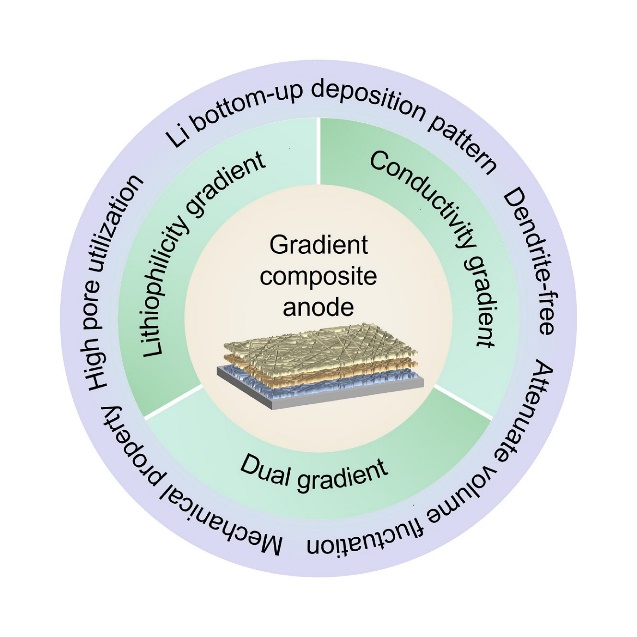
Advances in carbon-based composite anodes with gradients of lithiophilicity and conductivity used for stable lithium metal batteries
-
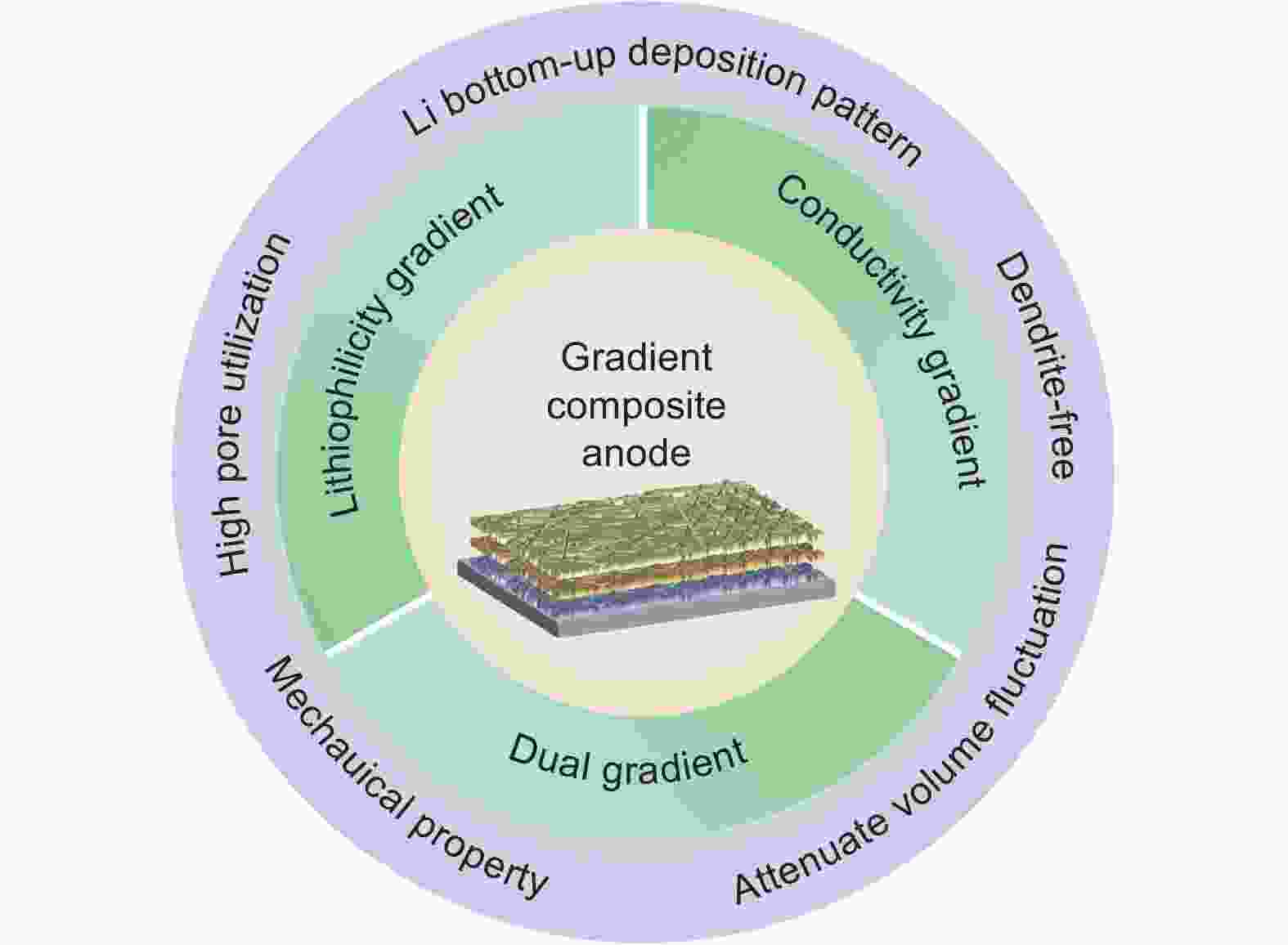
摘要: 金属锂是下一代高能量密度二次电池的理想负极材料。然而,循环期间不均匀的锂沉积/脱出过程和大体积形变,严重阻碍了金属锂负极的实用化。为了解决上述问题,引入三维骨架形成复合锂负极是一种有前景的策略,可以促进均匀的锂沉积/脱出并缓解负极体积形变。梯度复合锂负极可以引导锂自底向上沉积,从而最大限度地发挥复合锂负极的优势。由于碳骨架具有结构可调节性强、(电)化学稳定和重量轻等特点,碳基梯度复合锂负极受到了广泛关注。本文总结了碳基梯度复合锂负极的最新进展,将其分为梯度亲锂骨架、梯度导电骨架和双梯度骨架,讨论了各类梯度骨架的特点和研究进展。同时,对梯度复合锂负极的发展进行了展望,以望推动复合锂负极在高能量密度电池中的应用。Abstract: To address the issues of non-uniform Li plating/stripping and the large volume fluctuations during their repeated cycling, Li metal anodes, a composite Li anode with a three-dimensional (3D) host has been proposed as a promising strategy to improve the uniformity of Li plating/stripping and relieve volume fluctuations. One key strategy in this area is to develop 3D carbon hosts with gradients of lithiophilicity and conductivity to guide Li deposition from bottom to top, thus maximizing the positive effect of this composite Li anode. Such anodes have recently received significant attention due to the flexibility, adjustability, (electro)chemical stability and light weight of the carbon hosts. This review summarizes recent advances in these anodes, categorizing them into those that have hosts with a lithiophilicity gradient, hosts with a conductivity gradient, and those that have both. Latest research findings are discussed and these different anode categories are reviewed. Prospects for the design of such anodes to promote the use of Li metal anodes in high-energy-density rechargeable batteries are presented.
-
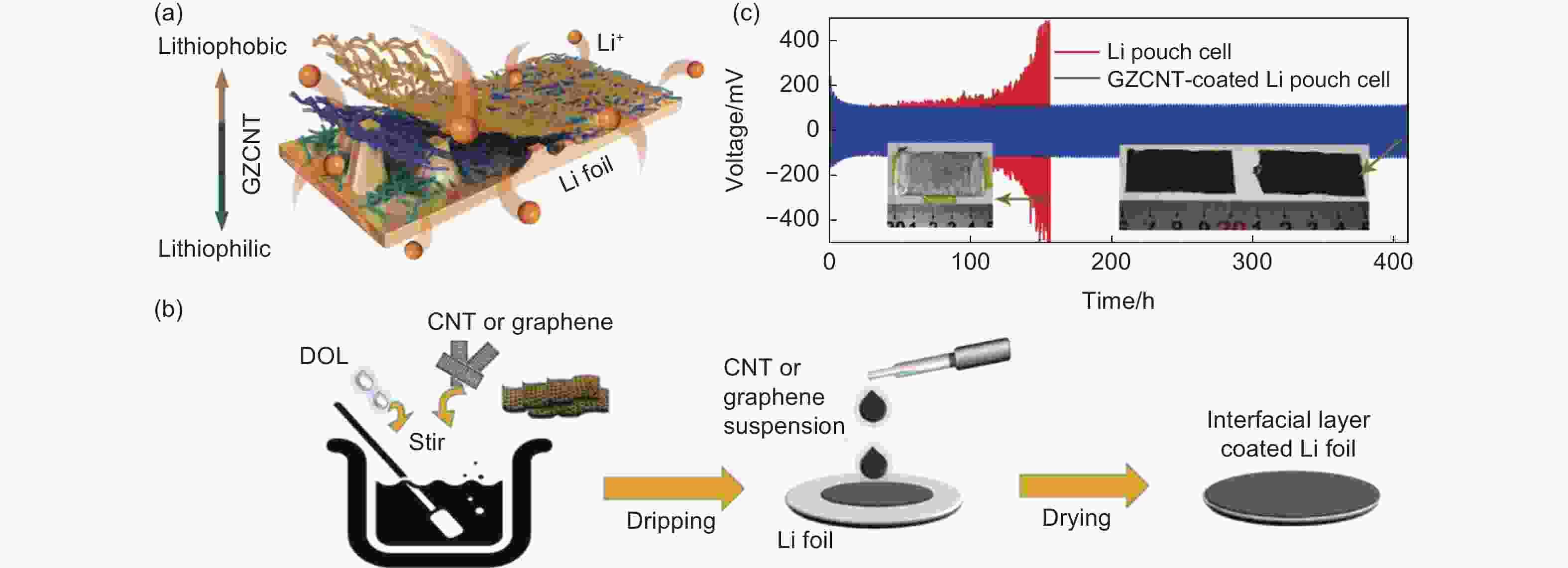
Figure 2. (a) Schematic diagram of the GZCNT host-coated Li foil[96]. (b) Fabrication of interfacial layer of GZCNT. (c) Cycling performance of symmetric pouch cells. Inset: digital photograph of Li foil, and GZCNT-coated Li foils in pouch cells after 100 and 200 cycles, respectively. (Reprinted with permission)
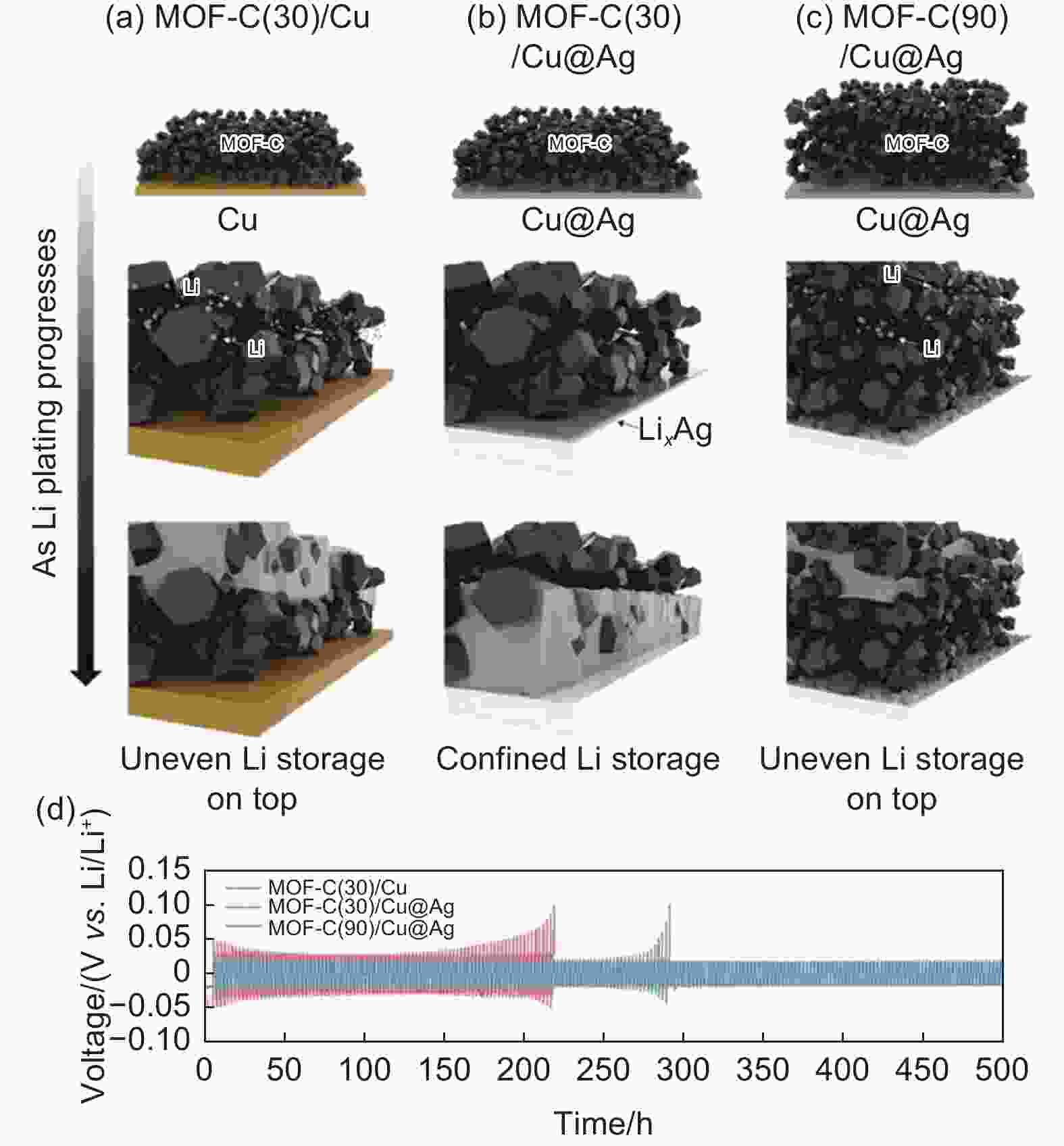
Figure 3. Schematic diagram of Li plating behaviors of (a) MOF-C(30)/Cu, (b) MOF-C(30)/Cu@Ag, and (c) MOF-C(90)/Cu@Ag[98]. (d) Cycling performance of MOF-C(t)/X//Li pouch cells at 0.4 mAh cm−2 and 0.4 mA cm−2. (Reprinted with permission)
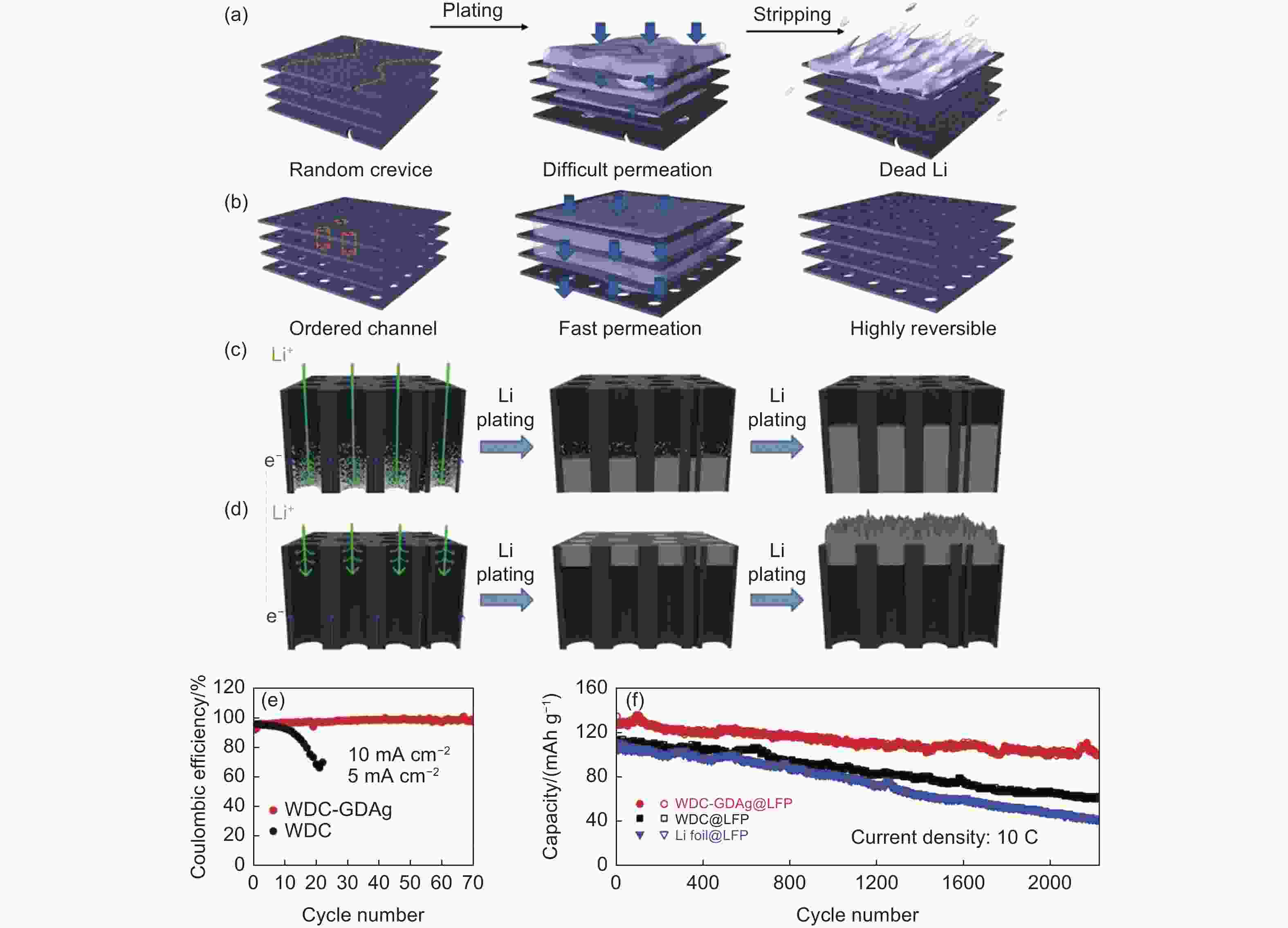
Figure 4. Schematic diagram of Li plating/stripping of (a) pure GO and (b) holey GO[102]. Schematic diagram of Li plating behaviors of (c) WDC-GDAg and (d) WDC[105]. (e) CE of WDC-GDAg and WDC half cells at 10.0 mA cm−2 and 5.0 mAh cm−2. (f) Cycling performance of WDC-GDAg//LFP, WDC//LFP, and Li//LFP at 10 C. (Reprinted with permission)
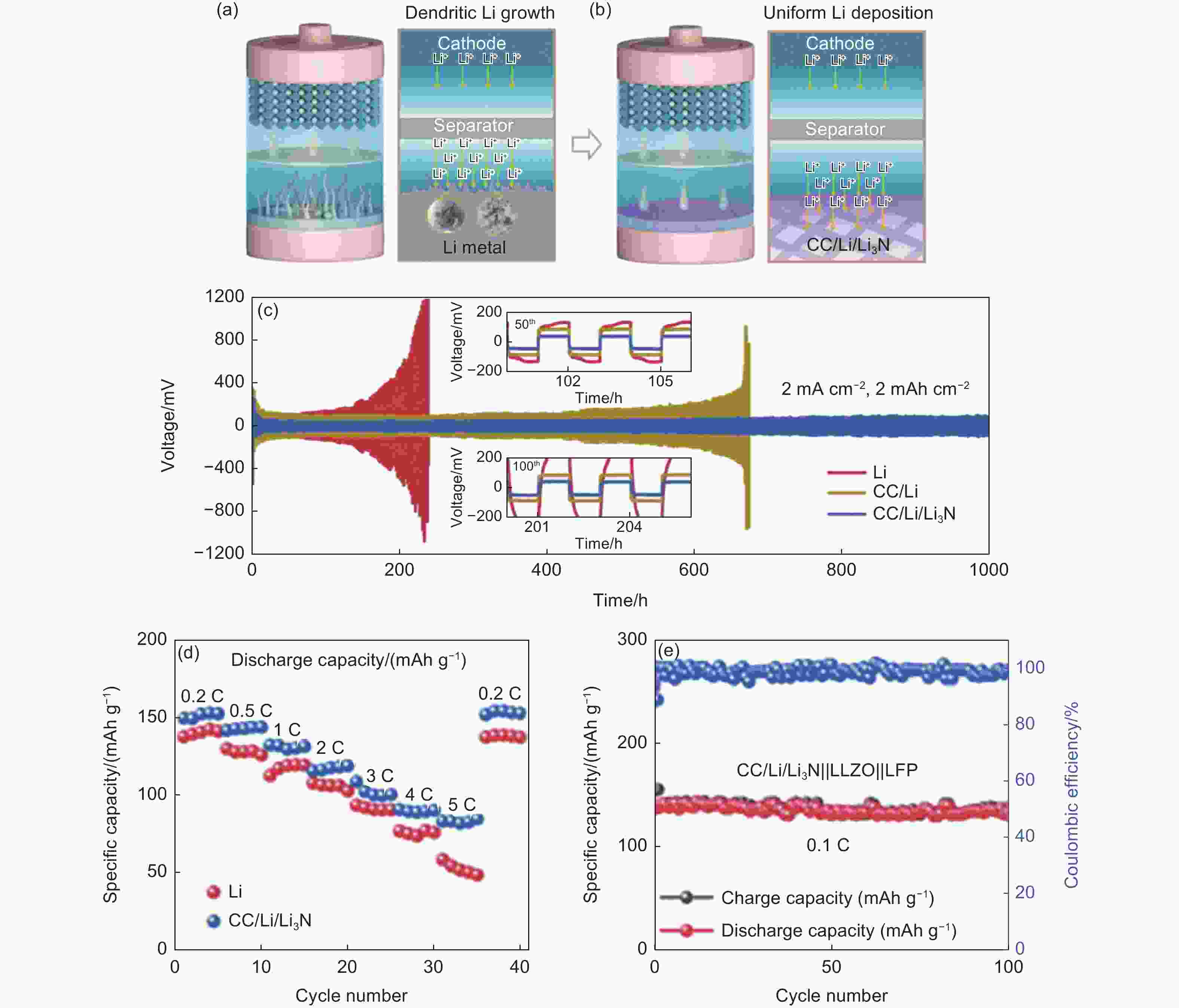
Figure 5. Schematic diagram of Li plating behaviors of (a) Li//LFP and (b) CC/Li/Li3N//LFP[106]. (c) Voltage-time curves of bare Li, CC/Li, and CC/Li/Li3N symmetric cells at 2.0 mA cm−2 and 2.0 mAh cm−2. (d) Rate performance of CC/Li/Li3N//LFP and bare Li//LFP full cell. (e) Cycling performance of CC/Li/Li3N//LLZO//LFP at 0.1 C for 100 cycles. (Reprinted with permission)
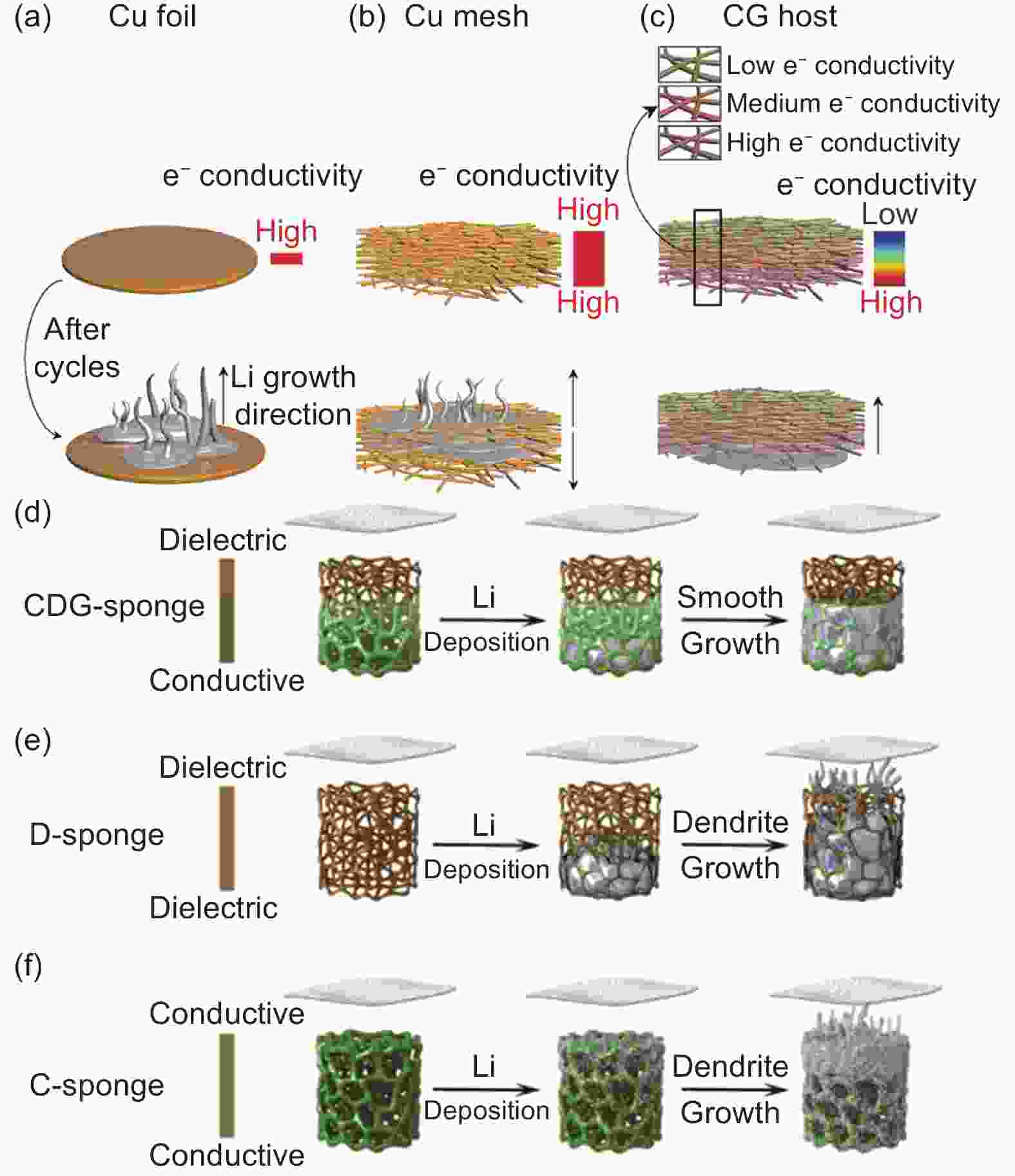
Figure 6. Schematic diagram of Li plating behaviors of (a) Cu foil, (b) Cu mesh, and (c) CG host[107]. Schematic diagram of Li plating behaviors of (d) CDG-sponge: bottom deposition without dendrite formation. (e) D-sponge: bottom deposition with dendrite formation. (f) C-sponge: top deposition with dendrite formation near the separator[108]. (Reprinted with permission)
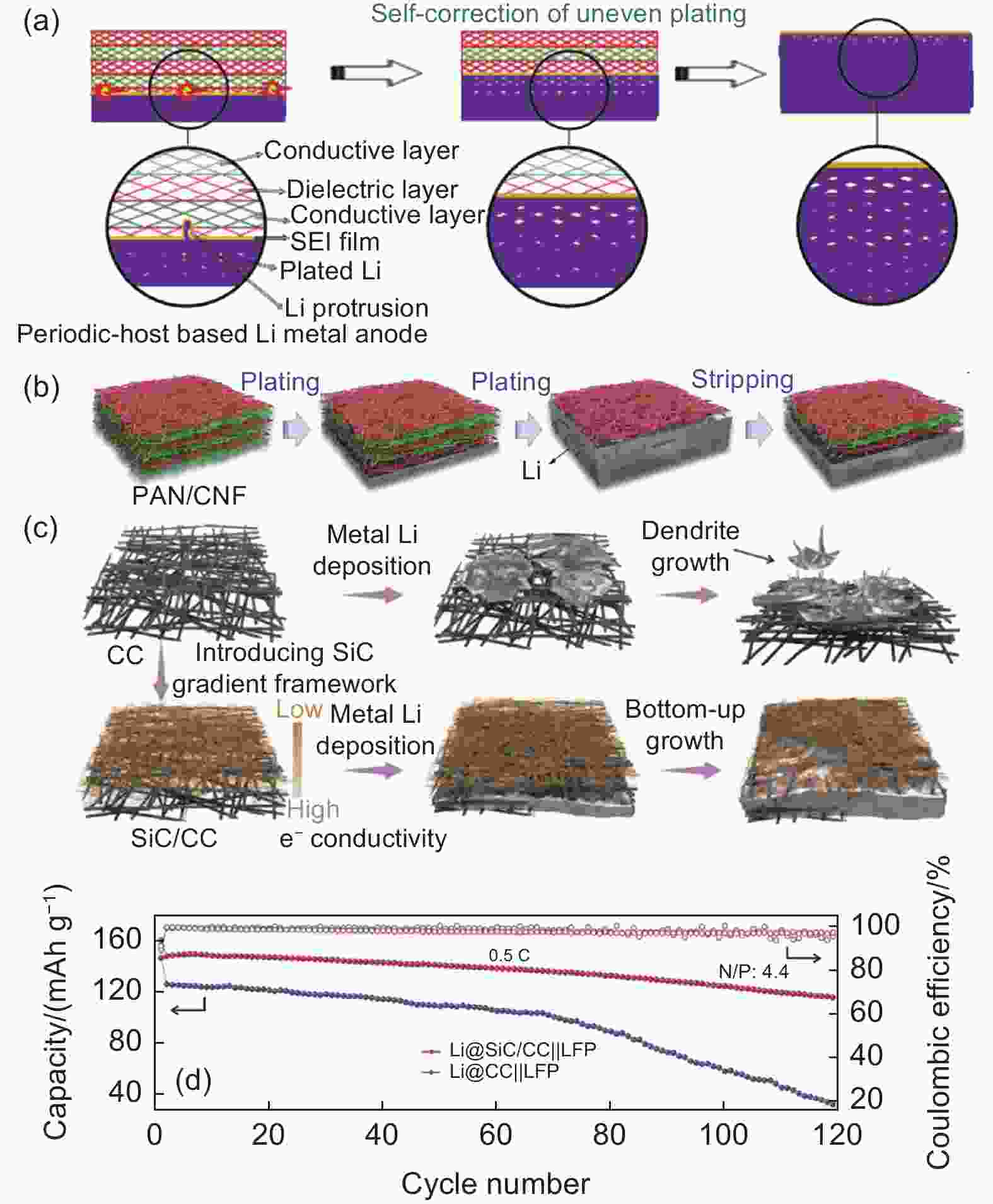
Figure 7. (a) Schematic diagram of Li metal evolution in periodic-host based Li metal anode showing a “self-correction” behavior, and (b) schematic diagram of Li plating/stripping of the periodic PAN/CNF host[109]. (c) Schematic diagram of Li plating behaviors of CC and SiC/CC host, and (d) cycling performance of the Li@Cu//LFP and Li@SiC/CC//LFP full cell at 0.5 C[110]. (Reprinted with permission)
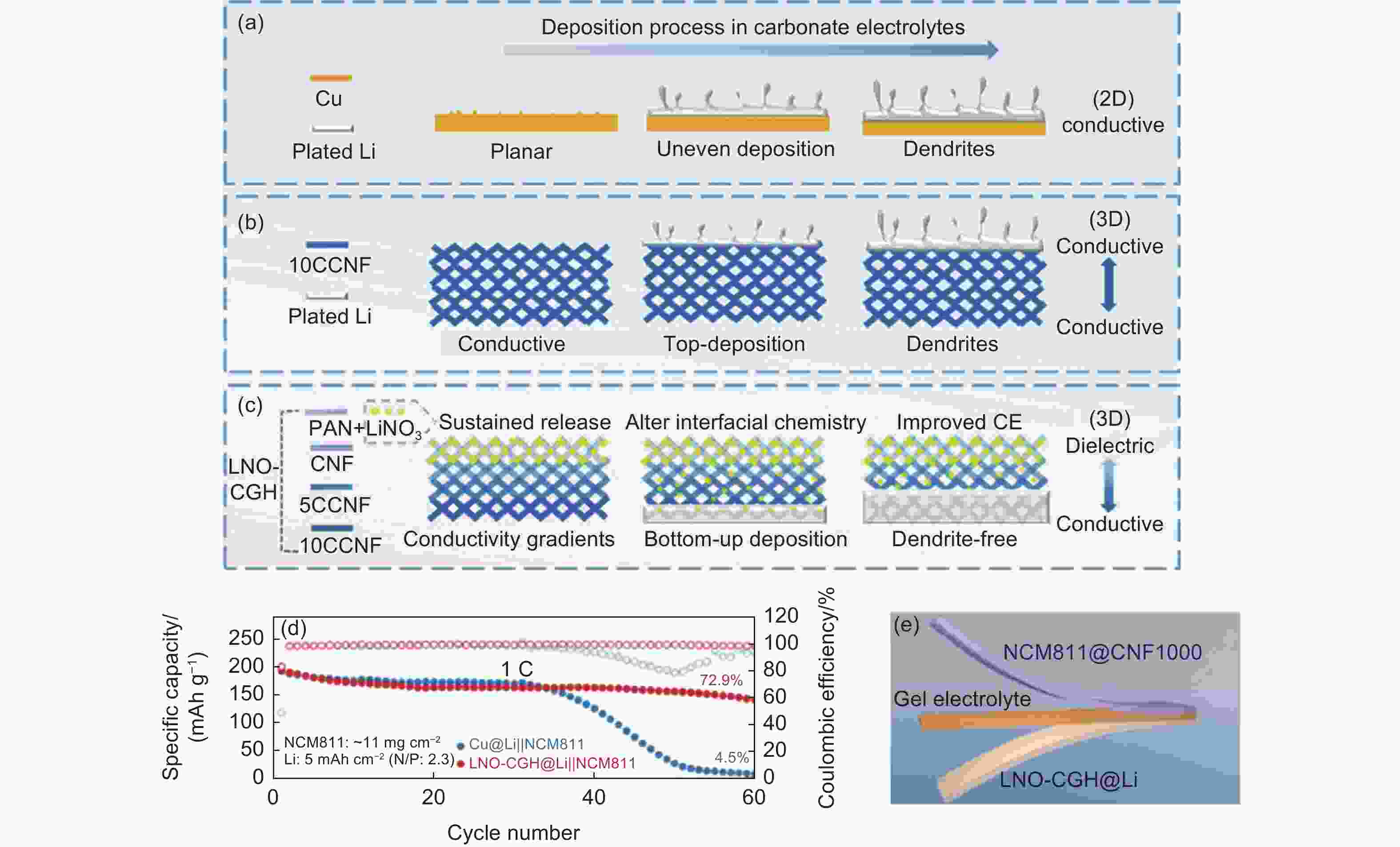
Figure 8. Schematic diagram of Li plating behaviors of (a) Cu foil, (b) 10CCNF and (c) LNO-CGH[112]. (d) Cycling performance of Cu@Li//NCM811 and LNO-CGH@Li//NCM811 full cell at 1 C. (e) Schematic of flexible quasi-solid Li metal battery (LNO-CGH@Li//Gel electrolytes//NCM811@PCNF1000). (Reprinted with permission)
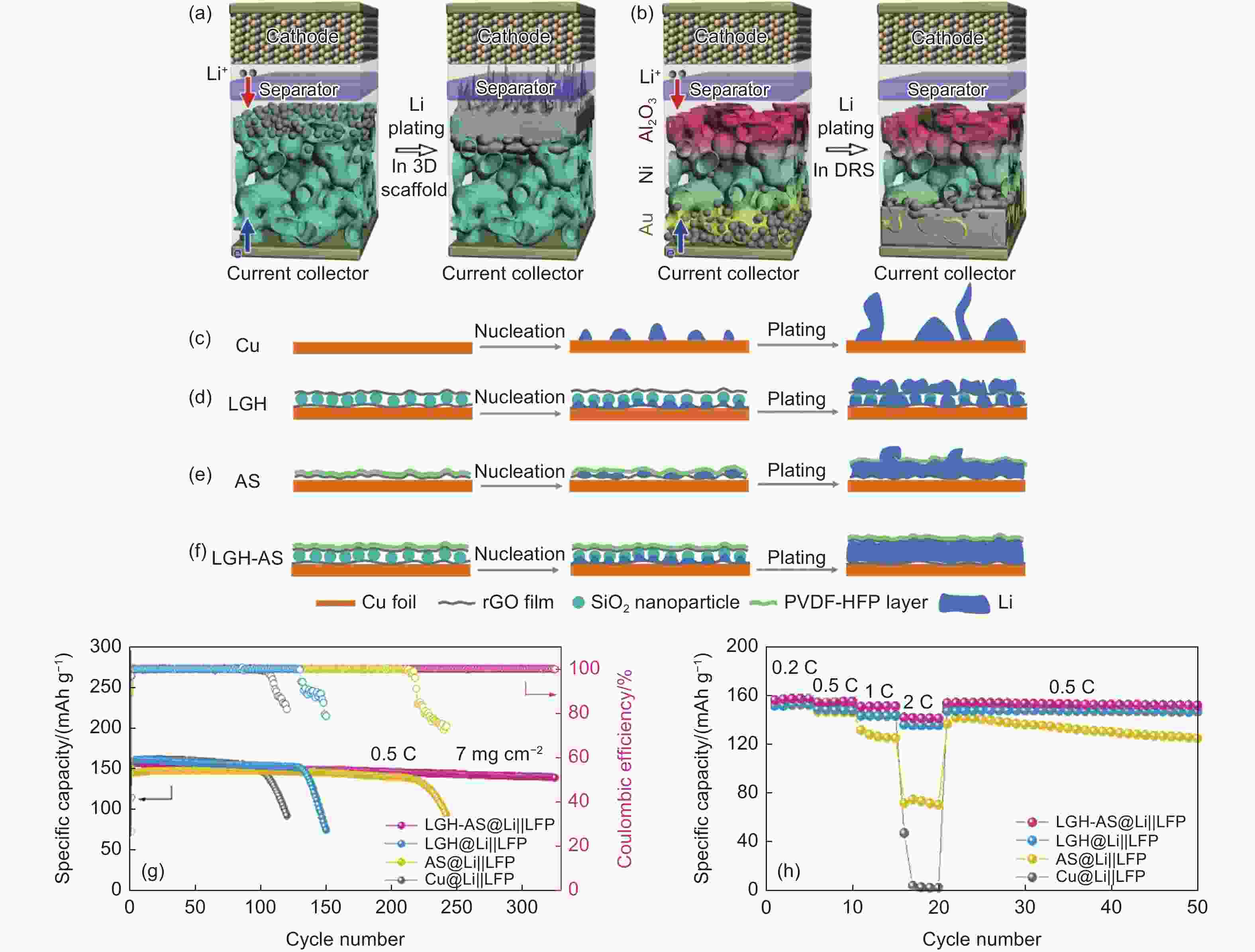
Figure 9. Schematic diagram of Li plating behaviors of (a) BNS following the top-growth mode and (b) DRS following the bottom–up mode[113]. Schematic diagram of Li plating behaviors of (c) Cu with uneven nucleation and dendrite. (d) LGH with inter deposition and low pore utilization. (e) AS with first deposition between rGO and PVDF-HFP layer and then on AS because of the fragile polymer coating, and (f) LGH-AS with Li deposition from bottom to top in SiO2-embedded interlayer[114]. (g) Cycling performance of full cells at 0.5 C. (h) Rate performance of full cells from 0.2 to 2.0 C. (Reprinted with permission)
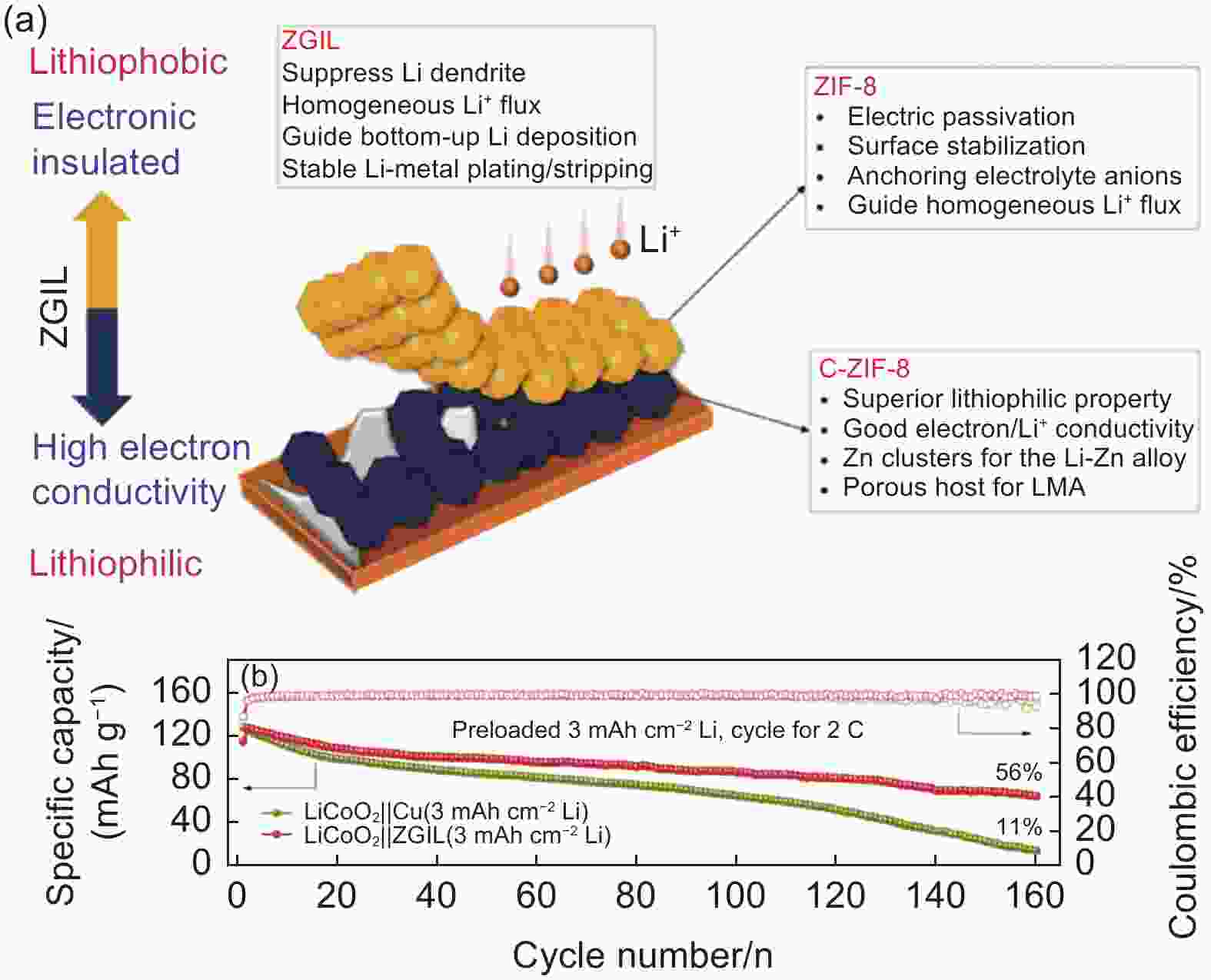
Figure 10. (a) Schematic diagram of ZGIL coated Cu foil. (b) Cycling performance of LiCoO2//ZGIL and LiCoO2//Cu full cell at 2 C[117]. (Reprinted with permission)
Table 1. Comparison of full cell and pouch cell performance of carbon-based gradient composite anodes
Type Sample Coin cell Pouch cell Electrolyte Ref. Gradient lithiophilicity GZCNT GZCNT-coated Li//S
(2.5 mg cm−2)
0.2 C, 200 cyclesSymmetric GZCNT-coated
Li pouch cell
1.0 mA cm−2,
1.0 mAh cm−2,
200 cycles0.6 mol L−1 LiTFSI in DOL/DME
(1∶1, w/w) with 0.4 mol L−1 LiNO3[96] GSCP GSCP@Li (5.0 mAh)//LTO
(~1.2 mg cm−2)
10 C, 5000 cyclesN.A. 1.0 mol L−1 LiTFSI in DOL/DME
(1∶1, v/v) with 1 wt% LiNO3[97] MOF-C(t)/X N.A. MOF-C(t)/X//Li
0.5 mA cm−2, 1 mAh cm−2,
nucleation overpotential of
MOF-C(30)/Cu@Ag was 6 mV1.0 mol L−1 LiTFSI in DOL/DME
(1∶1, v/v) with 1 wt% LiNO3[98] Holey GO/Li holey GO/Li//LFP
(2.0-2.4 mg cm−2)
1 C, 500 cycles
holey GO/Li//NCM811
(3.4-4.7 mg cm−2)
0.5 C, 150 cyclesHoley GO/Li//SPAN
(S: 9.75 mg)
0.2 C, 100 cycles1.0 mol L−1 LiPF6 in EC/DEC (1∶1, v/v)
with 1% VC and 10% FEC[102] WDC-GDAg WDC-GDAg//LFP (4 mg cm−2)
10 C, 2000 cyclesN.A. 1.0 mol L−1 LiTFSI in
DOL/Dimethoxymethane
(1∶1, v/v) with 1 wt% LiNO3[105] CC/Li/Li3N CC/Li/Li3N//LFP
(2.0 mg cm−2)
0.5 C, 150 cyclesSolid-state battery at
room temperature:
CC/Li/Li3N//LLZO//LFP
(2.0 mg cm−2)
0.1 C, 100 cycles1.0 mol L−1 LiPF6 in
EC/EMC (3∶7, v/v)[106] Gradient
conductivityCG CG@Li//NCM811
(~1.1 mAh cm−2, N/P ratio of 3.6)
1 C, 100 cyclesN.A. 1.0 mol L−1 LiPF6 in EC/DEC
(1∶1, v/v) with 1 wt% VC[107] CDG-sponge Li@CDG-sponge
(3.0 mAh cm−2)//LFP (4.0 mg cm−2)
1 C, 400 cyclesN.A. 1.0 mol L−1 LiTFSI in DOL/DME
(1∶1, v/v) with 2 wt% LiNO3[108] PAN/CNF Li@PAN/CNF//NCM811
(~20.0 mg cm−2)
1 C, 100 cyclesN.A. 1.0 mol L−1 LiPF6 in EC/EMC/DMC
(1∶1∶1, v/v/v)[109] SiC/CC Li@SiC/CC (3.0 mAh cm−2)//
LFP (4.5 mg cm−2)
0.5 C, 120 cyclesN.A. 1.0 mol L−1 LiTFSI in DOL/DME
(1∶1, v/v) with 2 wt% LiNO3[110] LNO-CGH LNO-CGH@Li (5.0 mAh cm−2)//
NCM811 (11 mg cm−2)
1 C, 60 cyclesFlexible quasi-solid Li
metal battery: LNO-
CGH@Li (5.0 mAh cm−2)//
Gel electrolyte//
NCM811@PCNF1000
(5.5 mg cm−2)
1 C, 100 cycles1.0 mol L−1 LiPF6 in EC/DEC
(1∶1, w/w) with 5% FEC[112] Dual gradient LGH-AS LGH-AS@Li (4.0 mAh cm−2)//
LFP (7.0 mg cm−2)
0.5 C, 300 cyclesN.A. 1.0 mol L−1 LiTFSI in DOL/DME
(1∶1, v/v) with 1% LiNO3[114] ZGIL ZGIL (3.0 mAh cm−2)//
LiCoO2 (4.6 mg cm−2)
2 C, 160 cyclesN.A. 1.0 mol L−1 LiPF6 in EC/DEC
(1∶1, w/w) with 30% FEC[117] G-HGA G-HGA@Li (3.0 mAh cm−2)//
LFP (2.7 mAh cm−2)
0.5 C, 175 cyclesN.A. 1.0 mol L−1 LiTFSI in DOL/DME
(1∶1, v/v) with 2% LiNO3[120] -
[1] Zhang X Q, Li T, Li B Q, et al. A sustainable solid electrolyte interphase for high-energy-density lithium metal batteries underpractical conditions[J]. Angewandte Chemie International Edition,2020,59(8):3252-3257. doi: 10.1002/anie.201911724 [2] Zhou M Y, Ding X Q, Ding J F, et al. Quantifying the apparent electron transfer number of electrolyte decomposition reactions in anode-free batteries[J]. Joule,2022,6(9):2122-2137. doi: 10.1016/j.joule.2022.07.003 [3] Wang Y Y, Zhang X Q, Zhou M Y, et al. Mechanism, quantitative characterization, and inhibition of corrosion in lithium batteries[J]. Nano Research Energy,2023,2:e9120046. doi: 10.26599/NRE.2023.9120046 [4] Schmuch R, Wagner R, Hörpel G, et al. Performance and cost of materials for lithium-based rechargeable automotive batteries[J]. Nature Energy,2018,3(4):267-278. doi: 10.1038/s41560-018-0107-2 [5] Yuan H D, Ding X F, Liu T F, et al. A review of concepts and contributions in lithium metal anode development[J]. Materials Today,2022,53:173-196. doi: 10.1016/j.mattod.2022.01.015 [6] Sun W, Li Q M, Xiao P, et al. Effect of a compressed separator on the electrochemical performance of Li-ion battery[J]. Journal of Power Sources,2023,563:232835. doi: 10.1016/j.jpowsour.2023.232835 [7] Bi C X, Hou L P, Li Z, et al. Protecting lithium metal anodes in lithium–sulfur batteries: A review[J]. Energy Material Advances,2023,4:0010. doi: 10.34133/energymatadv.0010 [8] Yuan S Y, Kong T Y, Zhang Y Y, et al. Advanced electrolyte design for high-energy-density Li-metal batteries under practical conditions[J]. Angewandte Chemie International Edition,2021,60(49):25624-25638. doi: 10.1002/anie.202108397 [9] Shen X, Zhang X Q, Ding F, et al. Advanced electrode materials in lithium batteries: Retrospect and prospect[J]. Energy Material Advances,2021,2021:1205324. [10] Fu W B, Kim D, Wang F J, et al. Stabilizing cathodes and interphases for next-generation Li-ion batteries[J]. Journal of Power Sources,2023,561:232738. doi: 10.1016/j.jpowsour.2023.232738 [11] Liu H, Cheng X B, Chong Y, et al. Advanced electrode processing of lithium ion batteries: A review of powder technology in battery fabrication[J]. Particuology,2021,57:56-71. doi: 10.1016/j.partic.2020.12.003 [12] Cheng X B, Liu H, Yuan H, et al. A perspective on sustainable energy materials for lithium batteries[J]. SusMat,2021,1(1):38-50. doi: 10.1002/sus2.4 [13] Zhang X, Yang Y A, Zhou Z. Towards practical lithium-metal anodes[J]. Chemical Society Reviews,2020,49(10):3040-3071. doi: 10.1039/C9CS00838A [14] Sun S Y, Yao N, Jin C B, et al. The crucial role of electrode potential of a working anode in dictating the structural evolution of solid electrolyte interphase[J]. Angewandte Chemie International Edition,2022,61(42):e202208743. [15] Shi P, Hou L P, Jin C B, et al. A successive conversion-deintercalation delithiation mechanism for practical composite lithium anodes[J]. Journal of the American Chemical Society,2022,144(1):212-218. doi: 10.1021/jacs.1c08606 [16] Zheng H F, Zhang Q F, Chen Q L, et al. 3D lithiophilic–lithiophobic–lithiophilic dual-gradient porous skeleton for highly stable lithium metal anode[J]. Journal of Materials Chemistry A,2020,8(1):313-322. doi: 10.1039/C9TA09505E [17] Nanda S, Gupta A, Manthiram A. Anode-free full cells: A pathway to high-energy density lithium-metal batteries[J]. Advanced Energy Materials,2021,11(2):2000804. doi: 10.1002/aenm.202000804 [18] Jin C B, Shi P, Zhang X Q, et al. Advances in carbon materials for stable lithium metal batteries[J]. New Carbon Materials,2022,37(1):1-24. doi: 10.1016/S1872-5805(22)60573-0 [19] Zhang H Y, Ju S L, Xia G L, et al. Identifying the positive role of lithium hydride in stabilizing Li metal anodes[J]. Science Advances,2022,8(3):eabl8245. doi: 10.1126/sciadv.abl8245 [20] Liu H, Cheng X B, Yan C, et al. A perspective on energy chemistry of low-temperature lithium metal batteries[J]. iEnergy,2022,1(1):72-81. doi: 10.23919/IEN.2022.0003 [21] Jiang Z P, Li A, Meng C Y, et al. Strategies and challenges of carbon materials in the practical applications of lithium metal anode: a review[J]. Physical Chemistry Chemical Physics,2022,24(43):26356-26370. doi: 10.1039/D2CP04032H [22] Bi C X, Zhao M, Hou L P, et al. Anode material options toward 500 Wh kg−1 lithium-sulfur batteries[J]. Advanced Science,2022,9(2):2103910. doi: 10.1002/advs.202103910 [23] Vincent C A. Lithium batteries: A 50-year perspective, 1959–2009[J]. Solid State Ionics,2000,134:159-167. doi: 10.1016/S0167-2738(00)00723-2 [24] Zhang Y F, Wu S C, Yang Q H. Revisiting lithium metal anodes from a dynamic and realistic perspective[J]. EnergyChem,2021,3(5):100063. doi: 10.1016/j.enchem.2021.100063 [25] Pu J, Li J C, Shen Z H, et al. Interlayer lithium plating in Au nanoparticles pillared reduced graphene oxide for lithium metal anodes[J]. Advanced Functional Materials,2018,28(41):1804133. doi: 10.1002/adfm.201804133 [26] Shen L, Shi P R, Hao X G, et al. Progress on lithium dendrite suppression strategies from the interior to exterior by hierarchical structure designs[J]. Small,2020,16(26):2000699. doi: 10.1002/smll.202000699 [27] Kim J Y, Chae O B, Wu M, et al. Extraordinary dendrite-free Li deposition on highly uniform facet wrinkled Cu substrates in carbonate electrolytes[J]. Nano Energy,2021,82:105736. doi: 10.1016/j.nanoen.2020.105736 [28] Zhao Q, Chen X, Hou W, et al. A facile, scalable, high stability Lithium metal anode[J]. SusMat,2022,2(1):104-112. doi: 10.1002/sus2.43 [29] Huang X Y, Xue J J, Xiao M, et al. Comprehensive evaluation of safety performance and failure mechanism analysis for lithium sulfur pouch cells[J]. Energy Storage Materials,2020,30:87-97. doi: 10.1016/j.ensm.2020.04.035 [30] Jeong J, Chun J, Lim W G, et al. Mesoporous carbon host material for stable lithium metal anode[J]. Nanoscale,2020,12(22):11818-11824. doi: 10.1039/D0NR02258F [31] Kim J, Lee J, Yun J, et al. Functionality of dual-phase lithium storage in a porous carbon host for lithium-metal anode[J]. Advanced Functional Materials,2020,30(15):1910538. doi: 10.1002/adfm.201910538 [32] Li J W, Kong Z, Liu X X, et al. Strategies to anode protection in lithium metal battery: A review[J]. InfoMat,2021,3(12):1333-1363. doi: 10.1002/inf2.12189 [33] Zhang X Q, Jin Q, Nan Y L, et al. Electrolyte structure of lithium polysulfides with anti-reductive solvent shells for practical lithium-sulfur batteries[J]. Angewandte Chemie International Edition,2021,133(28):15631-15637. doi: 10.1002/ange.202103470 [34] Hou L P, Yao N, Xie J, et al. Modification of nitrate ion enables stable solid electrolyte interphase in lithium metal batteries[J]. Angewandte Chemie International Edition,2022,61(20):e202201406. [35] Zhang Q K, Zhang X Q, Hou L P, et al. Regulating solvation structure in nonflammable amide-based electrolytes for long-cycling andsafe lithium metal batteries[J]. Advanced Energy Materials,2022,12(24):2200139. doi: 10.1002/aenm.202200139 [36] Li X Y, Feng S, Zhao C X, et al. Regulating lithium salt to inhibit surface gelation on an electrocatalyst for high-energy-density lithium–sulfur batteries[J]. Journal of the American Chemical Society,2022,144(32):14638-14646. doi: 10.1021/jacs.2c04176 [37] Wang Z, Hou L P, Li Z, et al. Highly soluble organic nitrate additives for practical lithium metal batteries[J]. Carbon Energy,2023,5:e283. [38] Guo F, Chen X, Hou Y H, et al. Improved cycling of Li| | NMC811 batteries under practical conditions by a localized high-concentration electrolyte[J]. Small, 2023, 19: 2207290. [39] Ye S F, Wang L F, Liu F F, et al. g-C3N4 derivative artificial organic/inorganic composite solid electrolyte interphase layer for stable lithium metal anode[J]. Advanced Energy Materials,2020,10(44):2002647. doi: 10.1002/aenm.202002647 [40] Beichel W, Skrotzki J, Klose P, et al. An artificial SEI layer based on an inorganic coordination polymer with self-healing ability for long-lived rechargeable lithium-metal batteries[J]. Batteries & Supercaps,2022,5(2):e202100347. [41] Chen C, Liang Q W, Wang G, et al. Grain-boundary-rich artificial SEI layer for high-rate lithium metal anodes[J]. Advanced Functional Materials,2022,32(4):2107249. doi: 10.1002/adfm.202107249 [42] Li Z, Hou L P, Zhang X Q, et al. A nafion protective layer for stabilizing lithium metal anodes in working lithium-sulfur batteries[J]. Battery Energy,2022,1(3):20220006. doi: 10.1002/bte2.20220006 [43] Zhao F F, Zhai P B, Wei Y, et al. Constructing artificial SEI layer on lithiophilic MXene surface for high-performance lithium metal anodes[J]. Advanced Science,2022,9(6):2103930. doi: 10.1002/advs.202103930 [44] Zhang R, Chen B, Shi C S, et al. Decreasing interfacial pitfalls with self-grown sheet-like Li2S artificial solid-electrolyte interphase for enhanced cycling performance of lithium metal anode[J]. Small, 2023, 19: 2208095. [45] Liu B, Zhang Y, Pan G X, et al. Ordered lithiophilic sites to regulate Li plating/stripping behavior for superior lithium metal anodes[J]. Journal of Materials Chemistry A,2019,7(38):21794-21801. doi: 10.1039/C9TA09502K [46] Luo L, Li J Y, Asl H Y, et al. A 3D lithiophilic Mo2N-modified carbon nanofiber architecture for dendrite-free lithium-metal anodes in a full cell[J]. Advanced Materials,2019,31(48):1904537. doi: 10.1002/adma.201904537 [47] Chen X R, Li B Q, Zhao C X, et al. Synergetic coupling of lithiophilic sites and conductive scaffolds for dendrite-free lithium metal anodes[J]. Small Methods,2020,4(6):1900177. doi: 10.1002/smtd.201900177 [48] Wu Q P, Zheng Y J, Guan X, et al. Dynamical SEI reinforced by open-architecture MOF film with stereoscopic lithiophilic sites for high-performance lithium-metal batteries[J]. Advanced Functional Materials,2021,31(28):2101034. doi: 10.1002/adfm.202101034 [49] Jeon Y, Kim J, Jang H, et al. Argentophilic pyridinic nitrogen for embedding lithiophilic silver nanoparticles in a three-dimensional carbon scaffold for reversible lithium plating/stripping[J]. Journal of Materials Chemistry A,2022,10(4):1768-1779. doi: 10.1039/D1TA07306K [50] Liu Y H, Sun J M, Hu X Q, et al. Lithiophilic sites dependency of lithium deposition in Li metal host anodes[J]. Nano Energy,2022,94:106883. doi: 10.1016/j.nanoen.2021.106883 [51] Ahn C H, Kim J J, Yang W S, et al. Multiple functional biomolecule-based metal-organic-framework-reinforced polyethylene oxide composite electrolytes for high-performance solid-state lithium batteries[J]. Journal of Power Sources,2023,557:232528. doi: 10.1016/j.jpowsour.2022.232528 [52] Cui J, Du Y F, Zhao L, et al. Thermal stable poly-dioxolane based electrolytes via a robust crosslinked network for dendrite-free solid-state Li-metal batteries[J]. Chemical Engineering Journal,2023,461:141973. doi: 10.1016/j.cej.2023.141973 [53] Guo Y, Wu S C, He Y B, et al. Solid-state lithium batteries: Safety and prospects[J]. eScience,2022,2(2):138-163. doi: 10.1016/j.esci.2022.02.008 [54] Wu L Q, Wang Y T, Guo X W, et al. Interface science in polymer-based composite solid electrolytes in lithium metal batteries[J]. SusMat,2022,2(3):264-292. doi: 10.1002/sus2.67 [55] Zhao T, Kou W J, Zhang Y F, et al. Laminar composite solid electrolyte with succinonitrile-penetrating metal-organic framework (MOF) for stable anode interface in solid-state lithium metal battery[J]. Journal of Power Sources,2023,554:232349. doi: 10.1016/j.jpowsour.2022.232349 [56] Wu J Y, Rao Z X, Liu X T, et al. Composite lithium metal anodes with lithiophilic and low-tortuosity scaffold enabling ultrahigh currents and capacities in carbonate electrolytes[J]. Advanced Functional Materials,2021,31(14):2009961. doi: 10.1002/adfm.202009961 [57] Chen W Y, Li S P, Wang C H, et al. Targeted deposition in a lithiophilic silver-modified 3D Cu host for lithium-metal anodes[J]. Energy & Environmental Materials, 2022, https://doi.org/10.1002/eem2.12412. [58] Liu P, Su H, Liu Y, et al. LiBr-LiF-rich solid-electrolyte interface layer on lithiophilic 3D framework for enhanced lithium metal anode[J]. Small Structures,2022,3(6):2200010. doi: 10.1002/sstr.202200010 [59] Yu S C, Wu Z H, Holoubek J, et al. A fiber-based 3D lithium host for lean electrolyte lithium metal batteries[J]. Advanced Science,2022,9(10):2104829. doi: 10.1002/advs.202104829 [60] Hu L, Deng J J, Liang Q H, et al. Engineering current collectors for advanced alkali metal anodes: A review and perspective[J]. EcoMat,2023,5(1):e12269. [61] Liu H R, Zhao M L, Bai X D, et al. An ultrafast rechargeable and high durability lithium metal battery using composite electrolyte with the three-dimensional inorganic framework by Li6.4La3Zr1.4Ta0.6O12 surface functionalization[J]. eTransportation,2023,16:100234. [62] Ni S Y, Tan S S, An Q Y, et al. Three dimensional porous frameworks for lithium dendrite suppression[J]. Journal of Energy Chemistry,2020,44:73-89. doi: 10.1016/j.jechem.2019.09.031 [63] Liu Y H, Li Y F, Sun J M, et al. Present and future of functionalized Cu current collectors for stabilizing lithium metal anodes[J]. Nano Research Energy,2023,2:e9120048. doi: 10.26599/NRE.2023.9120048 [64] Jiang Z P, Jin L, Zeng Z Q, et al. Facile preparation of a stable 3D host for lithium metal anodes[J]. Chemical Communications,2020,56(68):9898-9900. doi: 10.1039/D0CC03864D [65] Zhan Y X, Shi P, Zhang X Q, et al. The insights of lithium metal plating/stripping in porous hosts: Progress and perspectives[J]. Energy Technology,2021,9(2):2000700. doi: 10.1002/ente.202000700 [66] Liu F F, Jin Z Z, Hu Z X, et al. Constructing Co3O4 nanowires on carbon fiber film as a lithiophilic host for stable lithium metal anodes[J]. Chemistry – An Asian Journal,2020,15(7):1057-1066. doi: 10.1002/asia.201901668 [67] Cha E, Yun J H, Ponraj R, et al. A mechanistic review of lithiophilic materials: Resolving lithium dendrites and advancing lithium metal-based batteries[J]. Materials Chemistry Frontiers,2021,5(17):6294-6314. doi: 10.1039/D1QM00579K [68] Wang J P, Lan D N, Chen G Y, et al. Built-in stable lithiophilic sites in 3D current collectors for dendrite free Li metal electrode[J]. Small,2022,18(27):2106718. doi: 10.1002/smll.202106718 [69] Shi P, Zhang X Q, Shen X, et al. A review of composite lithium metal anode for practical applications[J]. Advanced Materials Technologies,2020,5(1):1900806. doi: 10.1002/admt.201900806 [70] Wang H, Wu J Y, Yuan L X, et al. Stable lithium metal anode enabled by 3D soft host[J]. ACS Applied Materials & Interfaces,2020,12(25):28337-28344. [71] Zhan Y X, Shi P, Jin C B, et al. Regulating the two-stage accumulation mechanism of inactive lithium for practical composite lithium metal anodes[J]. Advanced Functional Materials,2022,32(43):2206834. doi: 10.1002/adfm.202206834 [72] Zhan Y X, Shi P, Zhang R, et al. Deciphering the effect of electrical conductivity of hosts on lithium deposition in composite lithium metal anodes[J]. Advanced Energy Materials,2021,11(37):2101654. doi: 10.1002/aenm.202101654 [73] Yun J, Park B K, Won E S, et al. Bottom-up lithium growth triggered by interfacial activity gradient on porous framework for lithium-metal anode[J]. ACS Energy Letters,2020,5(10):3108-3114. doi: 10.1021/acsenergylett.0c01619 [74] Lai Y M, Zhao Y, Cai W P, et al. Constructing ionic gradient and lithiophilic interphase for high-rate li-metal anode[J]. Small,2019,15(47):1905171. doi: 10.1002/smll.201905171 [75] Fang Y J, Zhang S L, Wu Z P, et al. A highly stable lithium metal anode enabled by Ag nanoparticle–embedded nitrogen-doped carbon macroporous fibers[J]. Science Advances,2021,7(21):eabg3626. doi: 10.1126/sciadv.abg3626 [76] Ma Y, Wu F, Chen N, et al. Reversing the dendrite growth direction and eliminating the concentration polarization via an internal electric field for stable lithium metal anodes[J]. Chemical Science,2022,13(32):9277-9284. doi: 10.1039/D2SC03313E [77] Lu L L, Ge J, Yang J N, et al. Free-standing copper nanowire network current collector for improving lithium anode performance[J]. Nano Letters,2016,16(7):4431-4437. doi: 10.1021/acs.nanolett.6b01581 [78] Huang S B, Zhang H, Fan L Z. Confined lithium deposition triggered by an integrated gradient scaffold for a lithium-metal anode[J]. ACS Applied Materials & Interfaces,2022,14(15):17539-17546. [79] Xiang J W, Yuan L X, Shen Y, et al. Improved rechargeability of lithium metal anode via controlling lithium-ion flux[J]. Advanced Energy Materials,2018,8(36):1802352. doi: 10.1002/aenm.201802352 [80] Zhou S, Zhang Y F, Chai S M, et al. Incorporation of LiF into functionalized polymer fiber networks enabling high capacity and high rate cycling of lithium metal composite anodes[J]. Chemical Engineering Journal,2021,404:126508. doi: 10.1016/j.cej.2020.126508 [81] Tan Y H, Lu G X, Zheng J H, et al. Lithium fluoride in electrolyte for stable and safe lithium-metal batteries[J]. Advanced Materials,2021,33(42):2102134. doi: 10.1002/adma.202102134 [82] Pathak R, Chen K, Wu F, et al. Advanced strategies for the development of porous carbon as a Li host/current collector for lithium metal batteries[J]. Energy Storage Materials,2021,41:448-465. doi: 10.1016/j.ensm.2021.06.015 [83] Zhou J H, Wu F, Wei G L, et al. Lithium-metal host anodes with top-to-bottom lithiophilic gradients for prolonged cycling of rechargeable lithium batteries[J]. Journal of Power Sources,2021,495:229773. doi: 10.1016/j.jpowsour.2021.229773 [84] Lang J L, Jin Y, Luo X Y, et al. Surface graphited carbon scaffold enables simple and scalable fabrication of 3D composite lithium metal anode[J]. Journal of Materials Chemistry A,2017,5(36):19168-19174. doi: 10.1039/C7TA05997C [85] Liu L, Yin Y X, Li J Y, et al. Uniform lithium nucleation/growth induced by lightweight nitrogen-doped graphitic carbon foams for high-performance lithium metal anodes[J]. Advanced Materials,2018,30(10):1706216. doi: 10.1002/adma.201706216 [86] Wang A X, Tang S, Kong D B, et al. Bending-tolerant anodes for lithium-metal batteries[J]. Advanced Materials,2018,30(1):1703891. doi: 10.1002/adma.201703891 [87] Chen J Y, Wang Y Z, Li S J, et al. Porous metal current collectors for alkali metal batteries[J]. Advanced Science,2023,10(1):2205695. doi: 10.1002/advs.202205695 [88] Jin S, Sun Z W, Guo Y L, et al. High areal capacity and lithium utilization in anodes made of covalently connected graphite microtubes[J]. Advanced Materials,2017,29(38):1700783. doi: 10.1002/adma.201700783 [89] Ye H, Xin S, Yin Y X, et al. Stable Li plating/stripping electrochemistry realized by a hybrid Li reservoir in spherical carbon granules with 3D conducting skeletons[J]. Journal of the American Chemical Society,2017,139(16):5916-5922. doi: 10.1021/jacs.7b01763 [90] Yang Y, Zhao M, Geng H B, et al. Three-dimensional graphene/Ag aerogel for durable and stable Li metal anodes in carbonate-based electrolytes[J]. Chemistry-A European Journal,2019,25(19):5036-5042. doi: 10.1002/chem.201805941 [91] Mao H, Yu W, Cai Z Y, et al. Current-density regulating lithium metal directional deposition for long cycle-life Li metal batteries[J]. Angewandte Chemie International Edition,2021,60:19306. doi: 10.1002/anie.202105831 [92] Park S, Jin H J, Yun Y S. Advances in the design of 3D-structured electrode materials for lithium-metal anodes[J]. Advanced Materials,2020,32(51):2002193. doi: 10.1002/adma.202002193 [93] Guan W Q, Hu X Q, Liu Y H, et al. Advances in the emerging gradient designs of Li metal hosts[J]. Research, 2022, 2022: 9846537. [94] Chen X R, Chen X, Yan C, et al. Role of lithiophilic metal sites in lithium metal anodes[J]. Energy & Fuels,2021,35(15):12746-12752. [95] Chen X R, Zhao B C, Yan C, et al. Review on Li deposition in working batteries: from nucleation to early growth[J]. Advanced Materials,2021,33(8):2004128. doi: 10.1002/adma.202004128 [96] Zhang H M, Liao X B, Guan Y P, et al. Lithiophilic-lithiophobic gradient interfacial layer for a highly stable lithium metal anode[J]. Nature Communications,2018,9(1):3729. doi: 10.1038/s41467-018-06126-z [97] Yan X L, Zhang Q F, Xu W J, et al. Bottom-top channeling Li nucleation and growth by a gradient lithiophilic 3D conductive host for highly stable Li-metal anodes[J]. Journal of Materials Chemistry A,2020,8(4):1678-1686. doi: 10.1039/C9TA11311H [98] Yun J, Shin H R, Won E S, et al. Confined Li metal storage in porous carbon frameworks promoted by strong Li-substrate interaction[J]. Chemical Engineering Journal,2022,430:132897. doi: 10.1016/j.cej.2021.132897 [99] Lin D, Liu Y, Liang Z, et al. Layered reduced graphene oxide with nanoscale interlayer gaps as a stable host for lithium metal anodes[J]. Nature Nanotechnology,2016,11(7):626-632. doi: 10.1038/nnano.2016.32 [100] Chen H, Pei A, Wan J Y, et al. Tortuosity effects in lithium-metal host anodes[J]. Joule,2020,4(4):938-952. doi: 10.1016/j.joule.2020.03.008 [101] Huang Q K, Ni S Y, Jiao M L, et al. Aligned carbon-based electrodes for fast-charging batteries: a review[J]. Small,2021,17(48):2007676. doi: 10.1002/smll.202007676 [102] Ni S Y, Sheng J Z, Zhang C, et al. Dendrite-free lithium deposition and stripping regulated by aligned microchannels for stable lithium metal batteries[J]. Advanced Functional Materials,2022,32(21):2200682. doi: 10.1002/adfm.202200682 [103] Wang G, Xiong X H, Zou P J, et al. Lithiated zinc oxide nanorod arrays on copper current collectors for robust Li metal anodes[J]. Chemical Engineering Journal,2019,378:122243. doi: 10.1016/j.cej.2019.122243 [104] Tantratian K, Cao D X, Abdelaziz A, et al. Stable Li metal anode enabled by space confinement and uniform curvature through lithiophilic nanotube arrays[J]. Advanced Energy Materials,2020,10(5):1902819. doi: 10.1002/aenm.201902819 [105] Zhu Z X, Liu B, Qian Y, et al. Spatially distributed lithiophilic gradient in low-tortuosity 3D hosts via capillary action for high-performance Li metal anodes[J]. Advanced Energy Materials,2022,13(7):2203687. [106] Cao W Z, Chen W M, Lu M, et al. In situ generation of Li3N concentration gradient in 3D carbon-based lithium anodes towards highly-stable lithium metal batteries[J]. Journal of Energy Chemistry,2023,76:648-656. doi: 10.1016/j.jechem.2022.09.025 [107] Hong S H, Jung D H, Kim J H, et al. Electrical conductivity gradient based on heterofibrous scaffolds for stable lithium-metal batteries[J]. Advanced Functional Materials,2020,30(14):1908868. doi: 10.1002/adfm.201908868 [108] Li J, Zou P C, Chiang S W, et al. A conductive-dielectric gradient framework for stable lithium metal anode[J]. Energy Storage Materials,2020,24:700-706. doi: 10.1016/j.ensm.2019.06.019 [109] Zou P C, Chiang S W, Zhan H C, et al. A periodic “self-correction” scheme for synchronizing lithium plating/stripping at ultrahigh cycling capacity[J]. Advanced Functional Materials,2020,30(21):1910532. doi: 10.1002/adfm.201910532 [110] Sun B, Zhang Q, Xu W L, et al. A gradient topology host for a dendrite-free lithium metal anode[J]. Nano Energy,2022,94:106937. doi: 10.1016/j.nanoen.2022.106937 [111] Sun X J, Shao C Z, Zhang F, et al. SiC nanofibers as long-life lithium-ion battery anode materials[J]. Frontiers in Chemistry,2018,6:166. doi: 10.3389/fchem.2018.00166 [112] Zhou S, Fu C Y, Chang Z, et al. Conductivity gradient modulator induced highly reversible Li anodes in carbonate electrolytes for high-voltage lithium-metal batteries[J]. Energy Storage Materials,2022,47:482-490. doi: 10.1016/j.ensm.2022.02.033 [113] Pu J, Li J C, Zhang K, et al. Conductivity and lithiophilicity gradients guide lithium deposition to mitigate short circuits[J]. Nature Communications,2019,10(1):1896. doi: 10.1038/s41467-019-09932-1 [114] Cai Q C, Qin X Y, Lin K, et al. Gradient structure design of a floatable host for preferential lithium deposition[J]. Nano Letters,2021,21(24):10252-10259. doi: 10.1021/acs.nanolett.1c03207 [115] Guo B K, Shu J, Wang Z X, et al. Electrochemical reduction of nano-SiO2 in hard carbon as anode material for lithium ion batteries[J]. Electrochemistry Communications,2008,10(12):1876-1878. doi: 10.1016/j.elecom.2008.09.032 [116] Lai L S, Yeong Y F, Ani N C, et al. Effect of synthesis parameters on the formation of zeolitic imidazolate framework 8 (ZIF-8) nanoparticles for CO2 adsorption[J]. Particulate Science and Technology,2014,32(5):520-528. doi: 10.1080/02726351.2014.920445 [117] Shi Y B, Yang S H, Sun X R, et al. Metal-organic framework derived gradient interfacial layer for stable lithium metal anode[J]. Electrochimica Acta,2022,417:140333. doi: 10.1016/j.electacta.2022.140333 [118] Chen H, Yang Y F, Boyle D T, et al. Free-standing ultrathin lithium metal-graphene oxide host foils with controllable thickness for lithium batteries[J]. Nature Energy,2021,6(8):790-798. doi: 10.1038/s41560-021-00833-6 [119] Lin L D, Qin K, Hu Y S, et al. A better choice to achieve high volumetric energy density: anode-free lithium-metal batteries[J]. Advanced Materials,2022,34(23):2110323. doi: 10.1002/adma.202110323 [120] Yu Z, Yang Q Y, Xue W J, et al. Uniformizing the lithium deposition by gradient lithiophilicity and conductivity for stable lithium-metal batteries[J]. Nanoscale,2023,15(9):4529-4535. doi: 10.1039/D2NR06210K [121] Shen X, Zhang R, Shi P, et al. How does external pressure shape Li dendrites in Li metal batteries?[J]. Advanced Energy Materials,2021,11(10):2003416. doi: 10.1002/aenm.202003416 [122] Liu H, Sun X, Cheng X B, et al. Working principles of lithium metal anode in pouch cells[J]. Advanced Energy Materials,2022,12(47):2202518. doi: 10.1002/aenm.202202518 [123] Noh H J, Lee M H, Kim B G, et al. 3D carbon-based porous anode with a pore-size gradient for high-performance lithium metal batteries[J]. ACS Applied Materials & Interfaces,2021,13(46):55227-55234. [124] Liu H, Di J, Wang P, et al. A novel design of 3D carbon host for stable lithium metal anode[J]. Carbon Energy,2022,4(4):654-664. doi: 10.1002/cey2.193 [125] Zhan Y X, Shi P, Ma X X, et al. Failure mechanism of lithiophilic sites in composite lithium metal anode under practical conditions[J]. Advanced Energy Materials,2022,12(2):2103291. doi: 10.1002/aenm.202103291 -





 下载:
下载: