Graphene with the KI-modified pore structure and its electrochemical capacitor application
-
摘要: 较低的体积能量密度限制了当前电化学电容器的应用,而提高体积能量密度的关键在于发展具有致密化储能特性的多孔炭材料。目前,毛细致密化已成为平衡多孔炭密度和孔隙率从而提高材料体积比电容的主要方法,但仍在孔结构的精细调控方面存在不足,制约了毛细致密化多孔炭与高电压离子液体的兼容性。本文提出了碘化钾(KI)辅助的毛细致密化策略,通过在石墨烯网络中预载KI来控制毛细致密化过程,实现了对孔结构的有效调控。同时电化学性能表征结果表明KI具有增加离子到达表面积和提供赝电容的作用。基于此,所制碘化钾/石墨烯材料的密度达到0.96 g cm−3,在离子液体中的体积比电容为115 F cm−3。由该材料所组装的电化学电容器可以提供19.6 Wh L−1的体积能量密度。Abstract: The use of electrochemical capacitors is greatly limited by their poor volumetric energy density, and the key for improving it is to develop porous yet compact carbon materials. In recent years, the densification of porous carbons by capillary forces during drying has been used as a main method to balance the density and porosity of porous carbons for high volumetric performance. But there are still deficiencies in fine tuning the pore structure, which limits the compatibility of porous carbons with high-voltage ionic liquids. We report a KI-assisted method to prepare graphene-based porous carbons with high density and high capacitance. During the synthesis of the carbons, graphene oxide was dispersed in KI solutions with concentrations of 12.5-37.5 mmol/mL followed by hydrothermal treatment at 180 °C for 6 h. The obtained hydrogels were dried in 0.01 MPa of air at 60 °C for 72 h. By this procedure, KI was loaded into a matrix of compact graphene and formed crystals to suppress over shrinkage of pores during drying to produce densification and pseudo-capacitance. Electrochemical characterization of the resulting materials indicated that the ion-accessible surface area and pseudo-capacitance of the porous carbons were increased. The KI/graphene composite with an optimum concentration of KI of 25 mmol/mL achieved both a high electrode density of 0.96 g cm−3 and a high volumetric capacitance of 115 F cm−3 (at 1 A g−1) in an ionic liquid electrolyte (1-alkyl-3-methylimidazolium tetrafluoroborate). A symmetric cell assembled using this material had a high volumetric energy density of 19.6 Wh L−1.
-
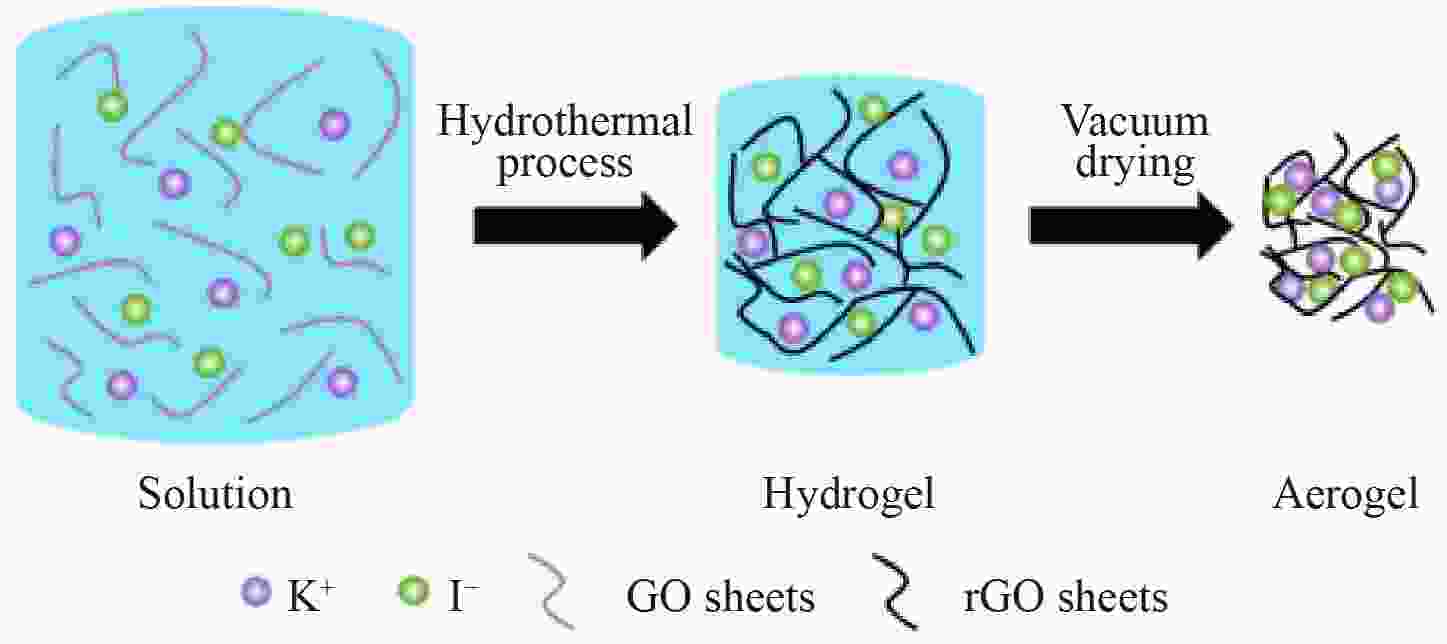
图 1 (a)KI与石墨烯(KIG)材料的制备路线示意图,(b)G、KIG-12.5、KIG-25和KIG-37.5的X射线光电子I 3d精细谱和(c)X射线衍射谱,(d)振实密度和KI含量关系图
Figure 1. (a) Schematic of the synthesis of KIG hybrids. (b) I 3d XPS fine spectra and (c) X-ray diffraction patterns of G, KIG-12.5, KIG-25 and KIG-37.5. (d) The relationship between tap density and the loading amount of KI
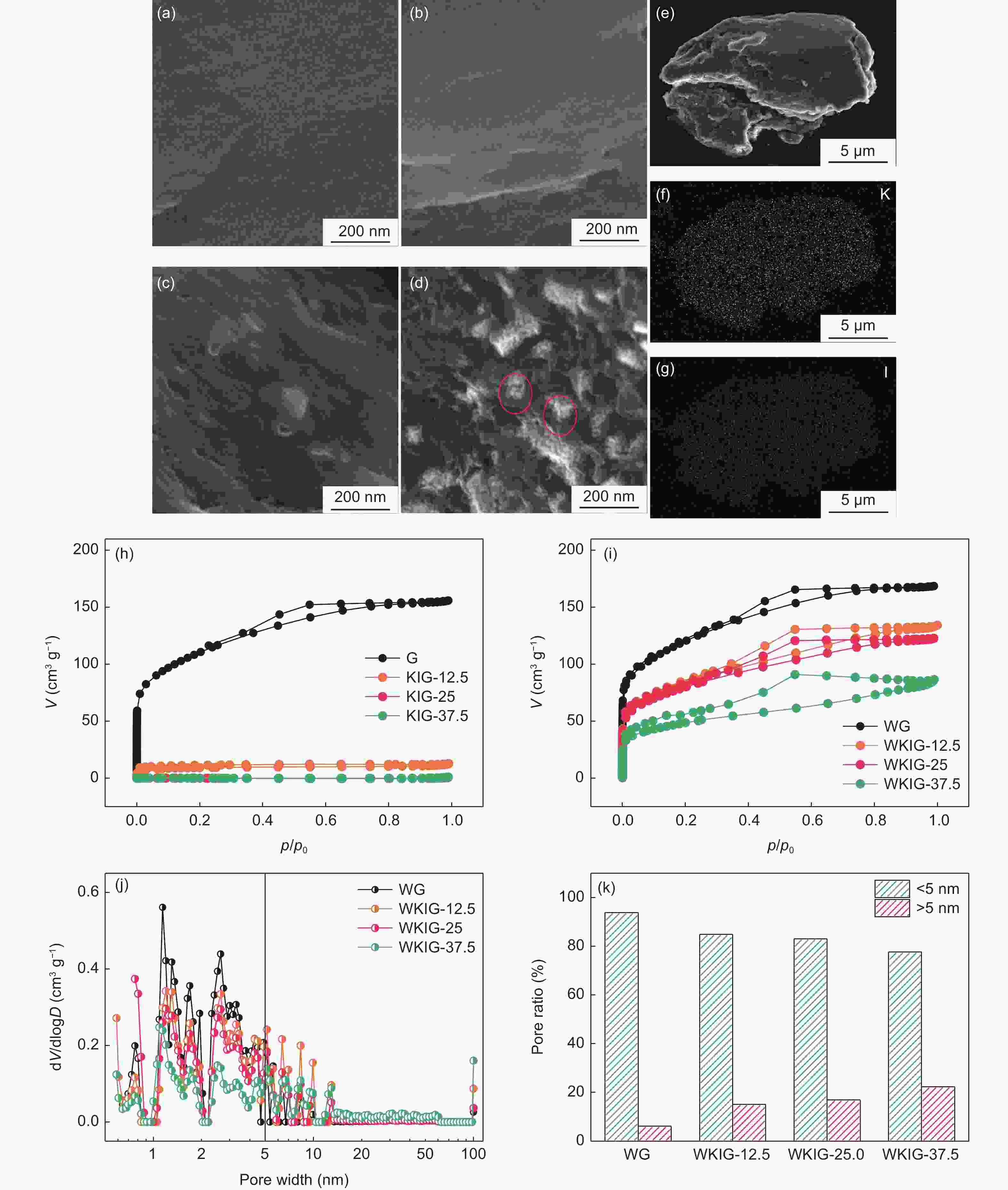
图 2 (a-d)G,KIG-12.5,KIG-25和KIG-37.5的扫描电镜照片,(e-g)KIG-25的扫描电镜照片与对应(K,I)元素分布. (h)G,KIG-12.5、KIG-25和KIG-37.5与(i)WG,WKIG-12.5、WKIG-25和WKIG-37.5的氮气吸脱附曲线和(j)对应孔径分布. (k)WG和WKIG样品的孔体积所占比例
Figure 2. (a-d) SEM images of G, KIG-12.5, KIG-25 and KIG-37.5, (e-g) SEM image and corresponding EDS elemental (K and I) maps of KIG-25, Nitrogen adsorption/desorption isotherms of (h) G, KIG-12.5, KIG-25 and KIG-37.5 and (i) WG, WKIG-12.5, WKIG-25 and WKIG-37.5, and (j) corresponding pore size distribution curves. (k) Pore volume ratio of WG and WKIG hybrids
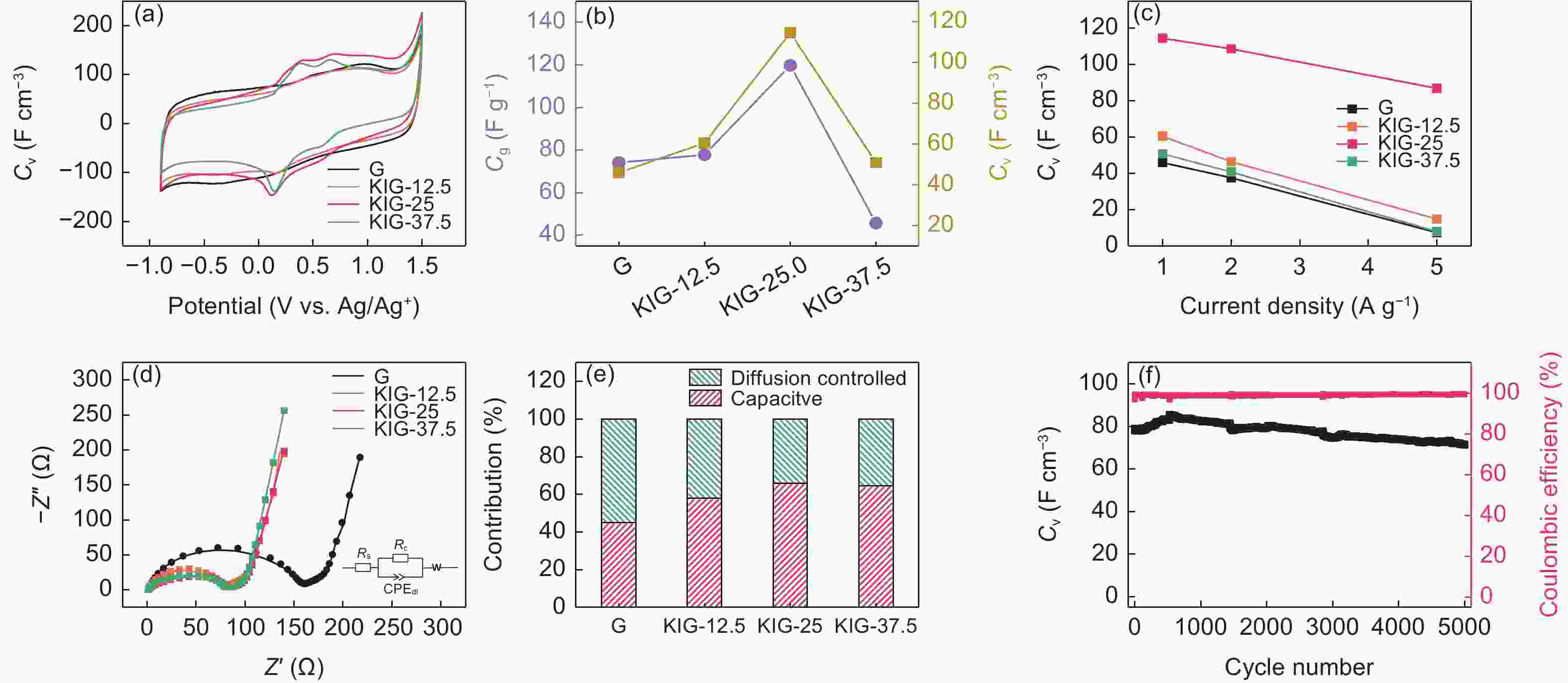
图 3 G,KIG-12.5、KIG-25和KIG-37.5的(a)循环伏安曲线(扫描速率为0.5 mV s−1),(b)质量比电容和体积比电容对比图(电流密度为1 A g−1),(c)倍率性能,(d)电化学交流阻抗谱和(e)电荷储存机制对比图. (f)KIG-25的循环性能(电流密度为1 A g−1)
Figure 3. (a) Cyclic voltammetry profiles at scan rate of 0.5 mV s−1, (b) the comparison of gravimetric and volumetric capacitance at current density of 1 A g−1, (c) rate performance, (d) Nyquist plots and (e) differentiation of charge storage mechanism of G, KIG-12.5, KIG-25 and KIG-37.5. (f) Cycling stability of KIG-25 at 1 A g−1
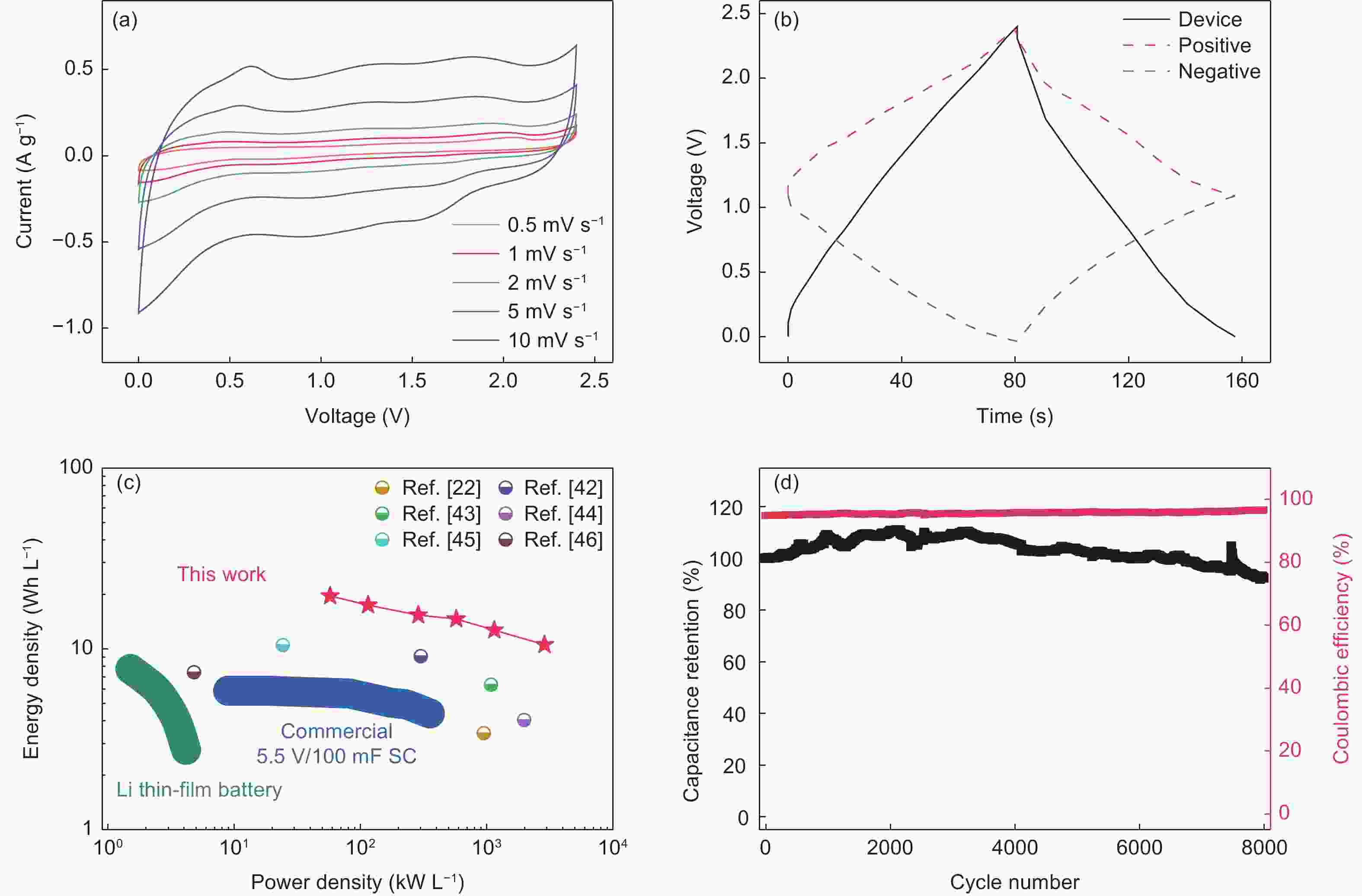
图 4 (a)所组装的KIG对称电化学电容器的循环伏安曲线,(b)器件和正负极的恒流充放电曲线(电流密度为1 A g−1). (c)所组装的KIG对称电化学电容器与已报道文献中器件的能量密度-功率密度关系图[22, 24, 45-49]. (d)所组装的KIG对称电化学电容器的循环性能(电流密度为1 A g−1)
Figure 4. (a) Cyclic voltammetry profiles of as-assembled KIG symmetric capacitor. (b) Galvanostatic charge–discharge curves of device, positive and negative electrodes at current density of 1 A g−1. (c) Ragone plots of the as-assembled KIG symmetric capacitor and previously reported devices[22, 24, 45-49]. (d) Cycling stability of the as-assembled KIG symmetric capacitor at current density of 1 A g−1
-
[1] Tarascon J M, Armand M. Issues and challenges facing rechargeable lithium batteries[J]. Nature,2001,414(6861):359-367. doi: 10.1038/35104644 [2] Zhang C, Lv W, Tao Y, et al. Towards superior volumetric performance: design and preparation of novel carbon materials for energy storage[J]. Energy & Environmental Science,2015,8(5):1390-1403. [3] Li H, Tao Y, Zheng X, et al. Ultra-thick graphene bulk supercapacitor electrodes for compact energy storage[J]. Energy & Environmental Science,2016,9(10):3135-3142. [4] Li H, Qi C, Tao Y, et al. Quantifying the volumetric performance metrics of supercapacitors[J]. Advanced Energy Materials,2019,9(21):1900079. doi: 10.1002/aenm.201900079 [5] Luo X Y, Chen Y, Mo Y. A review of charge storage in porous carbon-based supercapacitors[J]. New Carbon Materials,2021,36(1):49-68. doi: 10.1016/S1872-5805(21)60004-5 [6] Zhang W, Cheng R R, Bi H H, et al. A review of porous carbons produced by template methods for supercapacitor applications[J]. New Carbon Materials,2021,36(1):69-81. doi: 10.1016/S1872-5805(21)60005-7 [7] Wang Q, Yan J, Fan Z. Carbon materials for high volumetric performance supercapacitors: design, progress, challenges and opportunities[J]. Energy & Environmental Science,2016,9(3):729-762. [8] Li S J, Zhang M Y, Gao Y, et al. Preparation of a porous carbon from Enteromorpha prolifera with excellent electrochemical properties[J]. New Carbon Materials,2021,36(6):1158-1166. doi: 10.1016/S1872-5805(21)60068-9 [9] Xu K L, Liu J, Yan Z X, et al. Synthesis and use of hollow carbon spheres for electric double-layer capacitors[J]. New Carbon Materials,2021,36(4):794-809. doi: 10.1016/S1872-5805(20)60517-0 [10] Wang D W, Li F, Liu M, et al. 3D aperiodic hierarchical porous graphitic carbon material for high-rate electrochemical capacitive energy storage[J]. Angewandte Chemie International Edition,2008,47(2):373-376. doi: 10.1002/anie.200702721 [11] Gogotsi Y, Simon P. True performance metrics in electrochemical energy storage[J]. Science,2011,334(6058):917-918. doi: 10.1126/science.1213003 [12] Simon P, Gogotsi Y. Capacitive energy storage in nanostructured carbon-electrolyte systems[J]. Acc Chem Res,2013,46(5):1094-1103. doi: 10.1021/ar200306b [13] Gao F, Xie Y Q, Zang Y H, et al. A sustainable strategy to prepare porous carbons with tailored pores from shrimp shell for use as supercapacitor electrode materials[J]. New Carbon Materials,2022,37(4):752-763. [14] Wei Y C, Zhou J, Yang L, et al. N/S co-doped interconnected porous carbon nanosheets as high-performance supercapacitor electrode materials[J]. New Carbon Materials,2022,37(4):707-715. [15] Xu Y, Lin Z, Zhong X, et al. Holey graphene frameworks for highly efficient capacitive energy storage[J]. Nat Commun,2014,5(1):1-8. [16] Liu C, Yan X, Hu F, et al. Toward superior capacitive energy storage: Recent advances in pore engineering for dense electrodes[J]. Adv Mater,2018,30(17):1705713. doi: 10.1002/adma.201705713 [17] Bu Y, Sun T, Cai Y, et al. Compressing carbon nanocages by capillarity for optimizing porous structures toward ultrahigh-volumetric-performance supercapacitors[J]. Advanced Materials,2017,29(24):1700470. doi: 10.1002/adma.201700470 [18] Li Z N, Gadipelli S, Li H C, et al. Tuning the interlayer spacing of graphene laminate films for efficient pore utilization towards compact capacitive energy storage[J]. Nature Energy,2020,5(2):160-168. doi: 10.1038/s41560-020-0560-6 [19] Yang X, Cheng C, Wang Y, et al. Liquid-mediated dense integration of graphene materials for compact capacitive energy storage[J]. Science,2013,341(6145):534-537. doi: 10.1126/science.1239089 [20] Qi C S, Luo C, Tao Y, et al. Capillary shrinkage of graphene oxide hydrogels[J]. Science China Materials,2019,63(10):1870-1877. [21] Chen X, Han J, Lv X, et al. Dense yet highly ion permeable graphene electrodes obtained by capillary-drying of a holey graphene oxide assembly[J]. Journal of Materials Chemistry A,2019,7(20):12691-12697. doi: 10.1039/C9TA03698A [22] Li P, Li H, Han D, et al. Packing activated carbons into dense graphene network by capillarity for high volumetric performance supercapacitors[J]. Advanced Science,2019,6(14):1802355. doi: 10.1002/advs.201802355 [23] Tao Y, Xie X, Lv W, et al. Towards ultrahigh volumetric capacitance: graphene derived highly dense but porous carbons for supercapacitors[J]. Scientific Reports,2013,3(1):1-8. [24] Li H, Tao Y, Zheng X, et al. Compressed porous graphene particles for use as supercapacitor electrodes with excellent volumetric performance[J]. Nanoscale,2015,7(44):18459-18463. doi: 10.1039/C5NR06113J [25] Huang J, Sumpter B G, Meunier V. A universal model for nanoporous carbon supercapacitors applicable to diverse pore regimes, carbon materials, and electrolytes[J]. Chemistry,2008,14(22):6614-6626. doi: 10.1002/chem.200800639 [26] Dyatkin B, Osti N C, Zhang Y, et al. Ionic liquid structure, dynamics, and electrosorption in carbon electrodes with bimodal pores and heterogeneous surfaces[J]. Carbon,2018,129:104-118. doi: 10.1016/j.carbon.2017.12.001 [27] Wang X, Wang Z, Chen L. Reduced graphene oxide film as a shuttle-inhibiting interlayer in a lithium-sulfur battery[J]. Journal of Power Sources,2013,242:65-69. doi: 10.1016/j.jpowsour.2013.05.063 [28] Pei S, Zhao J, Du J, et al. Direct reduction of graphene oxide films into highly conductive and flexible graphene films by hydrohalic acids[J]. Carbon,2010,48(15):4466-4474. doi: 10.1016/j.carbon.2010.08.006 [29] Przygocki P, Abbas Q, Béguin F. Capacitance enhancement of hybrid electrochemical capacitor with asymmetric carbon electrodes configuration in neutral aqueous electrolyte[J]. Electrochimica Acta,2018,269:640-648. doi: 10.1016/j.electacta.2018.03.016 [30] Frackowiak E, Meller M, Menzel J, et al. Redox-active electrolyte for supercapacitor application[J]. Faraday Discuss,2014,172:179-198. doi: 10.1039/C4FD00052H [31] Strydom C A, Van Staden J F, Strydom H J. An XPS investigation of the influence of bromide and iodide solutions on the surface of silver chloride coated ion-selective electrodes[J]. Electroanalysis,1991,3(3):197-201. doi: 10.1002/elan.1140030310 [32] Xu Y, Tao Y, Zheng X Y, et al. A metal-free supercapacitor electrode material with a record high volumetric capacitance over 800 F cm−3[J]. Advanced Materials,2015,27(48):8082-8087. doi: 10.1002/adma.201504151 [33] Brezesinski T, Wang J, Tolbert S H, et al. Ordered mesoporous alpha-MoO3 with iso-oriented nanocrystalline walls for thin-film pseudocapacitors[J]. Nature Materials,2010,9(2):146-151. doi: 10.1038/nmat2612 [34] Lindstrom H, Sodergren S, Solbrand A, et al. Li+ ion insertion in TiO2 (anatase). 1 chronoamperometry on CVD films and nanoporous films[J]. Journal of Physical Chemistry B,1997,101(39):7710-7716. doi: 10.1021/jp970489r [35] Aikens D A. Electrochemical methods, fundamentals and applications[J]. Journal of Chemical Education,1983,60(1):A25. [36] Liu T C, Pell W G, Conway B E, et al. Behavior of molybdenum nitrides as materials for electrochemical capacitors - comparison with ruthenium oxide[J]. Journal of the Electrochemical Society,1998,145(6):1882-1888. doi: 10.1149/1.1838571 [37] Wang J, Polleux J, Lim J, et al. Pseudocapacitive contributions to electrochemical energy storage in TiO2(anatase) nanoparticles[J]. Journal of Physical Chemistry C,2007,111(40):14925-14931. doi: 10.1021/jp074464w [38] Simon P, Gogotsi Y, Dunn B. Where do batteries end and supercapacitors begin?[J]. Science,2014,343(6176):1210-1211. doi: 10.1126/science.1249625 [39] Augustyn V, Simon P, Dunn B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage[J]. Energy & Environmental Science,2014,7(5):1597-1614. [40] Conway B E. Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications [M]. Springer Science & Business Media, 2013. [41] Lota G, Frackowiak E. Striking capacitance of carbon/iodide interface[J]. Electrochemistry Communications,2009,11(1):87-90. doi: 10.1016/j.elecom.2008.10.026 [42] Zhang G, Wang H, Zhang S, et al. Using core-shell interlinked polymer@C–iodine hollow spheres to synergistically depress polyiodide shuttle and boost kinetics for iodine-based batteries[J]. Journal of Materials Chemistry A,2018,6(19):9019-9031. doi: 10.1039/C8TA01470A [43] Lu K, Hu Z, Ma J, et al. A rechargeable iodine-carbon battery that exploits ion intercalation and iodine redox chemistry[J]. Nature Communications,2017,8(1):527. doi: 10.1038/s41467-017-00649-7 [44] Zhao Y, Wang L, Byon H R. High-performance rechargeable lithium-iodine batteries using triiodide/iodide redox couples in an aqueous cathode[J]. Nature Communications,2013,4(1):1-7. [45] Zhong J, Sun W, Wei Q W, et al. Efficient and scalable synthesis of highly aligned and compact two-dimensional nanosheet films with record performances[J]. Nature Communications,2018,9(1):3484. doi: 10.1038/s41467-018-05723-2 [46] Yu D, Goh K, Wang H, et al. Scalable synthesis of hierarchically structured carbon nanotube-graphene fibres for capacitive energy storage[J]. Nature Nanotechnology,2014,9(7):555. doi: 10.1038/nnano.2014.93 [47] Meng F, Ding Y. Sub-micrometer-thick all-solid-state supercapacitors with high power and energy densities[J]. Advanced Materials,2011,23(35):4098-4102. doi: 10.1002/adma.201101678 [48] Yang X, Zhu J, Ling Q, et al. Bioinspired effective prevention of restacking in multilayered graphene films: towards the next generation of high-performance supercapacitors[J]. Advanced Materials,2011,23(25):2833-2838. doi: 10.1002/adma.201100261 [49] El-Kady M F, Strong V, Dubin S, et al. Laser scribing of high-performance and flexible graphene-based electrochemical capacitors [J]. Science, 2012, 335(6074): 1326-1330. -
 20220189-Supporting information.pdf
20220189-Supporting information.pdf

-





 下载:
下载: