Recent progress in the research and development of natural graphite for use in thermal management, battery electrodes and the nuclear industry
-
摘要: 天然石墨具有高导热、高导电、耐高温、耐腐蚀和防辐射等诸多优点,已广泛应用于热管理、电池制造和核工业等众多领域。不过,矿物纯度即固定碳含量是影响天然石墨应用的一个重要因素,而要从高品位天然石墨矿物中分离出杂质难度较大。如何高效地提纯天然石墨矿物,以及提纯后的天然石墨有哪些材料化及高端应用,均为研究人员所关心的问题。因此,从天然石墨的类型和矿物资源储量出发,介绍了一些传统的石墨提纯工艺和获得高纯度石墨的新方法。重点综述了天然石墨特别是微晶石墨在热管理、电池制造和核工业等能源利用领域的最新研究进展。最后,对天然石墨的应用现状和未来趋势进行了总结和展望。Abstract: Natural graphite has many excellent properties such as high thermal and electrical conductivities, high temperature resistance, corrosion resistance, and radiation tolerance. It is widely used in many fields such as thermal management, battery electrodes, and the nuclear industry. The carbon content is an important factor that limits the applications of natural graphite minerals, but the impurities are difficult to remove from high-grade graphite minerals. This review discusses the types of natural graphite and mineral resources, followed by a discussion of traditional graphite purification processes and new methods to obtain high-purity graphite. Recent research on the development of natural graphite for use in thermal management, battery electrodes and the nuclear industry are summarized and the future applications of natural graphite are discussed.
-
Key words:
- Natural graphite /
- Purification /
- Materialization /
- Energy application
-
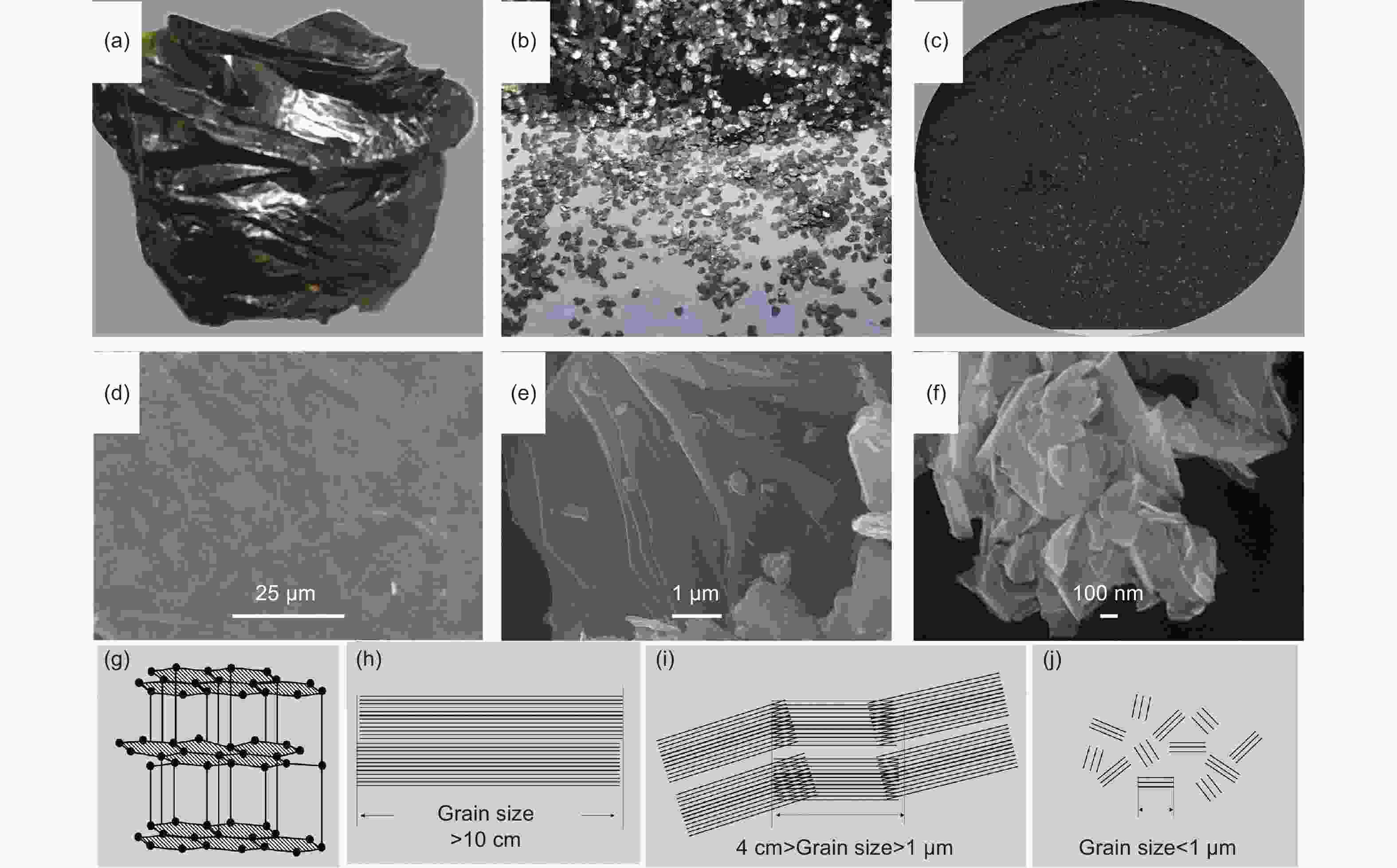
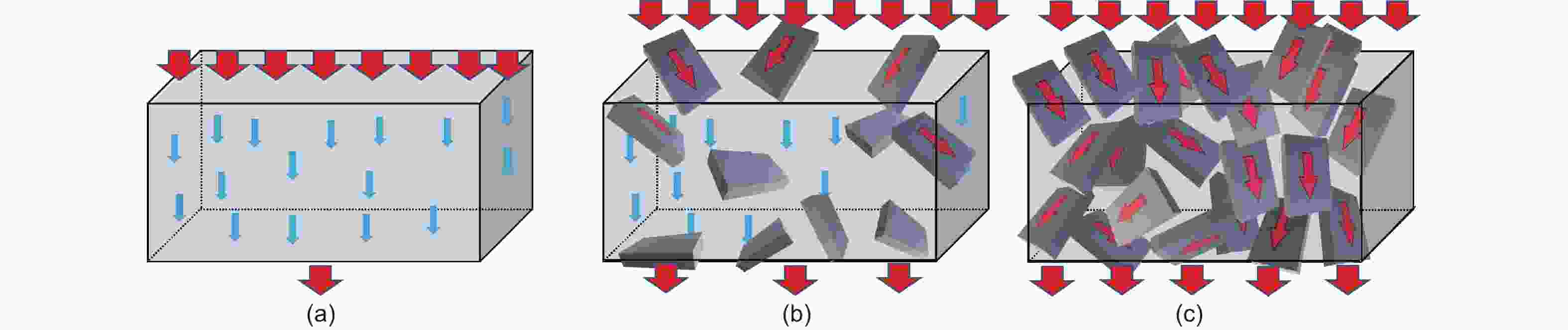
Figure 1. Digital photographs and micro-morphology of 3 kinds of natural graphite (a, b) vein graphite, (Open acces, Copyright 2021, Nature Publishing Group[8]); (b, e) flake graphite, (Reproduced with permission, Copyright 2019, IOP Publishing[18]); (c, f) microcrystalline graphite, (g) schematic diagram of the crystal structure and grain size (h) vein; (i) flake; (j) microcrystalline of graphite
Figure 2. (a) The preparation flow chart of flexible HOG/ polydimethylsiloxane (PDMS) composite material (Reproduced with permission, Copyright 2022, Elsevier[38]); (b) The structural diagram of graphite silver polyimide flexible foil (Reproduced with permission, Copyright 2020, Elsevier[39]); (c) The preparation flow chart of graphite nanosheet (GNP)/ polyurethane (PU) film (Reproduced with permission, Copyright 2021, Elsevier[40])
Figure 3. Preparation flow chart of graphite film reinforced aluminum matrix composite layer by layer (Reproduced with permission, Copyright 2018, Elsevier[41])
Figure 4. SEM image of graphite AlN heat dissipation substrate (Reproduced with permission, Copyright 2017, WILEY[49])
Figure 5. SEM images (Reproduced with permission, Copyright 2019, WILEY[42]) of (a) CCF-90-10-200, (b) CCF-50-50-200, (c) CCF-70-30-100 and (d) CCF-70-30-250
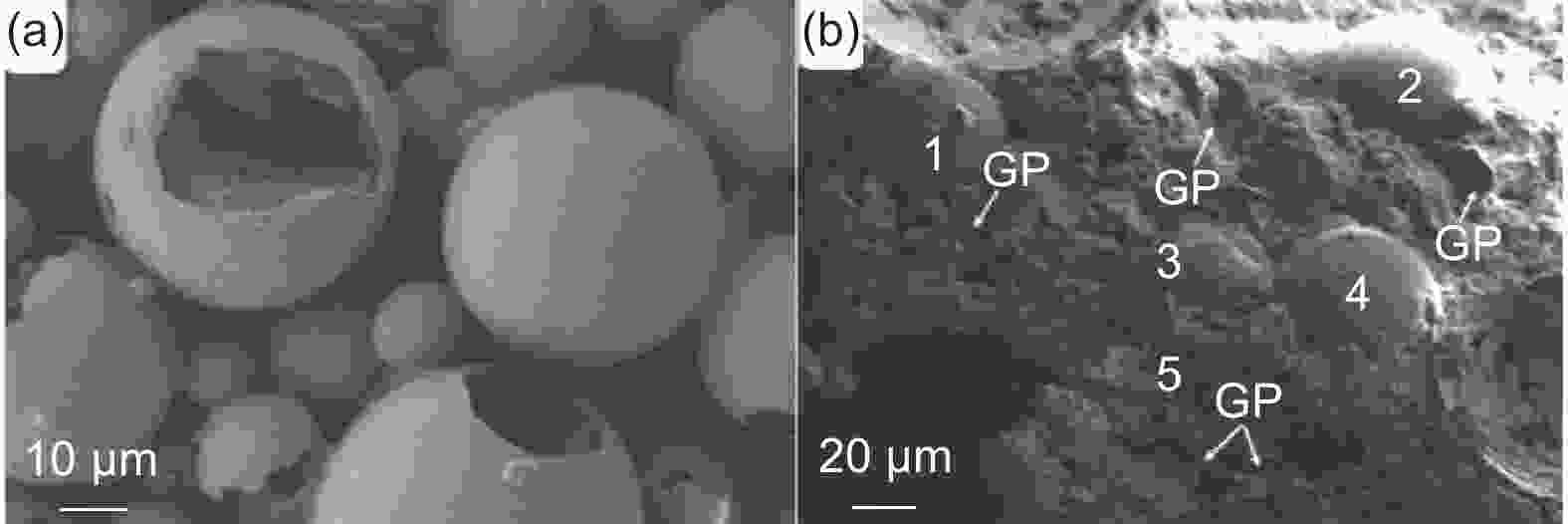
Figure 6. (a) SEM image of microcapsule phase change materials; (b) SEM image of cement-based composites doped with MPCM and GP (Reproduced with permission, Copyright 2022, Elsevier[55])
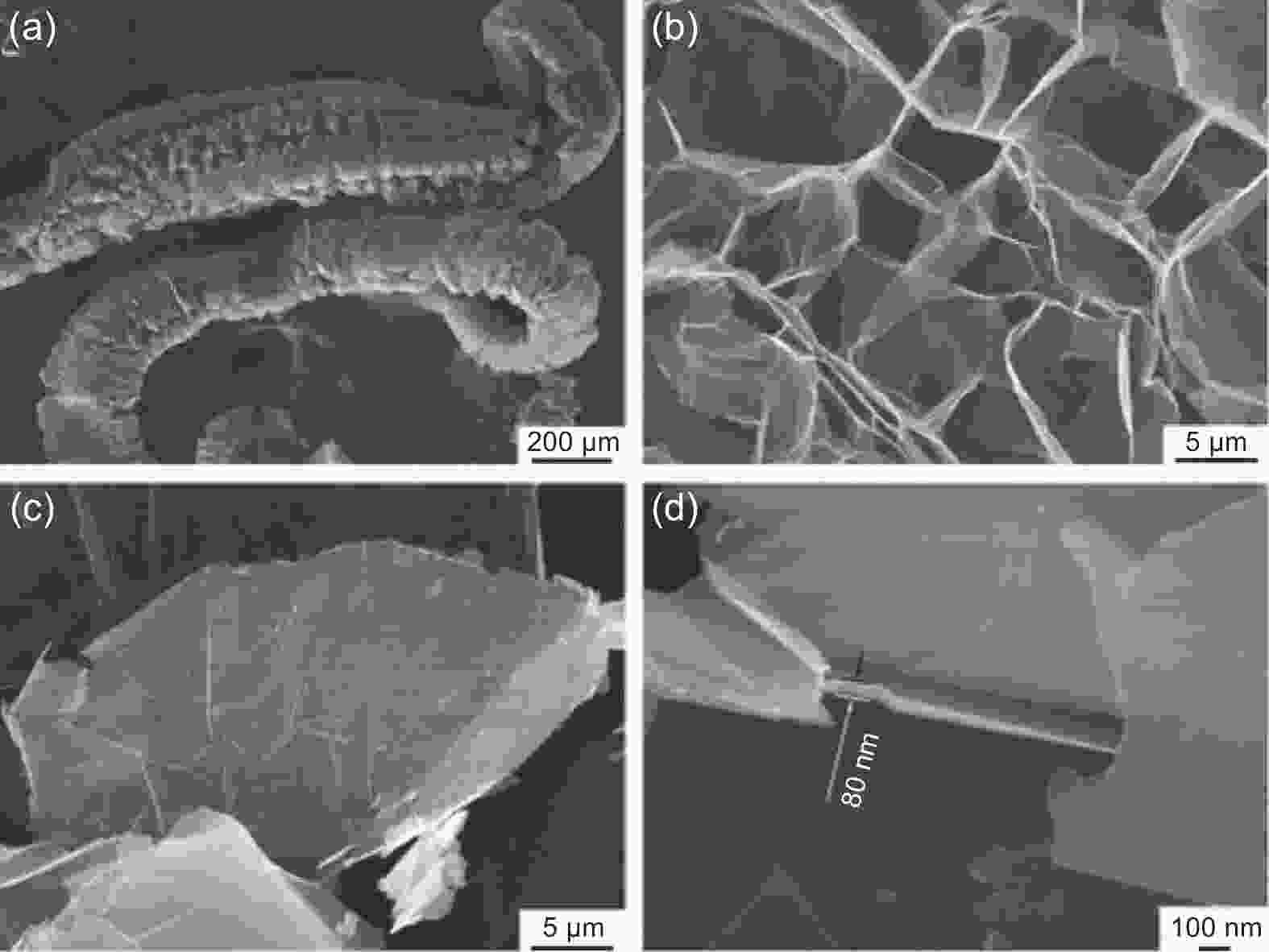
Figure 8. SEM images of EG (Reproduced with permission, Copyright 2019, Elsevier[58])
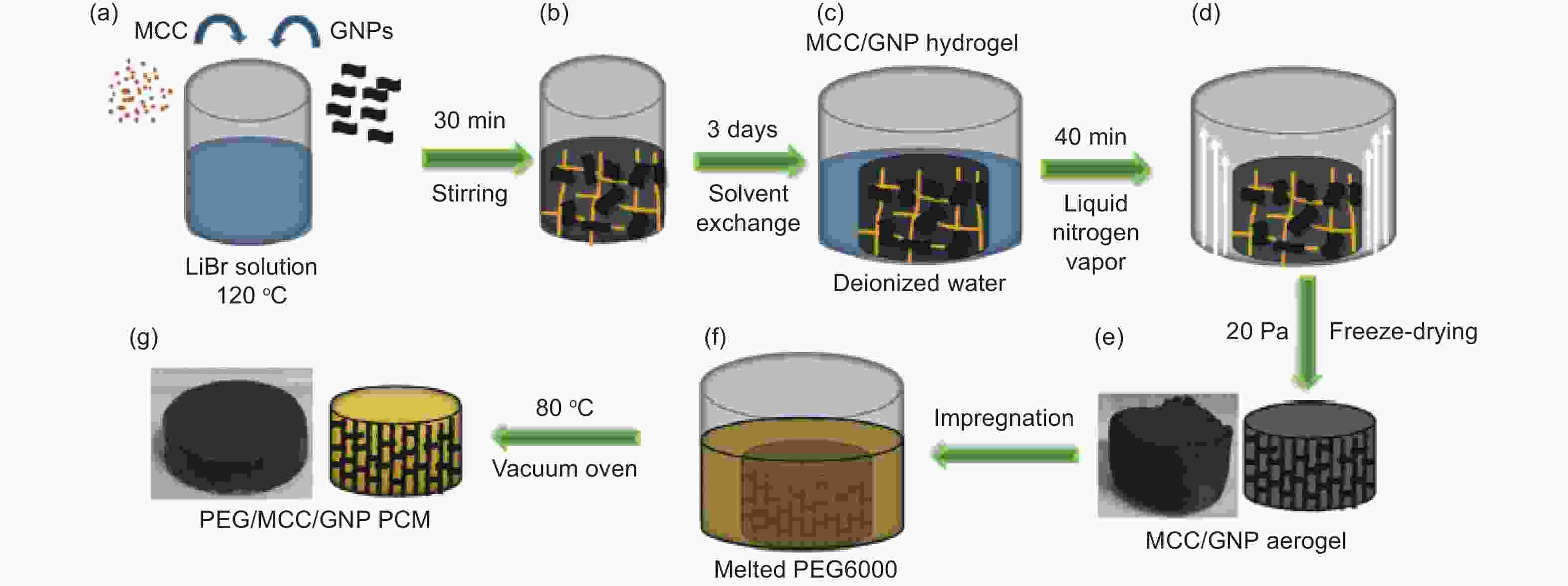
Figure 9. Schematic of the preparation of composite PCMs (Reproduced with permission, Copyright 2019, Elsevier[63])
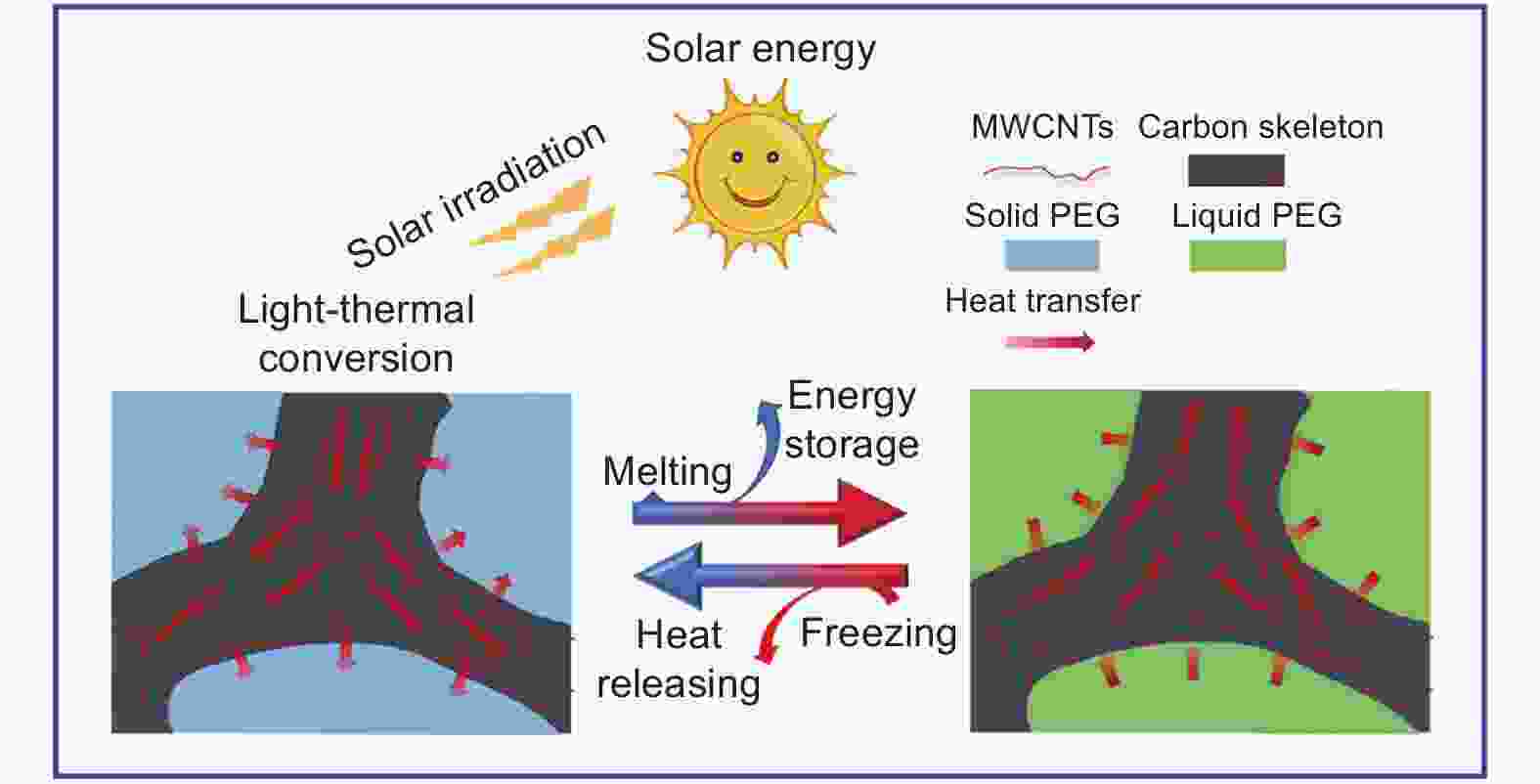
Figure 10. Schematic of light-thermal conversion and storage mechanism of composites (Reproduced with permission, Copyright 2021, Elsevier[65])
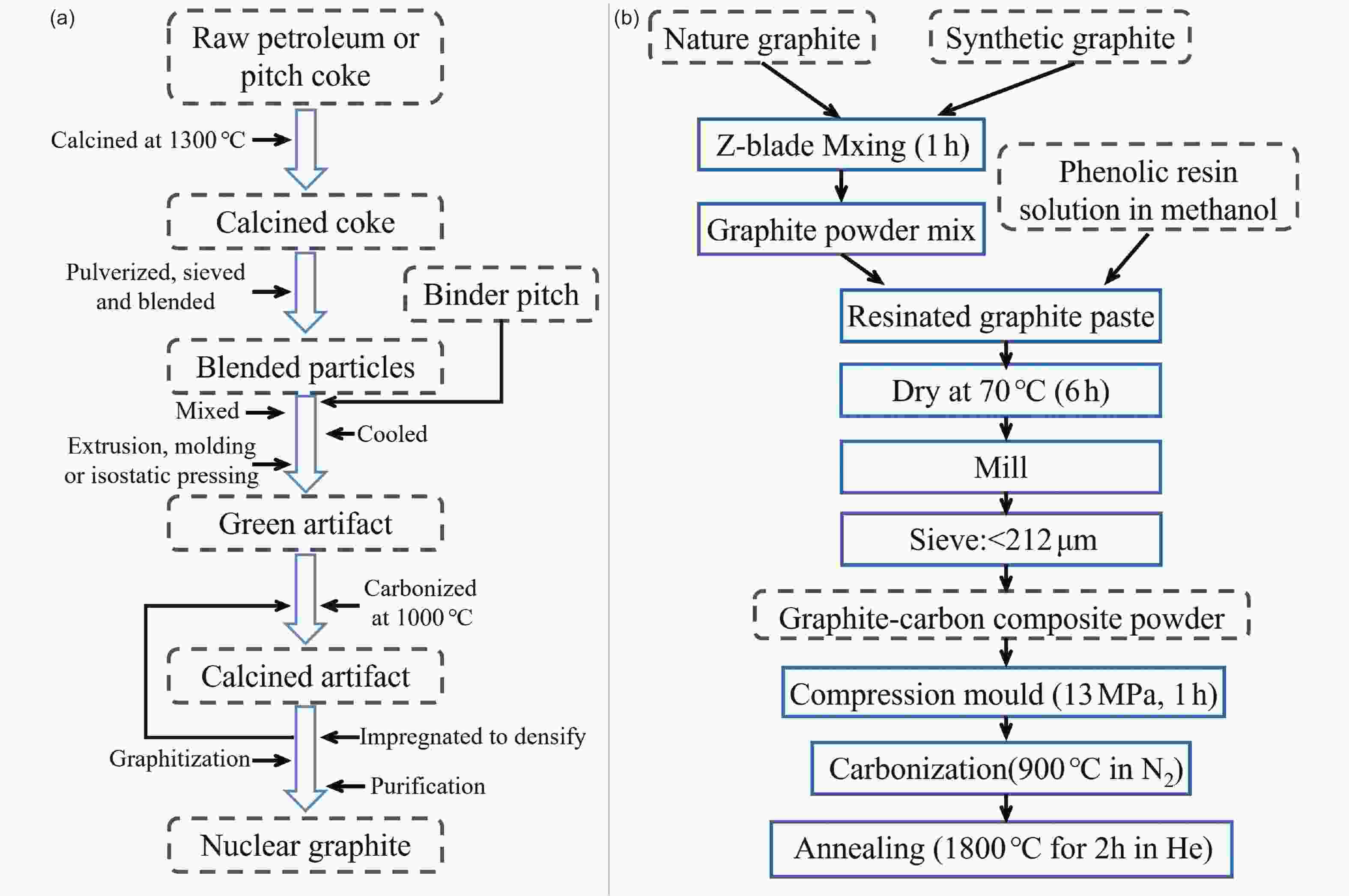
Figure 15. Several common graphite moderator components (Open access, Copyright 2016[95])
Table 1. Classifications and features of different natural graphites[10]
Graphite type Vein (lump) Flake Microcrystalline Morphology Interlocking
Aggregates of coarse graphite crystalsWell-developed crystal platelets of graphite Earthy to compact fine-grained graphite Ore grade 40%-90% 5%-50% 50%-90% Crystallinity Crystalline Crystalline Microcrystalline Grain size Up to 10 cm 1 μm-4 cm <1 μm Table 2. Chemical composition and major mineral impurities of nature graphite
Graphite type Chemical composition Major mineral impurities Flake C, O, Si, Al, Mg, Ca, Fe Quartz, feldspar, biotite, pyrite, pyrrhotite, rutile, monticellite etc. Microcrystalline C, O, Si, Al, Mg, Fe, Ca Quartz, chlorite, serpentine, montmorillonite, calcite, illite, mica,
chchalcopyrite, pyrite, kaolin, limonite, petalite etc.Vein C, O, Fe, Ca, Mg, Si, Al Quartz, pyroxene, pyrrhotite, pyrite, chalcopyrite, sphalerite, marcasite,
chlochlorite, calcite, siderite, dolomite, copper etc.[8, 19]Table 3. World production of nature graphite by country or locality (unit is ton)[20]
Country or locality Graphite category 2017 2018 2019 2020 2021 China flake 350000 416000 392000 762000 820000 amorphous 275000 277000 308000 Brazil flake 90000 95000 96000 63600 68000 Canada flake 40000 40000 11000 8000 8600 India flake 31500 31500 31500 6000 6500 amorphous 3500 3500 3500 Mozambique flake 1042 104000 107000 28000 30000 Russia flake 13200 13200 13100 25000 27000 amorphous 12000 12000 12000 Madagascar flake 14529 46900 48000 20900 22000 Ukraine flake 20000 20000 20000 16000 17000 Norway flake 11000 16000 16000 12000 13000 Korea, North flake 4500 4920 4920 8100 8700 amorphous 1000 1080 1080 Vietnam flake 5000 5000 5000 5000 5400 Sri Lanka vein 3900 4000 4000 4000 4300 Mexico amorphous 9000 9000 9000 3300 3500 Turkey amorphous 2300 2000 2000 2500 2700 Australia amorphous 1000 1000 1000 500 500 World total 880000 1110000 1100000 96600 1000000 Table 4. The thermal properties and applications of graphite or graphite-based composites reported in recent years
Materials Application Thermal conductivity (W·m−1·K−1) Ref. Graphite silver polyimide flexible foil Thermal interface materials 1600 [38] HOG/PDMS composites Selective thermal emitter 35.4 [39] GNP/ PU films Thermal interface materials 26.30 [40] Metal-based graphite composites Thermal management devices 743 [41] Graphite foam/PCMs Thermal energy storage systems 7.72 [42] Graphite/cement composites Geothermal reservoir exploitation 1.18 [43] EG/PCMs Thermal energy harvesting of electronics 4.4–35 [44] Graphene/PCMs Real-time and fast-charging solar-thermal energy conversion 8.87 [45] Table 5. Typical devices with graphite as electrodes and their latest reported properties
-
[1] Wang X, Li H, Yao H, et al. Network feature and influence factors of global nature graphite trade competition[J]. Resources Policy,2019,60:153-161. doi: 10.1016/j.resourpol.2018.12.012 [2] Kavanagh A, Schlögl R. The morphology of some natural and synthetic graphites[J]. Carbon,1988,26(1):23-32. doi: 10.1016/0008-6223(88)90005-X [3] Allah D J, Amha B, Girma W, et al. Purification, application and current market trend of natural graphite: A review[J]. International Journal of Mining Science and Technology,2019,29(5):671-689. doi: 10.1016/j.ijmst.2019.04.003 [4] Li Y, Tian X D, Song Y, et al. Preparation and lithium storage of anthracite-based graphite anode materials[J]. New Carbon Mater,2022,37(6):1163-1171. doi: 10.1016/S1872-5805(21)60057-4 [5] Douglas R III, Thomas C H. Carbon isotope geochemistry of graphite vein deposits from New Hampshire, U. S. A[J]. Geochimica Et Cosmochimica Acta,1986,50(6):1239-1247. doi: 10.1016/0016-7037(86)90407-2 [6] Rui X, Geng Y, Sun X, et al. Dynamic material flow analysis of natural graphite in China for 2001-2018[J]. Resources, Conservation & Recycling,2021,173:105732. [7] K. Soman; R. V. Lobzova; K. M. Sivadas. Geology, genetic types, and origin of graphite in South Kerala, India[J]. Economic Geology,1986,81(4):997-1002. doi: 10.2113/gsecongeo.81.4.997 [8] Gamaralalage R A K, Herath M G T A P, Buddika K, et al. Development of a chemical-free floatation technology for the purification of vein graphite and characterization of the products[J]. Scientific Reports,2021,11(1):22713. doi: 10.1038/s41598-021-02101-9 [9] Sun K K, Qiu Y S, Zhang L Y. Preserving flake size in an african flake graphite ore beneficiation using a modified grinding and pre-screening Process[J]. Minerals,2017,7(7):115. doi: 10.3390/min7070115 [10] Rakoto H A, Rajaomahefasoa R, Razafiarisera R, et al. Evaluation of flake graphite ore using self-potential (SP), electrical resistivity tomography (ERT) and induced polarization (IP) methods in east coast of Madagascar[J]. Journal of Applied Geophysics,2019,169:134-141. doi: 10.1016/j.jappgeo.2019.07.001 [11] Allah D J, Girma W, Amha B, et al. Mineralogical and petrographic analysis on the flake graphite ore from Saba Boru area in Ethiopia[J]. International Journal of Mining Science and Technology,2020,30(5):715-721. doi: 10.1016/j.ijmst.2020.05.025 [12] Albetran H M. Structural characterization of graphite nanoplatelets synthesized from graphite flakes[J]. Preprints,2020:2020080325. doi: 10.20944/preprints202008.032.v1 [13] Shen K, Cao X, Huang Z H, et al. Microstructure and thermal expansion behavior of natural microcrystalline graphite[J]. Carbon,2021,177:90-96. doi: 10.1016/j.carbon.2021.02.055 [14] Peng W, Li H, Hu Y, et al. Characterisation of reduced graphene oxides prepared from natural flaky, lump and amorphous graphites[J]. Materials Research Bulletin,2016,78:119-127. doi: 10.1016/j.materresbull.2016.02.034 [15] P R Solomon, D G Hamblen, R M Carangelo, et al. General model of coal devolatilization[J]. Energy Fuels,1988,2(4):405-422. doi: 10.1021/ef00010a006 [16] Li H, Lin B, Hong Y, et al. Effects of in-situ stress on the stability of a roadway excavated through a coal seam[J]. International Journal of Mining Science and Technology,2017,27(6):917-927. doi: 10.1016/j.ijmst.2017.06.013 [17] Stiller A H, Jefferson St, Morgantown WV, et al. Method of producing high quality, high purity, isotropic graphite from coall: US, US5705139 A[P]. 1998-01-06. [18] Duan S Z, Wu X W, Min X, et al. Effect of purity and proportion of microcrystalline graphite ore on the electrical, mechanical and tribological performance of copper-carbon composites[J]. Materials Research Express,2019,6(12):125604. doi: 10.1088/2053-1591/ab5380 [19] Hewathilake H P T S, Balasooriya N W B, Nakamura Y, et al. Geochemical, structural and morphological characterization of vein graphite deposits of Sri Lanka: Witness to carbon rich fluid activity[J]. Journal of Mineralogical Petrological Sciences,2018,113(2):96-105. doi: 10.2465/jmps.170721 [20] U. S. Geological Survey, Mineral Commodity Summaries[Z]. https://www.usgs.gov/centers/national-minerals-information-center/mineral-commodity-ummaries, 2021. [21] Liu C, Zhao T, Tong A N. Development situation and future trend of graphite industry in China[J]. Journal of Guilin University of Technology,2018,38(2):245-249. [22] Zhang Y F, An Z Z, Liang S, et al. Distribution characteristics, genetic types and prospecting progress of graphite deposits[J]. Geology in China,2022,49(1):135-150. [23] Zhang S J, Wang N, Cui L W, et al. Analysis of supply and demand situation of graphite resources at home and abroad[J]. Inorganic Chemicals Industry,2021,53(7):1-11. [24] Ca N A, Cifci F, Yan D. Optimization of process parameters for producing graphite concentrate using response surface methodology[J]. Separation and Purification Technology,2008,59(No.1):9-16. doi: 10.1016/j.seppur.2007.05.022 [25] Cui W Y, Chen J H. Insight into mineral flotation fundamentals through the DFT method[J]. International Journal of Mining Science and Technology,2021,31(6):983-994. doi: 10.1016/j.ijmst.2021.10.001 [26] Liu M X, Yu H, Zhang H Q, et al. Roles of the hydrophobic and hydrophilic groups of collectors in the flotation of different-sized mineral particles[J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects,2022,637:128262. doi: 10.1016/j.colsurfa.2022.128262 [27] Ren X C, Zhang G X. Study on preparation of high-purity graphite by stepwise purification with sulfuric acid/hydrofluoric acid[J]. Non-Metallic Mines,2007,30(3):68-70. [28] Zhang X, Aldahri T, Tan X, et al. Efficient co-extraction of lithium, rubidium, cesium and potassium from lepidolite by process intensification of chlorination roasting[J]. Chemical Engineering & Processing,2020,147:107777. [29] Li Y F, Zhu S F, Wang L. Purification of natural graphite by microwave assisted acid leaching[J]. Carbon,2013,55:377-378. [30] Bao C G, Shi K, Xu P, et al. Purification effect of the methods used for the preparation of the ultra-high purity graphite[J]. Diamond Related Materials,2021,120:108704. doi: 10.1016/j.diamond.2021.108704 [31] Duan S Z, Wu X W, Cheng Y F, et al. Study on the structure and composition of high-temperature purification precipitates of natural graphite after pickling [J]. Carbon Techniques, 2023, In Press. [32] Hu X L, Tang X, Zhou Y B, et al. A continuous high-temperature purification reactor for graphite using Freon-12[J]. Carbon,2017,100(114):753-754. [33] Shen K, Chen X, Shen W, et al. Thermal and gas purification of natural graphite for nuclear applications[J]. Carbon,2020,173(5):769-781. [34] Fu Y X, He Z X, Mo D C, et al. Thermal conductivity enhancement with different fillers for epoxy resin adhesives[J]. Applied Thermal Engineering,2014,66(1-2):493-498. doi: 10.1016/j.applthermaleng.2014.02.044 [35] Jakob A, Tobias E, Matthias K, et al. The success story of graphite as a lithium-ion anode material-fundamentals, remaining challenges, and recent developments including silicon (oxide) composites[J]. Sustainable Energy & Fuels,2020,4(11):5387-5416. [36] Li B, Zheng J, Zhang H, et al. Electrode materials, electrolytes, and challenges in nonaqueous lithium-ion capacitors[J]. Advanced Materials,2018,30(17):1705670. doi: 10.1002/adma.201705670 [37] Shen K, Huang Z H, Hu K, et al. Advantages of natural microcrystalline graphite filler over petroleum coke in isotropic graphite preparation[J]. Carbon,2015,90:197-206. doi: 10.1016/j.carbon.2015.03.068 [38] Zhang F, Ren D, Zhang Y, et al. Production of highly-oriented graphite monoliths with high thermal conductivity[J]. Chemical Engineering Journal,2022,431:134102. doi: 10.1016/j.cej.2021.134102 [39] Fan D, Jin M, Wang J, et al. Enhanced heat dissipation in graphite-silver-polyimide structure for electronic cooling[J]. Applied Thermal Engineering,2020,168:114676. doi: 10.1016/j.applthermaleng.2019.114676 [40] Wu X, Wang H, Wang Z, et al. Highly conductive thermal interface materials with vertically aligned graphite-nanoplatelet filler towards: High power density electronic device cooling[J]. Carbon,2021,182:445-453. doi: 10.1016/j.carbon.2021.06.048 [41] Chang J, Zhang Q, Lin Y F, et al. Layer by layer graphite film reinforced aluminum composites with an enhanced performance of thermal conduction in the thermal management applications[J]. Journal of Alloys and Compounds,2018,742:601-609. doi: 10.1016/j.jallcom.2018.01.332 [42] Wilson P, Vijayan S, Prabhakaran K. Thermally conducting microcellular carbon foams as a superior host for wax-based phase change materials[J]. Advanced Engineering Materials,2019,21:1801139. doi: 10.1002/adem.201801139 [43] Wang S, Jian L, Shu Z, et al. A high thermal conductivity cement for geothermal exploitation application[J]. Natural Resources Research,2020,29(6):3675-3687. doi: 10.1007/s11053-020-09694-4 [44] Si W, Ting X L, Zhen T, et al. High‐performance thermally conductive phase change composites by large‐size oriented graphite sheets for scalable thermal energy harvesting[J]. Advanced Materials,2019,31(49):1905099. doi: 10.1002/adma.201905099 [45] Min P, Liu J, Li X, et al. Thermally conductive phase change composites featuring anisotropic graphene aerogels for real‐time and fast‐charging solar‐thermal energy conversion[J]. Advanced Functional Materials,2018,28(51):1805365. doi: 10.1002/adfm.201805365 [46] Wang X, Su Y, OuYang Q, et al. Fabrication, mechanical and thermal properties of copper coated graphite films reinforced copper matrix laminated composites via ultrasonic-assisted electroless plating and vacuum hot-pressing sintering[J]. Materials Science Engineering:A,2021,824:141768. doi: 10.1016/j.msea.2021.141768 [47] Asalieva E, Sineva L, Slnichkina S, et al. Exfoliated graphite as a heat-conductive frame for a new pelletized Fischer-Tropsch synthesis catalyst[J]. Applied Catalysis A:General,2020,601:117639. doi: 10.1016/j.apcata.2020.117639 [48] Liu B, Zhang D, Li X, et al. Effect of graphite flakes particle sizes on the microstructure and properties of graphite flakes/copper composites[J]. Journal of Alloys and Compounds,2018,766:382-390. doi: 10.1016/j.jallcom.2018.06.129 [49] Chou T T, Tuan W H, Nishikawa H, et al. Brazing graphite to aluminum nitride for thermal dissipation purpose[J]. Advanced Engineering Materials,2017,19:1600876. doi: 10.1002/adem.201600876 [50] Liu X, Lin F, Zhang X, et al. Paraffin/Ti3C2Tx Mxene@Gelatin aerogels composite phase-change materials with high solar-thermal conversion efficiency and enhanced thermal conductivity for thermal energy storage[J]. Energy & Fuels,2021,35(3):2805-2814. [51] Lin F K, Liu X J, Leng G Q, et al. Grid structure phase change composites with effective solar/electro-thermal conversion for multi-functional thermal application[J]. Carbon,2023,201:1001-1010. doi: 10.1016/j.carbon.2022.09.077 [52] Duan S Z, Wu X W, Zeng K Q, et al. Simple routes from natural graphite to graphite foams: Preparation, structure and properties[J]. Carbon,2020,159:527-541. doi: 10.1016/j.carbon.2019.12.091 [53] Liu M, Zhang X, Liu X, et al. Multienergy-triggered composite phase-change materials based on graphite foams synthesized from graphite extracted from spent lithium-ion batteries[J]. ACS Sustainable Chemistry & Engineering,2022,10(24):8051-8063. [54] Duan S Z, Feng J, Yu W H, et al. The influences of ball milling processing on the morphology and thermal properties of natural graphite-based porous graphite and their phase change composites[J]. Journal of energy storage,2022,55:105800. doi: 10.1016/j.est.2022.105800 [55] Xiong T, Shah K W, Kua H W. Application of graphite platelets for heat transfer enhancement of cementitious composites containing microencapsulated phase change materials[J]. Construction Building Materials,2022,318:126024. doi: 10.1016/j.conbuildmat.2021.126024 [56] Frc M, Pichór W, Szodra P, et al. Cement composites with expanded graphite/paraffin as storage heater[J]. Construction Building Materials,2021,275:122126. doi: 10.1016/j.conbuildmat.2020.122126 [57] Frc M, Szudek W, Szodra P, et al. Grouts with highly thermally conductive binder for low-temperature geothermal applications[J]. Construction Building Materials,2021,295:123680. doi: 10.1016/j.conbuildmat.2021.123680 [58] Li M, Mu B. Effect of different dimensional carbon materials on the properties and application of phase change materials: A review[J]. Applied Energy,2019,242:695-715. doi: 10.1016/j.apenergy.2019.03.085 [59] Wang F, Hou R. Numerical study of nano-particle composite paraffin phase change heat storage capsule [J]. Journal of Physics: Conference Series, 2022, 2194(1). DOI: 10.1088/1742-6596/2194/1/012011 [60] Song Y, Zhang N, Yuan Y, et al. Prediction of the solid effective thermal conductivity of fatty acid/carbon material composite phase change materials based on fractal theory[J]. Energy,2019,170:752-762. doi: 10.1016/j.energy.2018.12.162 [61] Zhang S, Wu W, Wang S. Experimental investigations of Alum/expanded graphite composite phase change material for thermal energy storage and its compatibility with metals[J]. Energy,2018,161:508-516. doi: 10.1016/j.energy.2018.07.075 [62] Zhang T, Zhang T D, Zhang J, et al. Design of stearic acid/graphene oxide-attapulgite aerogel shape-stabilized phase change materials with excellent thermophysical properties[J]. Renewable Energy,2021,165:504-513. doi: 10.1016/j.renene.2020.11.030 [63] Wei X, Xue F, Qi X, et al. Photo-and electro-responsive phase change materials based on highly anisotropic microcrystalline cellulose/graphene nanoplatelet structure [J]. Applied energy, 2019, 236: 70-80. [64] Jian F, Liu X J, Lin F K, et al. Aligned channel Gelatin@nanoGraphite aerogel supported form-stable phase change materials for solar-thermal energy conversion and storage[J]. Carbon,2023,201:756-764. doi: 10.1016/j.carbon.2022.09.064 [65] Lin F K, Zhang X G, Liu X J, et al. Polyethylene glycol/modified carbon foam composites for efficient light-thermal conversion and storage[J]. Polymer,2021,228:123894. doi: 10.1016/j.polymer.2021.123894 [66] Luo P, Zheng C, He J, et al. Structural engineering in graphite‐based metal‐ion batteries[J]. Advanced Functional Materials,2021,32(9):2107277. [67] Zou L, Kang F, Zheng Y-P, et al. Modified natural flake graphite with high cycle performance as anode material in lithium ion batteries[J]. Electrochimica Acta,2009,54(15):3930-3934. doi: 10.1016/j.electacta.2009.02.012 [68] Lin Y, Huang Z-H, Yu X, et al. Mildly expanded graphite for anode materials of lithium ion battery synthesized with perchloric acid[J]. Electrochimica Acta,2014,116:170-174. doi: 10.1016/j.electacta.2013.11.057 [69] Yang X, Zhan C, Ren X, et al. Nitrogen-doped hollow graphite granule as anode materials for high-performance lithium-ion batteries[J]. Journal of Solid State Chemistry,2021,303:122500. doi: 10.1016/j.jssc.2021.122500 [70] Placke T, Rothermel S, Fromm O, et al. Influence of graphite characteristics on the electrochemical intercalation of bis(trifluoromethanesulfonyl) imide anions into a graphite-based cathode[J]. Journal of the Electrochemical Society,2013,160(11):A1979-A1991. doi: 10.1149/2.027311jes [71] Read J A, Cresce A V, Ervin M H, et al. Dual-graphite chemistry enabled by a high voltage electrolyte[J]. Energy & Environmental Science,2014,7(2):617-620. [72] Wan H, Ju X, He T, et al. Sulfur-doped porous carbon as high-capacity anodes for lithium and sodium ions batteries[J]. Journal of Alloys and Compounds,2021,863:158078. doi: 10.1016/j.jallcom.2020.158078 [73] Li P, Kim H, Myung S-T, et al. Diverting exploration of silicon anode into practical way: A review focused on silicon-graphite composite for lithium ion batteries[J]. Energy Storage Materials,2021,35:550-576. doi: 10.1016/j.ensm.2020.11.028 [74] Ratnakumar B V, Smart M C, Surampudi S. Effects of SEI on the kinetics of lithium intercalation[J]. Journal of Power Sources,2001,97-8:137-139. [75] Matsuo Y, Fumita K, Fukutsuka T, et al. Butyrolactone derivatives as electrolyte additives for lithium-ion batteries with graphite anodes[J]. Journal of Power Sources,2003,119:373-377. [76] Tarascon J M, Armand M. Issues and challenges facing rechargeable lithium batteries[J]. Nature,2001,414(6861):359-367. doi: 10.1038/35104644 [77] Choi N S, Profatilova I A, Kim S S, et al. Thermal reactions of lithiated graphite anode in LiPF6-based electrolyte[J]. Thermochim Acta,2008,480(1-2):10-14. doi: 10.1016/j.tca.2008.09.017 [78] Wen Y, He K, Zhu Y J, et al. Expanded graphite as superior anode for sodium-ion batteries[J]. Nature Communications,2014,5(1):1-10. [79] Ma J C, Yang C, Ma X J, et al. Improvement of alkali metal ion batteries via interlayer engineering of anodes: from graphite to graphene[J]. Nanoscale,2021,13(29):12521-12533. doi: 10.1039/D1NR01946E [80] Hu M X, Zhou H J, Gan X, et al. Ultrahigh rate sodium ion storage with nitrogen-doped expanded graphite oxide in ether-based electrolyte[J]. Journal of Materials Chemistry A,2018,6(4):1582-1589. doi: 10.1039/C7TA09631C [81] Zou L, Kang F, Li X, et al. Investigations on the modified natural graphite as anode materials in lithium ion battery[J]. Journal of Physics and Chemistry of Solids,2008,69(5):1265-1271. [82] Yang X, Zhan C, Xu D, et al. SiOx@Si-graphite microspheres for high-stable anode of lithium-ion batteries[J]. Electrochimica Acta,2022,426:140795. doi: 10.1016/j.electacta.2022.140795 [83] Carlin R T, De Long H C, Fuller J, et al. Dual intercalating molten electrolyte batteries[J]. Journal of The Electrochemical Society,1994,141(7):L73-L76. doi: 10.1149/1.2055041 [84] Hu Z, Liu Q, Zhang K, et al. All carbon dual ion batteries[J]. ACS Applied Materials & Interfaces,2018,10(42):35978-35983. [85] Yang H, Shi X, Deng T, et al. Carbon-based dual-ion battery with enhanced capacity and cycling stability[J]. ChemElectroChem,2018,5(23):3612-3618. doi: 10.1002/celc.201801108 [86] Kim K, Tang L, Muratli J M, et al. A graphite∥PTCDI aqueous dual-ion battery[J]. ChemSusChem,2022,15(5):e202102394. [87] Wang Q, Liu W, Wang S, et al. High cycling stability graphite cathode modified by artificial CEI for potassium-based dual-ion batteries[J]. Journal of Alloys and Compounds,2022,918:165436. doi: 10.1016/j.jallcom.2022.165436 [88] Ren X, Wang Y, Liu A, et al. Current progress and performance improvement of Pt/C catalysts for fuel cells[J]. Journal of Materials Chemistry A,2020,8(46):24284-24306. doi: 10.1039/D0TA08312G [89] Nitkiewicz Z, Dudek A, Wlodarczyk R. Corrosion analysis of aintered material used for low-temperature fuel cell plates[J]. Archives of Metallurgy and Materials,2011,56(1):181-186. [90] Zhang W, Jiao Z, Zhang C, et al. Diffusion of fission products in nuclear graphite: A review[J]. Nuclear Materials and Energy,2021,29:101100. doi: 10.1016/j.nme.2021.101100 [91] Zhang L, She D, Shi L. Influence of graphitization degree of nuclear graphite on HTGR reactor physics calculation[J]. Annals of Nuclear Energy,2020,143:107458. doi: 10.1016/j.anucene.2020.107458 [92] Magampa P P, Manyala N, Focke W W. Properties of graphite composites based on natural and synthetic graphite powders and a phenolic novolac binder[J]. Journal of Nuclear Materials,2013,436(1):76-83. [93] Shen K, Huang Z H, Shen W, et al. Homogenous and highly isotropic graphite produced from mesocarbon microbeads[J]. Carbon,2015,94:18-26. doi: 10.1016/j.carbon.2015.06.034 [94] Zhou X W, Tang Y P, Lu Z M, et al. Nuclear graphite for high temperature gas-cooled reactors[J]. New Carbon Materials,2017,32:193-204. doi: 10.1016/S1872-5805(17)60116-1 [95] Marsden B J, Haverty M, Bodel W, et al. Dimensional change, irradiation creep and thermal/mechanical property changes in nuclear graphite[J]. International Materials Reviews,2016,61:1-28. doi: 10.1179/1743280415Y.0000000012 [96] Paul R M, Arregui-Mena J D, Contescu C I, et al. Effect of microstructure and temperature on nuclear graphite oxidation using the 3D Random Pore Model[J]. Carbon,2022,191:132-145. doi: 10.1016/j.carbon.2022.01.041 [97] Tang C, Tang Y, Zhu J, et al. Design and manufacture of the fuel element for the 10 MW high temperature gas-cooled reactor[J]. Nuclear Engineering and Design,2002,218(1):91-102. [98] Zhou X W, Zhang K H, Yang Y, et al. Properties and microstructures of a matrix graphite for fuel elements of pebble-bed reactors after high temperature purification at different temperatures[J]. New Carbon Materials,2021,36(5):987-993. doi: 10.1016/S1872-5805(21)60048-3 [99] Wang P, Cui C, Yang D R, et al. Seed-assisted growth of cast-mono silicon for photovoltaic application: challenges and strategies[J]. Solar Rrl,2020,4(5):1-20. [100] Pupazan V, Negrila R, Bunoiu O, et al. Effects of crucible coating on the quality of multicrystalline silicon grown by a Bridgman technique[J]. Journal of Crystal Growth,2014,401:720-726. doi: 10.1016/j.jcrysgro.2014.02.038 [101] Gurusamy A, Manickam S, Perumalsamy R. Quality improvement of multi-crystalline silicon ingot by the Hot-Zone modification[J]. Journal of Crystal Growth,2022,592:126720. doi: 10.1016/j.jcrysgro.2022.126720 [102] Maboudian R, Carraro C, Senesky D G, et al. Advances in silicon carbide science and technology at the micro- and nanoscales[J]. Journal of Vacuum Science & Technology A-Vacuum Surfaces and Films,2013,31(5):50805. [103] Nakamura D, Shigetoh K. Fabrication of large-sized TaC-coated carbon crucibles for the low-cost sublimation growth of large-diameter bulk SiC crystals[J]. Japenese Journal of Applied Physics,2017,56(8):085504. doi: 10.7567/JJAP.56.085504 [104] Lee D H, Lee H T, Bae B J, et al. Effect of TaC-coated crucible on SiC single crystal growth[J]. Materials Science Forum,2014,778-780:26-30. doi: 10.4028/www.scientific.net/MSF.778-780.26 [105] Nakamura D. Simple and quick enhancement of SiC bulk crystal growth using a newly developed crucible material[J]. Applied Physics Express,2016,9(5):55507. doi: 10.7567/APEX.9.055507 -






 下载:
下载: