et al. Graphdiyne: structure of fluorescent quantum dots[J]. Angewandte Chemie International Edition,2020,59(38):16712-16716. doi: 10.1002/anie.202006891 [71] Thangavel S, Krishnamoorthy K, Krishnaswamy V, et al. Graphdiyne–ZnO nanohybrids as an advanced photocatalytic material[J]. The Journal of Physical Chemistry C,2015,119(38):22057-22065. doi: 10.1021/acs.jpcc.5b06138 [72] Dong Y, Zhao Y, Chen Y, et al. Graphdiyne-hybridized N-doped TiO2 nanosheets for enhanced visible light photocatalytic activity[J]. Journal of Materials Science,2018,53(12):8921-8932. doi: 10.1007/s10853-018-2210-y [73] Lin Y, Liu H, Yang C, et al. Gama-graphyne as photogenerated electrons transfer layer enhances photocatalytic performance of silver phosphate[J]. Applied Catalysis B:Environmental,2020,264:118479. doi: 10.1016/j.apcatb.2019.118479 [74] Huo B, Meng F, Yang J, et al. High efficiently piezocatalysis degradation of tetracycline by few-layered MoS2/GDY: Mechanism and toxicity evaluation[J]. Chemical Engineering Journal,2022,436:135173. doi: 10.1016/j.cej.2022.135173 [75] Zhang J, Bai Q, Bi X, et al. Piezoelectric enhanced peroxidase-like activity of metal-free sulfur doped graphdiyne nanosheets for efficient water pollutant degradation and bacterial disinfection[J]. Nano Today,2022,43:101429. doi: 10.1016/j.nantod.2022.101429 [76] Li J, Zhong L, Tong L, et al. Atomic Pd on graphdiyne/graphene heterostructure as efficient catalyst for aromatic nitroreduction[J]. Advanced Functional Materials,2019,29(43):1905423. doi: 10.1002/adfm.201905423 [77] Xu F, Meng K, Zhu B, et al. Graphdiyne: A new photocatalytic CO2 reduction cocatalyst[J]. Advanced Functional Materials,2019,29(43):1904256. doi: 10.1002/adfm.201904256 [78] Cao S, Wang Y, Zhu B, et al. Enhanced photochemical CO2 reduction in the gas phase by graphdiyne[J]. Journal of Materials Chemistry A,2020,8(16):7671-7676. doi: 10.1039/D0TA02256J [79] Shi G, Xie Y, Du L, et al. Constructing Cu-C bonds in a graphdiyne-regulated Cu single-atom electrocatalyst for CO2 reduction to CH4[J]. Angewandte Chemie International Edition,2022,61(23):e202203569. [80] Sun M, Huang B. Flexible modulations on selectivity of syngas formation via CO2 reduction on atomic catalysts[J]. Nano Energy,2022,99:107382. doi: 10.1016/j.nanoen.2022.107382 [81] Chang Y-B, Zhang C, Lu X-L, et al. Graphdiyene enables ultrafine Cu nanoparticles to selectively reduce CO2 to C2+ products[J]. Nano Research,2021,15(1):195-201. [82] Khan S, Sajid H, Ayub K, et al. High sensitivity of graphdiyne nanoflake toward detection of phosgene, thiophosgene and phosogenoxime; a first-principles study[J]. Journal of Molecular Graphics & Modelling,2020,100:107658. [83] Yan H, Guo S, Wu F, et al. Carbon atom hybridization matters: ultrafast humidity response of graphdiyne oxides[J]. Angewandte Chemie International Edition,2018,57(15):3922-3926. doi: 10.1002/anie.201709417 [84] Mashhadzadeh A H, Vahedi A M, Ardjmand M, et al. Investigation of heavy metal atoms adsorption onto graphene and graphdiyne surface: A density functional theory study[J]. Superlattices and Microstructures,2016,100:1094-1102. doi: 10.1016/j.spmi.2016.10.079 [85] Li Y, Huang H, Cui R, et al. Electrochemical sensor based on graphdiyne is effectively used to determine Cd2+ and Pb2+ in water[J]. Sensors and Actuators: B. Chemical,2021,332:129519. doi: 10.1016/j.snb.2021.129519 [86] Guo X, Li Y, Huang H, et al. Triazine-graphdiyne with well-defined two kinds of active sites for simultaneous detection of Pb2+ and Cd2+[J]. Journal of Environmental Chemical Engineering,2022,10(2):107159. doi: 10.1016/j.jece.2022.107159 [87] Wu L, Gao J, Lu X, et al. Graphdiyne: A new promising member of 2D all-carbon nanomaterial as robust electrochemical enzyme biosensor platform[J]. Carbon,2020,156:568-575. doi: 10.1016/j.carbon.2019.09.086 [88] Niu K, Gao J, Wu L, et al. Nitrogen-doped graphdiyne as a robust electrochemical biosensing platform for ultrasensitive detection of environmental pollutants[J]. Analytical Chemistry,2021,93(24):8656-8662. doi: 10.1021/acs.analchem.1c01800 [89] Gu Q, Wang Z, Qiao L, et al. Nitrogen-doped graphdiyne quantum dots for electrochemical chloramphenicol quantification in water[J]. ACS Applied Nano Materials,2021,4(11):12755-12765. doi: 10.1021/acsanm.1c03404 [90] Feng X, Zong Z, Elsaidi S K, et al. Kr/Xe Separation over a chabazite zeolite membrane[J]. Journal of the American Chemical Society,2016,138(31):9791-9794. doi: 10.1021/jacs.6b06515 [91] Liu J, Thallapally P K, Strachan D. Metal-organic frameworks for removal of Xe and Kr from nuclear fuel reprocessing plants[J]. Langmuir,2012,28(31):11584-11589. doi: 10.1021/la301870n [92] Zhang P, Song Q, Zhuang J, et al. First-principles study of gas adsorption on γ-graphyne[J]. Chemical Physics Letters,2017,689:185-189. doi: 10.1016/j.cplett.2017.10.026 [93] Fang L, Cao Z. Isoelectronic doping and external electric field regulate the gas-separation performance of graphdiyne[J]. The Journal of Physical Chemistry C,2020,124(4):2712-2720. doi: 10.1021/acs.jpcc.9b11062 [94] Chen X, Gao P, Guo L, et al. High-efficient physical adsorption and detection of formaldehyde using Sc- and Ti-decorated graphdiyne[J]. Physics Letters A,2017,381(9):879-885. doi: 10.1016/j.physleta.2017.01.009 [95] Vazhappilly T, Ghanty T K. The effect of doping on adsorption of Xe and Kr on graphyne and graphdiyne[J]. Materials Today Communications,2020,22:100738. doi: 10.1016/j.mtcomm.2019.100738 [96] Zhou Z, Tan Y, Yang Q, et al. Gas permeation through graphdiyne-based nanoporous membranes[J]. Nature Communications,2022,13(1):4031. doi: 10.1038/s41467-022-31779-2 [97] Gao X, Zhou J, Du R, et al. Robust superhydrophobic foam: A graphdiyne-based hierarchical architecture for oil/water separation[J]. Advanced Materials,2016,28(1):168-173. doi: 10.1002/adma.201504407 [98] Banan Baghbani N, Azamat J, Erfan-Niya H, et al. Molecular insights into water desalination performance of pristine graphdiyne nanosheet membrane[J]. Journal of Molecular Graphics and Modelling,2020,101:107729. doi: 10.1016/j.jmgm.2020.107729 [99] Qiu H, Xue M, Shen C, et al. Graphynes for water desalination and gas separation[J]. Advanced Materials,2019,31(42):e1803772. doi: 10.1002/adma.201803772 [100] Li J, Chen Y, Gao J, et al. Graphdiyne sponge for direct collection of oils from water[J]. ACS Applied Materials & Interfaces,2019,11(3):2591-2598. [101] Zhao F, Li X, He J, et al. Preparation of hierarchical graphdiyne hollow nanospheres as anode for lithium-ion batteries[J]. Chemical Engineering Journal,2021,413:127486. doi: 10.1016/j.cej.2020.127486 [102] Gao L, Ge X, Zuo Z, et al. High quality pyrazinoquinoxaline-based graphdiyne for efficient gradient storage of lithium ions[J]. Nano Letters,2020,20(10):7333-7341. doi: 10.1021/acs.nanolett.0c02728 [103] Farokh Niaei A H, Hussain T, Hankel M, et al. Sodium-intercalated bulk graphdiyne as an anode material for rechargeable batteries[J]. Journal of Power Sources,2017,343:354-363. doi: 10.1016/j.jpowsour.2017.01.027 [104] Zhang S, He J, Zheng J, et al. Porous graphdiyne applied for sodium ion storage[J]. Journal of Materials Chemistry A,2017,5(5):2045-2051. doi: 10.1039/C6TA09822C [105] Yi Y, Li J, Zhao W, et al. Temperature-mediated engineering of graphdiyne framework enabling high-performance potassium storage[J]. Advanced Functional Materials,2020,30(31):2003039. doi: 10.1002/adfm.202003039 [106] Wang F, Xiong Z, Jin W, et al. Graphdiyne oxide for aqueous zinc ion full battery with ultra-long cycling stability[J]. Nano Today,2022,44:101463. doi: 10.1016/j.nantod.2022.101463 [107] Li J, Chen Y, Guo J, et al. Graphdiyne oxide‐based high‐performance rechargeable aqueous Zn–MnO2 battery[J]. Advanced Functional Materials,2020,30(42):2004115. doi: 10.1002/adfm.202004115 [108] Yang Q, Li L, Hussain T, et al. Stabilizing interface ph by N-modified graphdiyne for dendrite-free and high-rate aqueous Zn-ion batteries[J]. Angewandte Chemie International Edition,2022,61(6):e202112304. [109] Wang F, Zuo Z, Shang H, et al. Ultrafastly interweaving graphdiyne nanochain on arbitrary substrates and its performance as a supercapacitor electrode[J]. ACS Applied Materials & Interfaces,2019,11(3):2599-2607. [110] Yue Y, Xu Y, Kong F, et al. Bulk-synthesis and supercapacitive energy storage applications of nanoporous triazine-based graphdiyne[J]. Carbon,2020,167:202-208. doi: 10.1016/j.carbon.2020.06.001 [111] Jin Z, Yuan M, Li H, et al. Graphdiyne: An efficient hole transporter for stable high-performance colloidal quantum dot solar cells[J]. Advanced Functional Materials,2016,26(29):5284-5289. doi: 10.1002/adfm.201601570 [112] Li J, Jian H, Chen Y, et al. Studies of graphdiyne-ZnO nanocomposite material and application in polymer solar cells[J]. Solar RRL,2018,2(11):1800211. doi: 10.1002/solr.201800211 [113] Gao Y, Xue Y, Li Y. Two-dimensional graphdiyne/metal hydroxide heterojunction for high-efficiency oxygen evolution reaction[J]. Scientia Sinica Chimica,2021,52(2):321-329. [114] Yin X P, Luo S W, Tang S F, et al. In situ synthesis of a nickel boron oxide/graphdiyne hybrid for enhanced photo/electrocatalytic H2 evolution[J]. Chinese Journal of Catalysis,2021,42(8):1379-1386. doi: 10.1016/S1872-2067(20)63601-4 [115] Yao Y, Zhu Y, Pan C, et al. Interfacial sp C-O-Mo hybridization originated high-current density hydrogen evolution[J]. Journal of the American Chemical Society,2021,143(23):8720-8730. doi: 10.1021/jacs.1c02831 [116] Xing C, Xue Y, Huang B, et al. Fluorographdiyne: A metal-free catalyst for applications in water reduction and oxidation[J]. Angewandte Chemie International Edition,2019,58(39):13897-13903. doi: 10.1002/anie.201905729 [117] Gao Y, Cai Z, Wu X, et al. Graphdiyne-supported single-atom-sized Fe catalysts for the oxygen reduction reaction: DFT predictions and experimental validations[J]. ACS Catalysis,2018,8(11):10364-10374. doi: 10.1021/acscatal.8b02360 [118] Liu J, Shen X, Baimanov D, et al. Immobilized ferrous ion and glucose oxidase on graphdiyne and its application on one-step glucose detection[J]. ACS Applied Materials & Interfaces,2019,11(3):2647-2654. [119] Liu J, Chen C, Zhao Y. Progress and prospects of graphdiyne-based materials in biomedical applications[J]. Advanced Materials,2019,31(42):e1804386. doi: 10.1002/adma.201804386 [120] Zhang Y, Liu W, Li Y, et al. 2D graphdiyne oxide serves as a superior new generation of Antibacterial Agents[J]. iScience,2019,19:662-675. doi: 10.1016/j.isci.2019.08.019 [121] Qin S, Xie M, Cao S, et al. Insight into the antibacterial resistance of graphdiyne functionalized by silver nanoparticles[J]. Cell Proliferation,2022,55(5):e13236. [122] Bai Q, Liang M, Wu W, et al. Plasmonic nanozyme of graphdiyne nanowalls wrapped hollow copper sulfide nanocubes for rapid bacteria‐killing[J]. Advanced Functional Materials,2022,32(20):2112683. doi: 10.1002/adfm.202112683 [123] Bai Q, Luo H, Shi S, et al. AuAg nanocages/graphdiyne for rapid elimination and detection of trace pathogenic bacteria[J]. Journal of Colloid and Interface Science,2022,613:376-383. doi: 10.1016/j.jcis.2022.01.046 [124] Li S, Chen Y, Liu H, et al. Graphdiyne materials as nanotransducer for in vivo photoacoustic imaging and photothermal therapy of tumor[J]. Chemistry of Materials,2017,29(14):6087-6094. doi: 10.1021/acs.chemmater.7b01965 [125] Jin J, Guo M, Liu J, et al. Graphdiyne nanosheet-based drug delivery platform for photothermal/chemotherapy combination treatment of cancer[J]. ACS Applied Materials & Interfaces,2018,10(10):8436-8442. [126] Min H, Qi Y, Zhang Y, et al. A graphdiyne oxide-based iron sponge with photothermally enhanced tumor-specific fenton chemistry[J]. Advanced Materials,2020,32(31):e2000038. doi: 10.1002/adma.202000038 [127] Guo M, Liu J, Chen X, et al. Graphdiyne oxide nanosheets reprogram immunosuppressive macrophage for cancer immunotherapy[J]. Nano Today,2022,45:101543. doi: 10.1016/j.nantod.2022.101543 [128] Xie J, Wang N, Dong X, et al. Graphdiyne nanoparticles with high free radical scavenging activity for radiation protection[J]. ACS Applied Materials & Interfaces,2019,11(3):2579-2590. -





 下载:
下载: