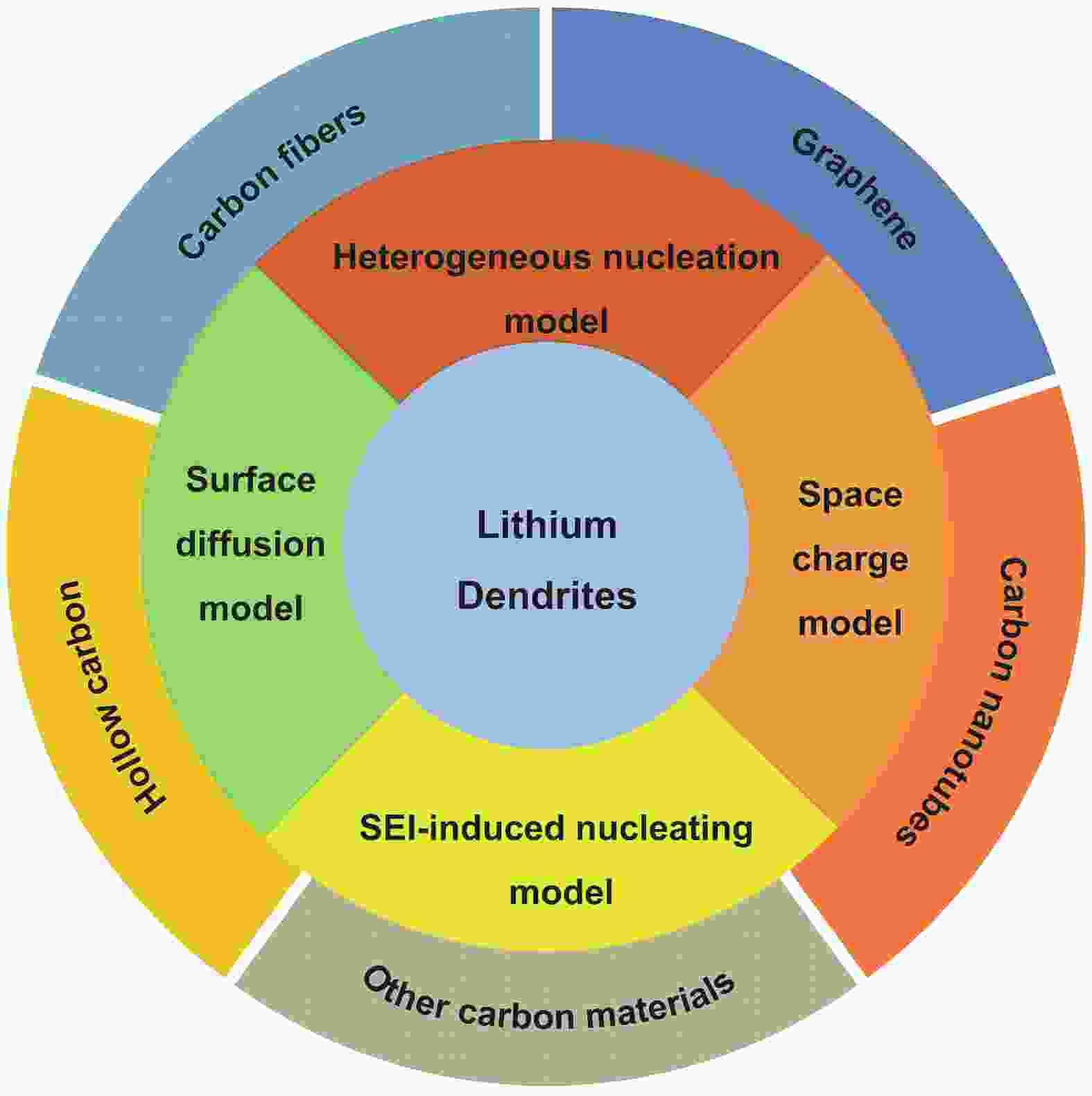
The role of carbon materials in suppressing dendrite formation in lithium metal batteries
-
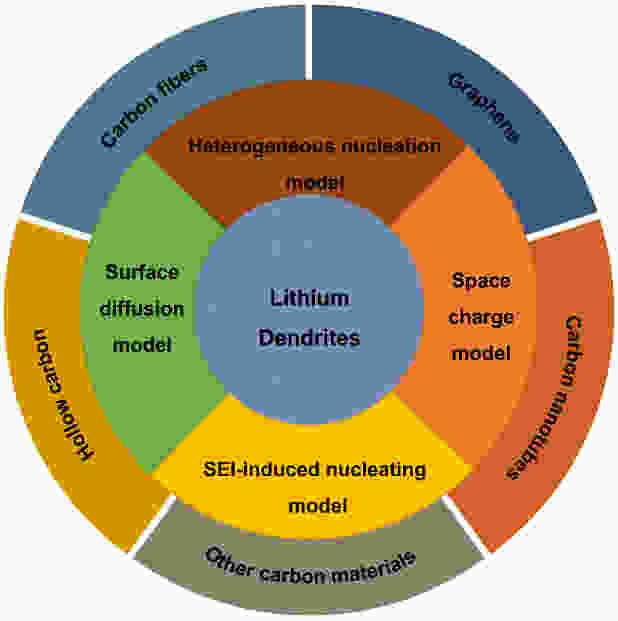
摘要: 锂金属有低还原电位和高比容量,是高能量密度电池负极材料的最终选择。然而,不可控的锂枝晶导致差的循环寿命和安全问题,对锂金属电池的实际应用构成了重大挑战。对锂枝晶形成机制的理解对于指导抑制枝晶生长的有效策略的发展至关重要。本文首先整理了近年来为理解锂枝晶形成过程而提出的几个具有代表性和影响力的模型。炭材料凭借其优异的导电性、电化学稳定性、机械性能和可塑性,已被广泛开发用于防止锂枝晶的形成。基于对上述主流锂枝晶形成模型的全面理解和认识,进一步全面汇总了近年来石墨烯、碳纳米管、炭纤维和空心炭等先进炭材料在应对锂枝晶形成方面的优势和策略。最后,对炭材料抑制锂枝晶的局限性和未来研究方向进行了总结和展望,为进一步开发高性能金属锂电极用先进炭材料提供了参考。Abstract: This review highlights several recent models of Li dendrite formation that have been proposed. Based on the comprehensive understanding and insight gained from these models, carbon materials have been developed to prevent the formation of Li dendrites by virtue of their exceptional electrical conductivity, electrochemical stability, mechanical properties and mouldability. A comprehensive review of the advantages of using carbon materials, such as graphene, carbon nanotubes, carbon fibers and hollow carbons, to deal with the formation of Li dendrites in recent years is provided. Finally, the limitations of carbon materials and future research directions for inhibiting Li dendrite formation are summarized as a reference for the development of new carbon materials for high-performance Li metal anodes.
-
Key words:
- Lithium metal batteries /
- Lithium dendrite /
- Carbon materials /
- SEI /
- Substrates
-
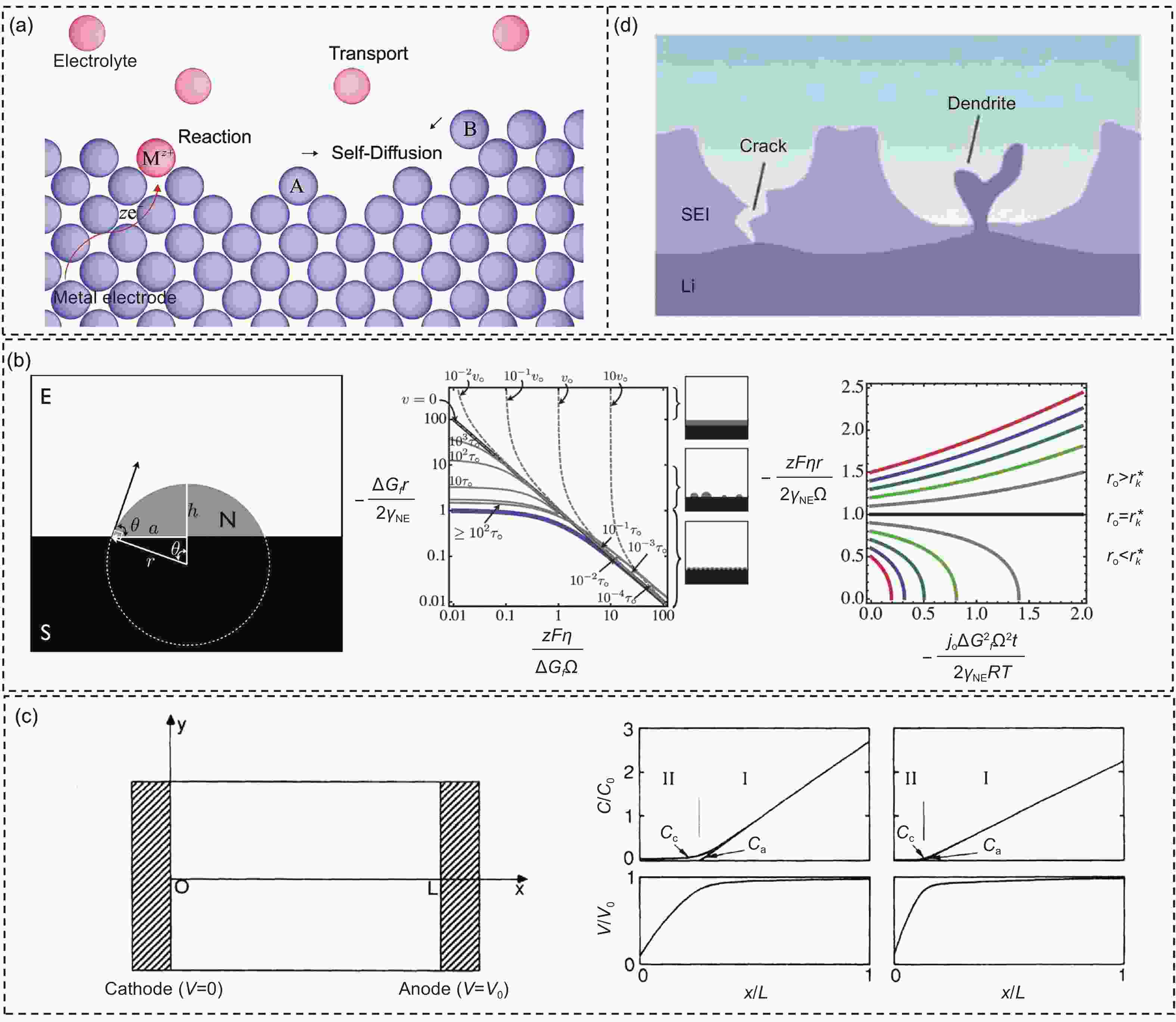
Figure 2. (a) Schematic illustration of electrodeposition[47]. (b) Schematic illustration of a nucleus with a spherical cap shape. Behavioral patterns during the early stages of nucleation and growth, and the growth electrodeposit size varies with time[50]. (c) Scheme of the cell. The relationship between ion concentration Cc, and Ca, and the electrostatic potential[51]. (d) A nonuniform SEI renders irregular Li deposition[56]. (Reprinted with permission)
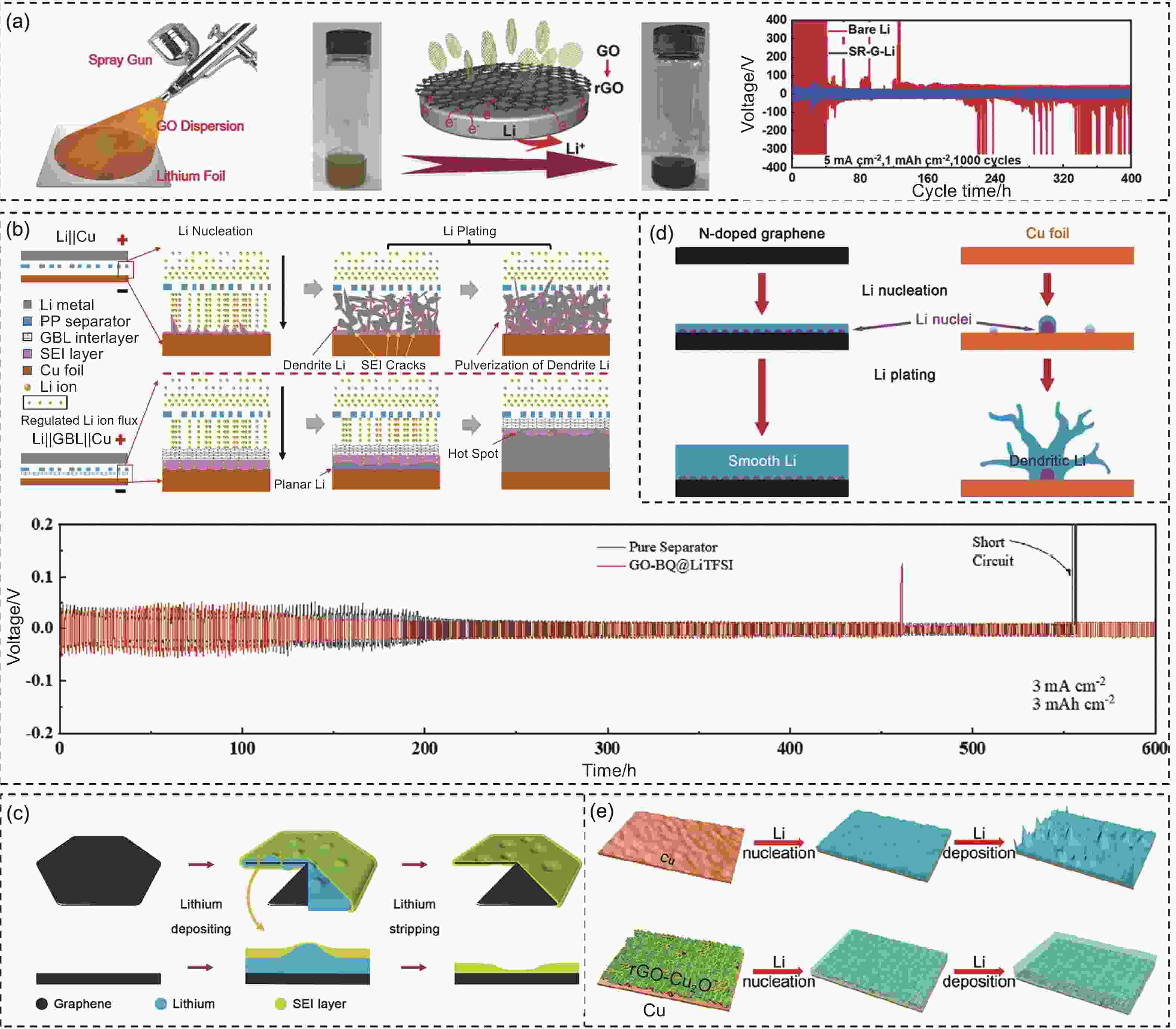
Figure 3. (a) Schematic diagram of the spray process and optical image of the reduction process for the preparation of SR-G-Li. The cycling stability of the bare Li and SR-G-Li electrodes at 5 mA cm−2[72]. (b) Schematic illustration of Li deposition on Cu and GBL||Cu. The cycling performance at 3.0 mA cm−2 with 3 mAh cm−2[74]. (c) Schematic illustration of Li depositing and stripping on graphene substrate[75]. (d) Comparative diagram of Li nucleation and plating on N-doped graphene and copper foil[77]. (e) Schematic diagram of Li nucleation and deposition on the Cu foil and rGO−Cu2O/Cu[80]. (Reprinted with permission)
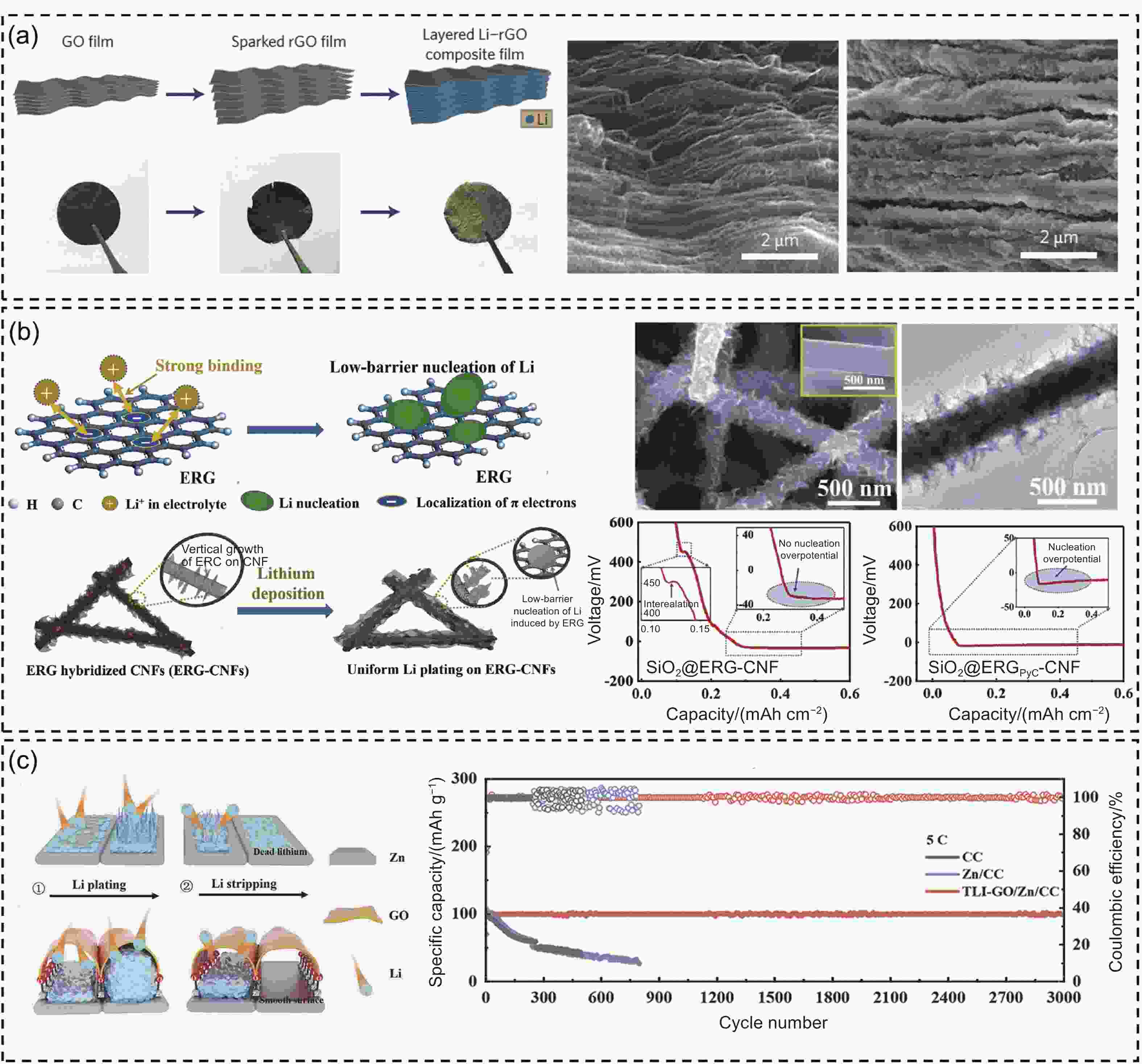
Figure 4. (a) Schematic diagram of preparation of the sparked rGO films and layered Li–rGO composite film, and the corresponding SEM images[81]. (b) Schematic diagram of the 0 V overpotential Li nucleation on ERG and uniform Li plating on the ERG-hybridized 3D CNF substrate. Deposition curves of Li at 0.2 mA cm−2 on SiO2@ERG-CNF and SiO2@ERGPyC-CNF[86]. (c) Schematic illustration of Li plating and stripping on bare Zn and the Zn with tent-like nanocavities interface anchored by Zn-O-C bond. The cycling stability of Li||LFP cells with different Li anodes at 5 C[7]. (Reprinted with permission)
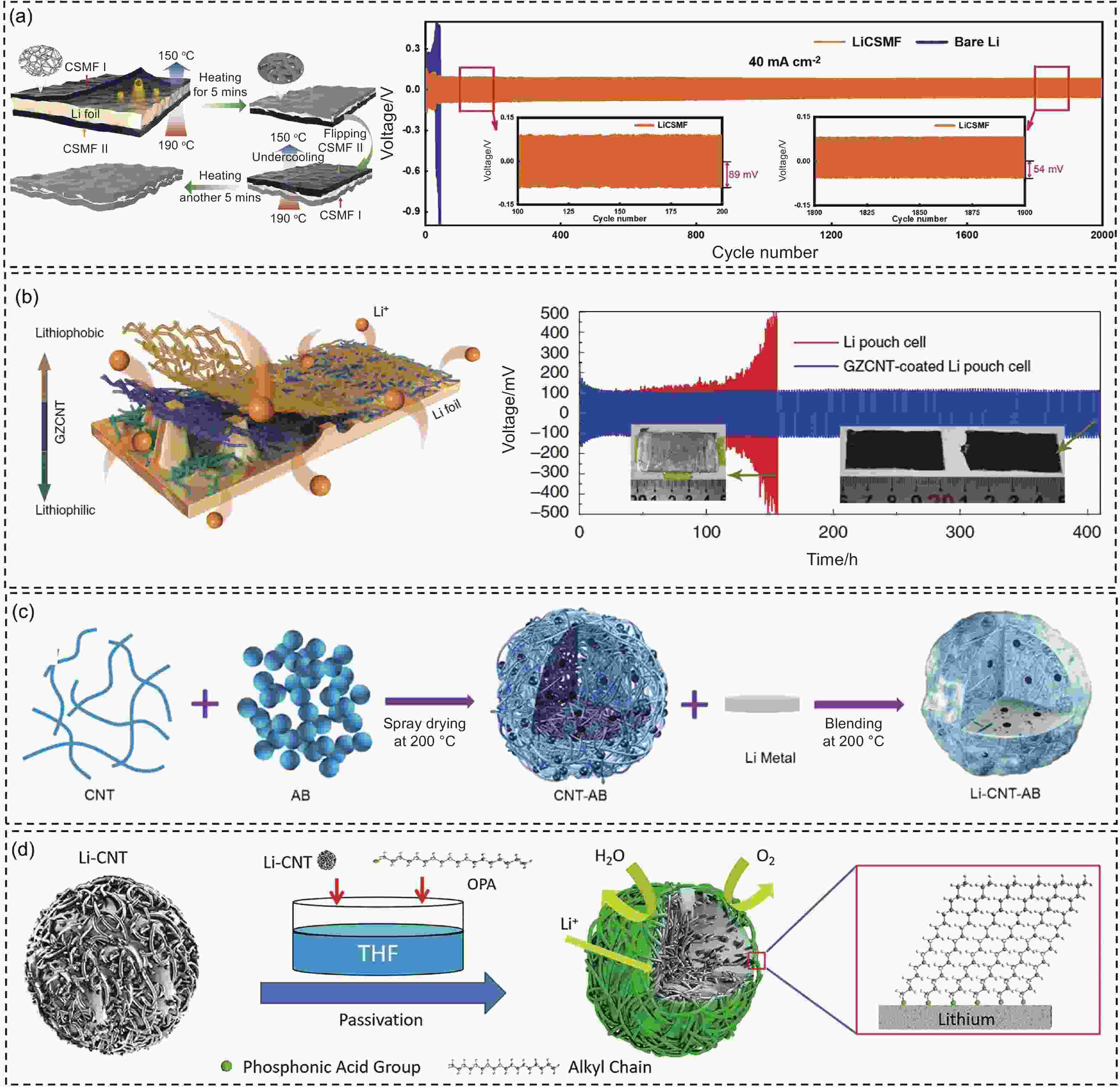
Figure 5. (a) Schematic diagram of preparation of the LiCSMF electrode. Cycling performance at 40 mA cm−2 with 2 mAh cm−2[88]. (b) Schematic illustration of Li deposition on Li foils with GZCNT interface, and the cycling stability of the pouch cells of Li foils with/without GZCNT at 1 mA cm–2 with 1 mAh cm–2[91]. (c) Schematic of preparation of the Li-CNT-AB[93]. (d) Schematic diagram of the preparation of OPA−Li−CNT sphere by molecular self-assembly [94]. (Reprinted with permission)
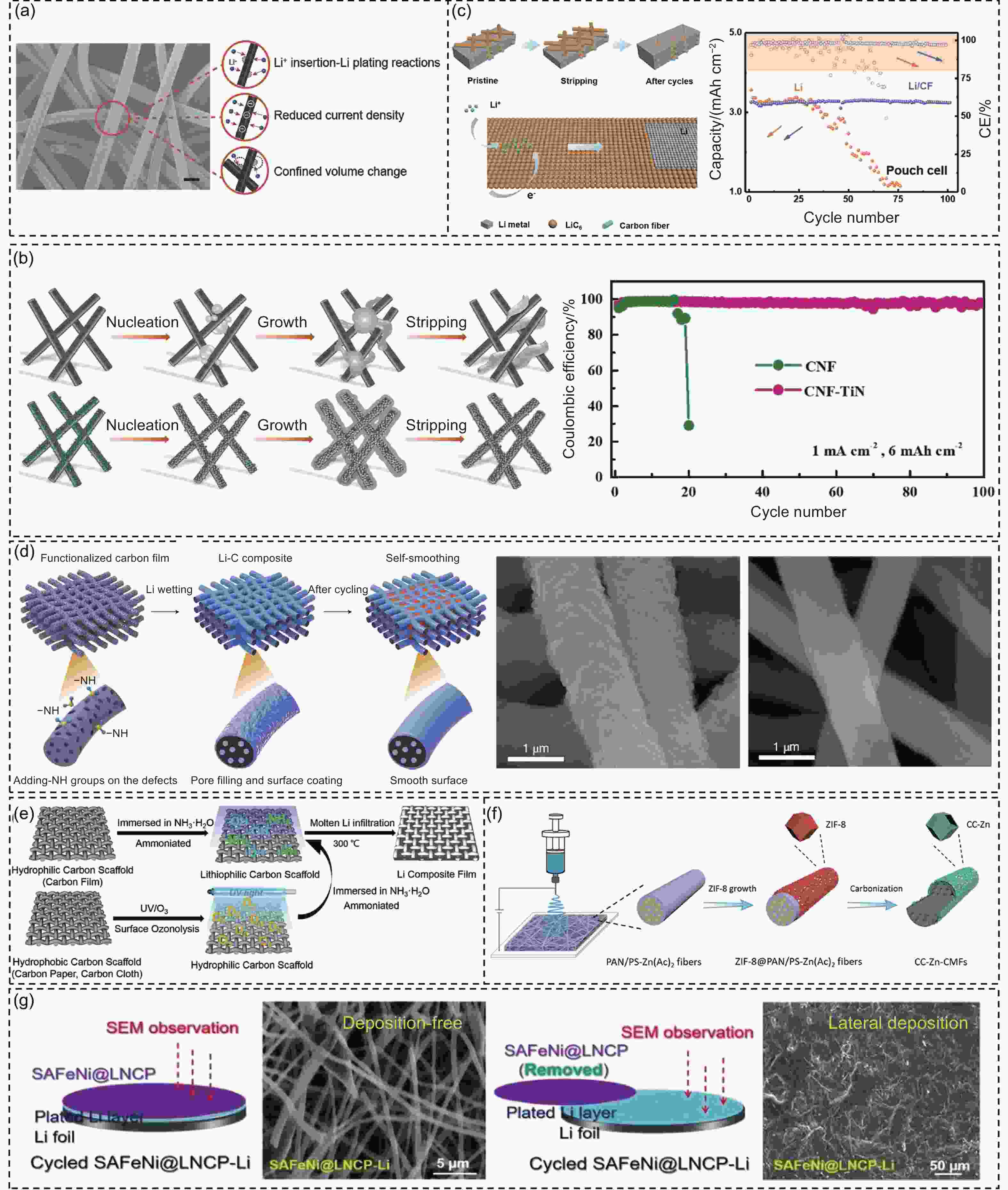
Figure 6. (a) SEM image and the multifunctionality of GCF electrode[96]. (b) Schematic illustration of Li deposition and stripping on the CNF and CNF-TiN, and the corresponding CE profiles at 1 mA cm−2 with 6 mAh cm−2[98]. (c) Schematic diagram of Li plating/stripping behavior on Li/CF composite anode, and the performance of pouch cells with S cathodes[99]. (d) Schematic representation of the self-smoothness of the Li-C electrode in cycling. SEM images of the pristine and cycled Li–C electrode[100]. (e) Schematic of the Li composite film prepared with hydrophilic or hydrophobic carbon scaffolds[101]. (f) Schematic diagram of the preparation of CC-Zn-CMFs[102]. (g) Schematic and SEM image of the cycled electrode with/without upper SAFeNi@LNCP modulation layer[103]. (Reprinted with permission)
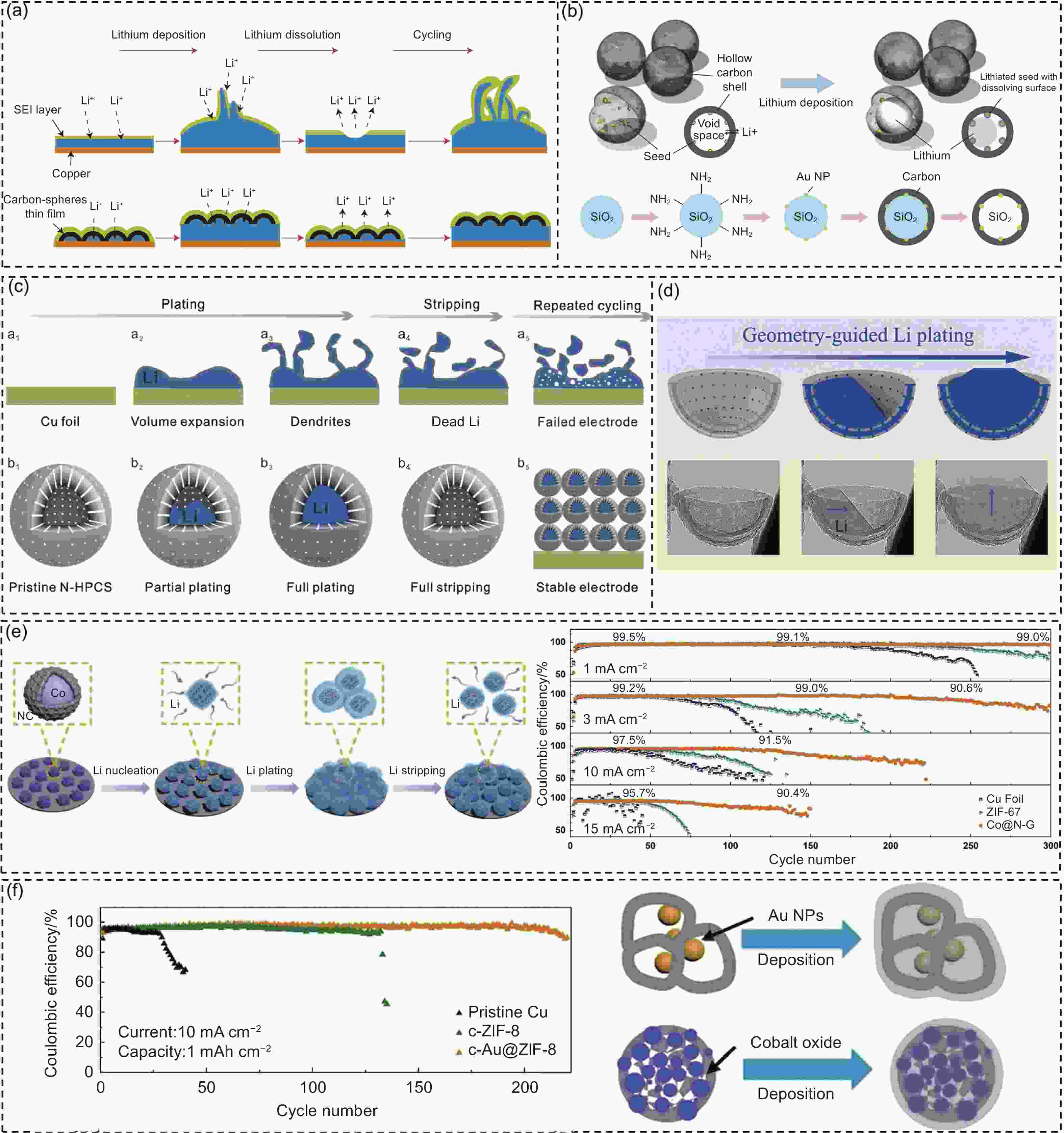
Figure 7. (a) Schematic illustration of the Li plating/stripping behavior on different structures[104]. (b) Schematic illustration of Li metal nanocapsules design and preparation of hollow carbon with Au NPs[105]. (c) Schematic diagrams of the Li plating and stripping on Cu foil and N-HPCSs[107]. (d) Schematic illustration and SEM images of Li plating on the CB[108]. (e) Schematic illustration of the Li nucleation and plating/stripping on the Co@N-G substrate. Coulombic efficiencies of different electrodes at 1, 3, 10, 15 mA cm−2 with 1 mAh cm−2[109]. (f) Schematic diagrams of the Li deposition on c-Au@ZIF-8 and TA-ZIF-67 host based electrodes. Coulombic efficiencies of different electrodes at 10 mA cm−2 with 1 mAh cm−2[111]. (Reprinted with permission)
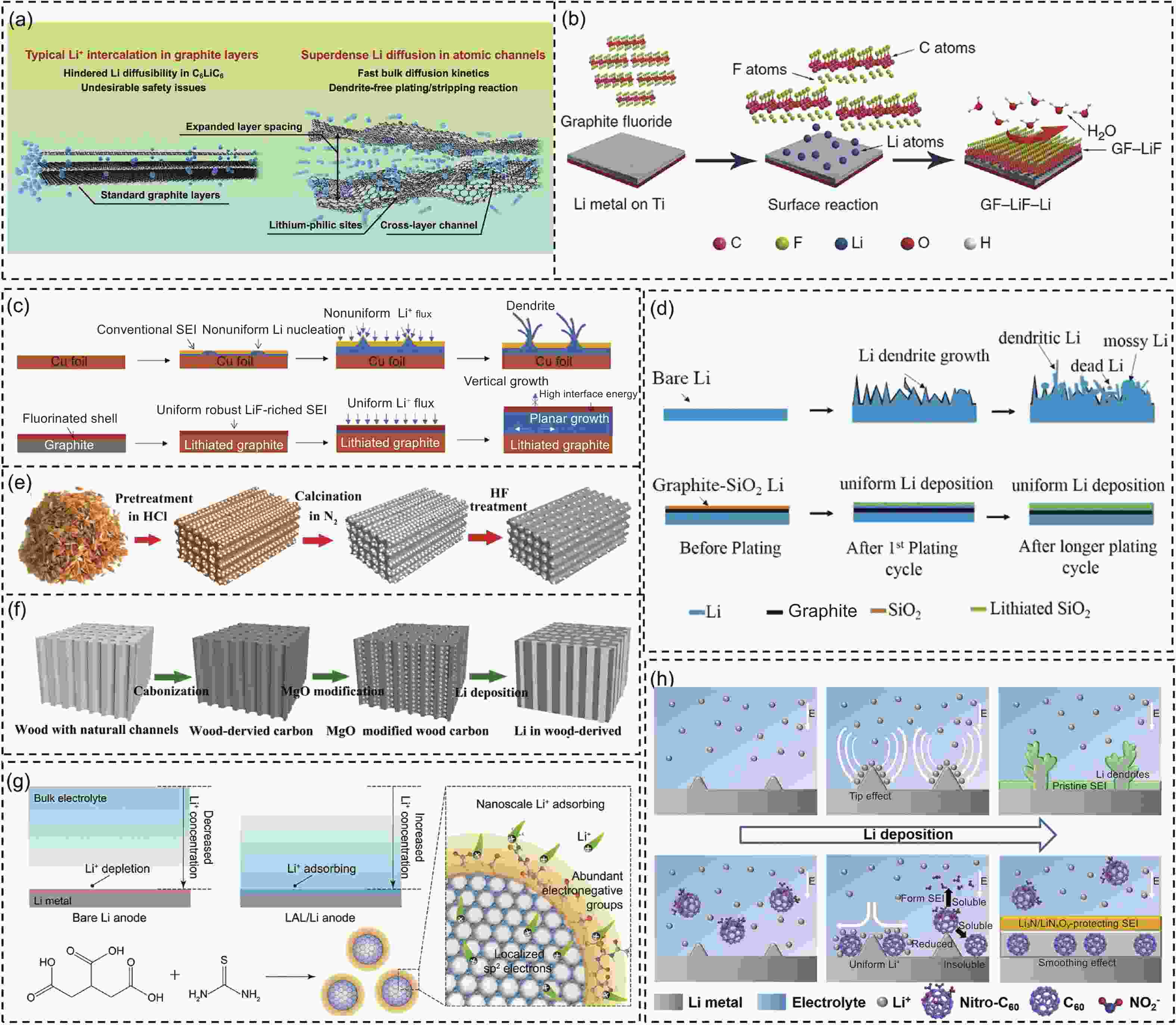
Figure 8. (a) Schematic diagram of typical Li+ intercalation in graphite layers and superdense Li diffusion in atomic channels[112]. (b) Schematic diagram of the preparation and protective mechanism of GF–LiF–Li[113]. (c) Comparative schematic illustration of the Li deposition on Cu foil and MCMB-F electrode[114]. (d) Comparative schematic diagrams of the Li plating on Li with or without graphite–SiO2 bilayer-modified [115]. (e) Schematic diagram of the preparation of RC[116]. (f) Schematic diagram of the preparation of MgO@WC/Li composite electrode[117]. (g) Comparative schematic diagrams of the Li+ in bare Li anode and LAL/Li anode upon Li deposition. And the preparation of the LAL[118]. (h) Schematic of the bifunctional effects of nitro-C60 additive[119]. (Reprinted with permission)
Table 1. Summary of the electrochemical performance of electrodes based on different advanced carbon materials
Electrode Symmetric cell Full cell Reference SR-G-Li 1000 cycles at 5 mA cm−2, 1 mAh cm−2 LiFePO4||SR-G-Li, 1 C, 128.8 mAh g−1 after 300 cycles [72] GBL 600 h at 3 mA cm−2, 3 mAh cm−2 LiFePO4||GBL||Li, 1C, 100 mAh g−1 after 1600 cycles [74] NG basedLi metal anodes 150 cycles at 1 mA cm−2, 0.042 mAh cm−2 - [77] rGO−Cu2O/Cu@Li 300 h at 0.5 mA cm−2 LiFePO4||rGO−Cu2O/Cu@Li, 2 C, 94.3 mA h g−1 [80] Layered Li–rGO electrodes Beyond 100 cycles at 3 mA cm−2, mAh cm−2 LiCoO2||Li–rGO, 10 C, ~70 mAh g–1 [81] Li-SiO2@ERG-CNF 1000 h at 0.5 mA cm−2, 1 mAh cm−2 LiFePO4||Li-SiO2@ERG-CNF, 1 C,
106.9 mAh g−1 after 1000 cycles[86] Li@TLI-GO/Zn/CC >1600 h at 1 mA cm−2, 1 mAh cm-2 LiFePO4|| Li@TLI-GO/Zn/CC, 5 C, capacity
retention of 94.6% after 3000 cycles[7] LiCSMF 2000 cycles at 40 mA cm−2, 2 mAh cm−2 S-CSMF||LiCSMF, 1 C, 200 cycles [88] GZCNT-coated Li 100 h at 10 mA cm−2 S||GZCNT-coated Li, 0.2 C, 1.73 mAh cm–2 after 200 cycles [91] Li-CNT-AB 100 cycles at 3 mA cm−2, 1 mAh cm−2 LiFePO4||Li-CNT-AB, CE of ~ 98.7% after 700 cycles. [93] OPA−Li−CNT 200 cycles at 3 mA cm−2, 0.5 mAh cm−2 LiFePO4||OPA−Li−CNT, 1 C, 250 cycles [94] GCF@Li over 300 h at 2 mA cm−2 LiFePO4||GCF@Li, 300 cycles with a capacity retention of 80% [96] CNF-TiN 200 cycles at 3 mA cm−2 LiFePO4||CNF-TiN, 122.4 mAh g−1 after 250 cycles [98] Li/CF 90 h with a small polarizationvoltage of 120 mV S||Li/CF, 3.25 mAh cm−2, 0.1 C, a capacity
retention rate of 98% after 100 cycles[99] Li-C anode 500 h at 1 mA cm−2, 1 mAh cm−2 NMC||Li-C, 350–380 Wh kg−1 for 200 cycles [100] Li@CF 270 cycles at 3 mA cm−2, 1 mAh cm−2 Mg/Ti-LiNiO2||Li@CF, 0.5 C, 127 mAh g−1 after 140 cycles [101] CC-Zn-CMFs 2000 h at 1 mA cm−2, 1 mAh cm−2 Mg/Ti-LiNiO2||CC-Zn-CMFs-Li, 200 cycles
without obvious capacity decay[102] SAFeNi@LNCP-Li 650 h at 5 mA cm−2, 20 mAh cm−2 SAFeNi@LNCP||SAFeNi@LNCP-Li, 5 C, 856 mA h g−1 [103] N-HPCSs - N-HPCSs/S||Li/Cu@N-HPCSs, 1 C, 907 mAh g−1,
a capacity retention rate of 80.1% after 400 cycles[107] Li-Co@N-G 1000 h at 1 mA cm−2, 1 mAh cm−2 NCM||Li-Co@N-G, 1 C, a capacity retention of 92% after 100 cycles [109] BDLC 2000 h at 1 mA cm−2, 1 mAh cm−2 100% capacity retention is achieved over 370 cycles [112] Li@MCMB-F - LiFePO4||Li@MCMB-F, 2.4 mAh cm−2 for 110 times
at a capacity decay of 0.01% per cycle[114] MgO@WC - LiCoO2||MgO@WC/Li, 1 C, 300 cycles [117] LAL/Li >1000 h at 60 mA cm−2, 60 mAh cm−2 LAL/Li-air, stably cycled for over 450 cycles [118] Nitro-C60 >400 h at 1 mA cm−2, 1 mAh cm−2 S||Li, retention of 63.2% over 100 cycles [119] -
[1] Zhu Z, Jiang T, Ali M, et al. Rechargeable batteries for grid scale energy storage[J]. Chemical Reviews,2022,122(22):16610-16751. doi: 10.1021/acs.chemrev.2c00289 [2] Shin C H, Lee H Y, Gyan-Barimah C, et al. Magnesium: Properties and rich chemistry for new material synthesis and energy applications[J]. Chemical Society Reviews,2023,52(6):2145-2192. doi: 10.1039/D2CS00810F [3] Quilty C D, Wu D, Li W, et al. Electron and ion transport in lithium and lithium-ion battery negative and positive composite electrodes[J]. Chemical Reviews,2023,123(4):1327-1363. doi: 10.1021/acs.chemrev.2c00214 [4] Kim S, Park G, Lee S J, et al. Lithium-metal batteries: From fundamental research to industrialization [J]. Advanced Materials, 2022: e2206625. [5] Piao Z, Gao R, Liu Y, et al. A Review on regulating Li+ solvation structures in carbonate electrolytes for lithium metal batteries[J]. Advanced Materials,2023,35(15):e2206009. [6] Zhang H, Qiao L, Armand M. Organic electrolyte design for rechargeable batteries: From lithium to magnesium[J]. Angewandte Chemie International Edtion,2022,61(52):e202214054. [7] Zhang N, Du L, Zhang J, et al. Self-assembled tent-like nanocavities for space-confined stable lithium metal anode[J]. Advanced Functional Materials,2023,33(16):2210862. doi: 10.1002/adfm.202210862 [8] Liu Y, Ju Z, Zhang B, et al. Visualizing the sensitive lithium with atomic precision: Cryogenic electron microscopy for batteries[J]. Accounts of Chemical Research,2021,54(9):2088-2099. doi: 10.1021/acs.accounts.1c00120 [9] Paul P P, Mcshane E J, Colclasure A M, et al. A review of existing and emerging methods for lithium detection and characterization in Li-ion and Li-metal batteries[J]. Advanced Energy Materials,2021,11(17):2100372. doi: 10.1002/aenm.202100372 [10] Zhang X, Yang Y, Zhou Z. Towards practical lithium-metal anodes[J]. Chemical Society Reviews,2020,49(10):3040-3071. doi: 10.1039/C9CS00838A [11] Chen X R, Zhao B C, Yan C, et al. Review on Li deposition in working batteries: From nucleation to early growth[J]. Advanced Materials,2021,33(8):e2004128. doi: 10.1002/adma.202004128 [12] Wang Z, Sun Z, Li J, et al. Insights into the deposition chemistry of Li ions in nonaqueous electrolyte for stable Li anodes[J]. Chemical Society Reviews,2021,50(5):3178-3210. doi: 10.1039/D0CS01017K [13] Santos E, Schmickler W. The crucial role of local excess charges in dendrite growth on lithium electrodes[J]. Angewandte Chemie International Edtion,2021,60(11):5876-5881. doi: 10.1002/anie.202017124 [14] Xiao J. How lithium dendrites form in liquid batteries[J]. Science,2019,366(6464):426-427. doi: 10.1126/science.aay8672 [15] Zheng J, Kim M S, Tu Z, et al. Regulating electrodeposition morphology of lithium: towards commercially relevant secondary Li metal batteries[J]. Chemical Society Reviews,2020,49(9):2701-2750. doi: 10.1039/C9CS00883G [16] Wei C, Zhang Y, Tian Y, et al. Design of safe, long-cycling and high-energy lithium metal anodes in all working conditions: Progress, challenges and perspectives[J]. Energy Storage Materials,2021,38:157-89. doi: 10.1016/j.ensm.2021.03.006 [17] Li M, Wang C, Chen Z, et al. New concepts in electrolytes[J]. Chemical Reviews,2020,120(14):6783-6819. doi: 10.1021/acs.chemrev.9b00531 [18] Kim K, Ma H, Park S, et al. Electrolyte-additive-driven interfacial engineering for high-capacity electrodes in lithium-ion batteries: Promise and challenges[J]. ACS Energy Letters,2020,5(5):1537-1553. doi: 10.1021/acsenergylett.0c00468 [19] Jie Y, Ren X, Cao R, et al. Advanced liquid electrolytes for rechargeable Li metal batteries[J]. Advanced Functional Materials,2020,30(25):1910777. doi: 10.1002/adfm.201910777 [20] Sheng O, Jin C, Ding X, et al. A decade of progress on solid-state electrolytes for secondary batteries: Advances and contributions[J]. Advanced Functional Materials,2021,31(27):2100891. doi: 10.1002/adfm.202100891 [21] Wu J, Li X, Rao Z, et al. Electrolyte with boron nitride nanosheets as leveling agent towards dendrite-free lithium metal anodes[J]. Nano Energy,2020,72:104725. doi: 10.1016/j.nanoen.2020.104725 [22] Li S, Zhang W, Wu Q, et al. Synergistic dual-additive electrolyte enables practical lithium-metal batteries[J]. Angew Chem Int Ed Engl,2020,59(35):14935-41. doi: 10.1002/anie.202004853 [23] Chen J, Fan X, Li Q, et al. Electrolyte design for LiF-rich solid-electrolyte interfaces to enable high-performance microsized alloy anodes for batteries[J]. Nature Energy,2020,5(5):386-397. doi: 10.1038/s41560-020-0601-1 [24] Fan X, Ji X, Chen L, et al. All-temperature batteries enabled by fluorinated electrolytes with non-polar solvents[J]. Nature Energy,2019,4(10):882-890. doi: 10.1038/s41560-019-0474-3 [25] Liu F, Wang L, Zhang Z, et al. A mixed lithium-ion conductive Li2S/Li2Se protection layer for stable lithium metal anode[J]. Advanced Functional Materials,2020,30(23):2001607. doi: 10.1002/adfm.202001607 [26] Yu Z, Cui Y, Bao Z. Design principles of artificial solid electrolyte interphases for lithium-metal anodes[J]. Cell Reports Physical Science,2020,1(7):100119. doi: 10.1016/j.xcrp.2020.100119 [27] Yan C, Xu R, Xiao Y, et al. Toward critical electrode/electrolyte interfaces in rechargeable batteries[J]. Advanced Functional Materials,2020,30(23):1909887. doi: 10.1002/adfm.201909887 [28] Lu G, Nai J, Luan D, et al. Surface engineering toward stable lithium metal anodes[J]. Science Advances,2023,9(14):eadf1550. doi: 10.1126/sciadv.adf1550 [29] Fang R, Han Z, Li J, et al. Rationalized design of hyperbranched trans-scale graphene arrays for enduring high-energy lithium metal batteries[J]. Science Advances,2022,8(34):eadc9961. doi: 10.1126/sciadv.adc9961 [30] Zhao X, Xia S, Zhang X, et al. Highly lithiophilic copper-reinforced scaffold enables stable Li metal anode[J]. ACS Applied Materials & Interfaces,2021,13(17):20240-20250. [31] Zou P, Chiang S W, Zhan H, et al. A Periodic “self-correction” scheme for synchronizing lithium plating/stripping at ultrahigh cycling capacity[J]. Advanced Functional Materials,2020,30(21):1910532. doi: 10.1002/adfm.201910532 [32] Yang T, Li L, Wu F, et al. A Soft lithiophilic graphene aerogel for stable lithium metal anode[J]. Advanced Functional Materials,2020,30(30):2002013. doi: 10.1002/adfm.202002013 [33] Brissot C, Rosso M, Chazalviel J N, et al. Dendritic growth mechanisms in lithium/polymer cells[J]. Journal of power sources,1999,81-82:925-929. doi: 10.1016/S0378-7753(98)00242-0 [34] Chen J, Zhao J, Lei L, et al. Dynamic intelligent Cu current collectors for ultrastable lithium metal anodes[J]. Nano Letters,2020,20(5):3403-3410. doi: 10.1021/acs.nanolett.0c00316 [35] Zhang C, Lyu R, Lv W, et al. A lightweight 3D Cu nanowire network with phosphidation gradient as current collector for high-density nucleation and stable deposition of lithium[J]. Advanced Materials,2019,31(48):e1904991. doi: 10.1002/adma.201904991 [36] Jin C B, Shi P, Zhang X Q, et al. Advances in carbon materials for stable lithium metal batteries[J]. New Carbon Materials,2022,37(1):1-24. doi: 10.1016/S1872-5805(22)60573-0 [37] Yan X, Lin L, Chen Q, et al. Multifunctional roles of carbon-based hosts for Li-metal anodes: A review[J]. Carbon Energy,2021,3(2):303-329. doi: 10.1002/cey2.95 [38] Cheng Y, Chen J, Chen Y, et al. Lithium host: Advanced architecture components for lithium metal anode[J]. Energy Storage Materials,2021,38:276-298. doi: 10.1016/j.ensm.2021.03.008 [39] Zhang L, Qin X, Zhao S, et al. Advanced matrixes for binder-Free nanostructured electrodes in lithium-ion batteries[J]. Advanced Materials,2020,32(24):e1908445. doi: 10.1002/adma.201908445 [40] Myung S T, Hitoshi Y, Sun Y K. Electrochemical behavior and passivation of current collectors in lithium-ion batteries[J]. Journal of Materials Chemistry,2011,21(27):9891-9911. doi: 10.1039/c0jm04353b [41] Shi P, Zhang X Q, Shen X, et al. A review of composite lithium metal anode for practical applications[J]. Advanced Materials Technologies,2019,5(1):1900806. [42] Fang R, Chen K, Yin L, et al. The regulating role of carbon nanotubes and graphene in lithium-ion and lithium-sulfur batteries[J]. Advanced Materials,2019,31(9):e1800863. doi: 10.1002/adma.201800863 [43] Shen X, Zhang R, Shi P, et al. How does external pressure shape Li dendrites in Li metal batteries?[J]. Advanced Energy Materials,2021,11(10):2003416. doi: 10.1002/aenm.202003416 [44] Jana A, Woo S I, Vikrant K S N, et al. Electrochemomechanics of lithium dendrite growth[J]. Energy & Environmental Science,2019,12(12):3595-3607. [45] Lin D, Liu Y, Pei A, et al. Nanoscale perspective: Materials designs and understandings in lithium metal anodes[J]. Nano Research,2017,10(12):4003-4026. doi: 10.1007/s12274-017-1596-1 [46] Jäckle M, Helmbrecht K, Smits M, et al. Self-diffusion barriers: Possible descriptors for dendrite growth in batteries?[J]. Energy & Environmental Science,2018,11(12):3400-3407. [47] Hao F, Verma A, Mukherjee P P. Electrodeposition stability of metal electrodes[J]. Energy Storage Materials,2019,20:1-6. doi: 10.1016/j.ensm.2019.05.004 [48] Ling C, Banerjee D, Matsui M. Study of the electrochemical deposition of Mg in the atomic level: Why it prefers the non-dendritic morphology[J]. Electrochimica Acta,2012,76:270-274. doi: 10.1016/j.electacta.2012.05.001 [49] Aurbach D, Gofer Y, Schechter A, et al. A comparison between the electrochemical behavior of reversible magnesium and lithium electrodes[J]. Journal of Power Sources,2001,97-98:269-273. doi: 10.1016/S0378-7753(01)00622-X [50] Ely D R, García R E. Heterogeneous nucleation and growth of lithium electrodeposits on negative electrodes[J]. Journal of The Electrochemical Society,2013,160(4):A662-A668. doi: 10.1149/1.057304jes [51] Chazalviel J. Electrochemical aspects of the generation of ramified metallic electrodeposits[J]. Physical Review A,1990,42(12):7355-7367. doi: 10.1103/PhysRevA.42.7355 [52] Fleury V V, Chazalviel J, Rosso M. Theory and experimental evidence of electroconvection around electrochemical deposits[J]. Physical Review Letters,1992,68(16):2492-2495. doi: 10.1103/PhysRevLett.68.2492 [53] Jiang J, Pan Z, Kou Z, et al. Lithiophilic polymer interphase anchored on laser-punched 3D holey Cu matrix enables uniform lithium nucleation leading to super-stable lithium metal anodes[J]. Energy Storage Materials,2020,29:84-91. doi: 10.1016/j.ensm.2020.04.006 [54] Zhang X, Wang S, Xue C, et al. Self-suppression of lithium dendrite in all-solid-state lithium metal batteries with poly(vinylidene difluoride)-based solid electrolytes[J]. Advanced Materials,2019,31(11):e1806082. doi: 10.1002/adma.201806082 [55] Cheng X B, Yan C, Chen X, et al. Implantable solid electrolyte interphase in lithium-metal batteries[J]. Chem,2017,2(2):258-270. doi: 10.1016/j.chempr.2017.01.003 [56] Shen X, Zhang R, Chen X, et al. The failure of solid electrolyte interphase on Li metal anode: Structural uniformity or mechanical strength?[J]. Advanced Energy Materials,2020,10(10):1903645. doi: 10.1002/aenm.201903645 [57] Lu Y, Tu Z, Archer L A. Stable lithium electrodeposition in liquid and nanoporous solid electrolytes[J]. Nature materials,2014,13(10):961-969. doi: 10.1038/nmat4041 [58] Cao X, Ren X, Zou L, et al. Monolithic solid-electrolyte interphases formed in fluorinated orthoformate-based electrolytes minimize Li depletion and pulverization[J]. Nature Energy,2019,4(9):796-805. doi: 10.1038/s41560-019-0464-5 [59] Amanchukwu C V, Yu Z, Kong X, et al. A new class of ionically conducting fluorinated ether electrolytes with high electrochemical stability[J]. Journal of the American Chemical Society,2020,142(16):7393-7403. doi: 10.1021/jacs.9b11056 [60] Wan J, Xie J, Kong X, et al. Ultrathin, flexible, solid polymer composite electrolyte enabled with aligned nanoporous host for lithium batteries[J]. Nature Nanotechnology,2019,14(7):705-711. doi: 10.1038/s41565-019-0465-3 [61] Pathak R, Chen K, Gurung A, et al. Fluorinated hybrid solid-electrolyte-interphase for dendrite-free lithium deposition[J]. Nature Communications,2020,11(1):93. doi: 10.1038/s41467-019-13774-2 [62] Xu T, Gao P, Li P, et al. Fast-charging and ultrahigh-capacity lithium metal anode enabled by surface alloying[J]. Advanced Energy Materials,2020,10(8):1902343. doi: 10.1002/aenm.201902343 [63] Guo W, Han Q, Jiao J, et al. In situ Construction of robust biphasic surface layers on Li metal for Li-S batteries with long cycle life[J]. Angewandte Chemie International Edition,2021,133(13):7343-7350. [64] Lin Y, Plaza-Rivera C O, Hu L, et al. Scalable dry-pressed electrodes based on holey graphene[J]. Accounts of Chemical Research,2022,55(20):3020-3031. doi: 10.1021/acs.accounts.2c00457 [65] Pan L, Luo Z, Zhang Y, et al. Seed-free selective deposition of lithium metal into tough graphene framework for stable lithium metal anode[J]. ACS Applied Materials & Interfaces,2019,11(47):44383-44389. [66] Zhao B, Li B, Wang Z, et al. Uniform Li deposition sites provided by atomic layer deposition for the dendrite-free lithium metal anode[J]. ACS Applied Materials & Interfaces,2020,12(17):19530-19538. [67] Dong L, Nie L, Liu W. Water-stable lithium metal anodes with ultrahigh-rate capability enabled by a hydrophobic graphene architecture[J]. Advanced Materials,2020,32(14):e1908494. doi: 10.1002/adma.201908494 [68] Liu L, Yin Y X, Li J Y, et al. Uniform lithium nucleation/growth induced by lightweight nitrogen-doped graphitic carbon foams for high-performance lithium metal anodes[J]. Advanced Materials,2018,30(10):1706216. doi: 10.1002/adma.201706216 [69] Zhou Y, Zhang X, Ding Y, et al. Reversible deposition of lithium particles enabled by ultraconformal and stretchable graphene film for lithium metal batteries[J]. Advanced Materials,2020,32(48):e2005763. doi: 10.1002/adma.202005763 [70] Liu S, Xia X, Zhong Y, et al. 3D TiC/C core/shell nanowire skeleton for dendrite-free and long-life lithium letal anode[J]. Advanced Energy Materials,2018,8(8):1702322. doi: 10.1002/aenm.201702322 [71] Wang X, Huang R Q, Niu S Z, et al. Research progress on graphene-based materials for high-performance lithium-metal batteries[J]. New Carbon Material,2021,36(4):711-728. doi: 10.1016/S1872-5805(21)60081-1 [72] Bai M, Xie K, Yuan K, et al. A scalable approach to dendrite-free lithium anodes via spontaneous reduction of spray-coated graphene oxide layers[J]. Advanced Materials,2018,30(29):e1801213. doi: 10.1002/adma.201801213 [73] Gao Y, Yan Z, Gray J L, et al. Polymer-inorganic solid-electrolyte interphase for stable lithium metal batteries under lean electrolyte conditions[J]. Nature Materials,2019,18(4):384-389. doi: 10.1038/s41563-019-0305-8 [74] Xu L, Zhang Q, Niu S, et al. Organic eutectic mixture incorporated with graphene oxide sheets as lithiophilic artificial protective layer for dendrite-free lithium metal batteries[J]. Advanced Energy Materials,2023,13(12):2204214. doi: 10.1002/aenm.202204214 [75] Zhang R, Cheng X B, Zhao C Z, et al. Conductive nanostructured scaffolds render low local current density to inhibit lithium dendrite growth[J]. Advanced Materials,2016,28(11):2155-62. doi: 10.1002/adma.201504117 [76] Meng Q, Deng B, Zhang H, et al. Heterogeneous nucleation and growth of electrodeposited lithium metal on the basal plane of single-layer graphene[J]. Energy Storage Materials,2019,16:419-425. doi: 10.1016/j.ensm.2018.06.024 [77] Zhang R, Chen X R, Chen X, et al. Lithiophilic sites in doped graphene guide uniform lithium nucleation for dendrite-free lithium metal anodes[J]. Angewandte Chemie International Edtion,2017,56(27):7764-7768. doi: 10.1002/anie.201702099 [78] Li Z, Li X, Zhou L, et al. A synergistic strategy for stable lithium metal anodes using 3D fluorine-doped graphene shuttle-implanted porous carbon networks[J]. Nano Energy,2018,49:179-185. doi: 10.1016/j.nanoen.2018.04.040 [79] Chen X, Chen X R, Hou T Z, et al. Lithiophilicity chemistry of heteroatom-doped carbon to guide uniform lithium nucleation in lithium metal anodes[J]. Science Advances,2019,5(2):eaau7728. doi: 10.1126/sciadv.aau7728 [80] Chen M, Cheng L, Chen J, et al. Facile and scalable modification of a Cu current collector toward uniform Li deposition of the Li metal anode[J]. ACS Applied Materials & Interfaces,2020,12(3):3681-3687. [81] Lin D, Liu Y, Liang Z, et al. Layered reduced graphene oxide with nanoscale interlayer gaps as a stable host for lithium metal anodes[J]. Nature Nanotechnology,2016,11(7):626-632. doi: 10.1038/nnano.2016.32 [82] Wang H, Cao X, Gu H, et al. Improving lithium metal composite anodes with seeding and pillaring effects of silicon nanoparticles[J]. ACS Nano,2020,14(4):4601-4608. doi: 10.1021/acsnano.0c00184 [83] Fang Y, Zhang Y, Zhu K, et al. Lithiophilic three-dimensional porous Ti3C2Tx-rGO membrane as a stable scaffold for safe alkali metal (Li or Na) anodes[J]. ACS Nano,2019,13(12):14319-14328. doi: 10.1021/acsnano.9b07729 [84] Shi H, Zhang C J, Lu P, et al. Conducting and lithiophilic MXene/Graphene framework for high-capacity, dendrite-free lithium-metal anodes[J]. ACS Nano,2019,13(12):14308-14318. doi: 10.1021/acsnano.9b07710 [85] Ni S, Sheng J, Zhang C, et al. Dendrite-free lithium deposition and stripping regulated by aligned microchannels for stable lithium metal batteries[J]. Advanced Functional Materials,2022,32(21):2200682. doi: 10.1002/adfm.202200682 [86] Song Q, Yan H, Liu K, et al. Vertically grown edge-rich graphene nanosheets for spatial control of Li nucleation[J]. Advanced Energy Materials,2018,8(22):1800564. doi: 10.1002/aenm.201800564 [87] Zhai P, Wang T, Jiang H, et al. 3D artificial solid-electrolyte interphase for lithium metal anodes enabled by insulator-metal-insulator layered heterostructures[J]. Advanced Materials,2021,33(13):e2006247. doi: 10.1002/adma.202006247 [88] Wang Z Y, Lu Z X, Guo W, et al. A dendrite-free lithium/carbon nanotube hybrid for lithium-metal batteries[J]. Advanced Materials,2021,33(4):e2006702. doi: 10.1002/adma.202006702 [89] Sun Z, Jin S, Jin H, et al. Robust expandable carbon nanotube scaffold for ultrahigh-capacity lithium-metal anodes[J]. Advanced Materials,2018,30(32):e1800884. doi: 10.1002/adma.201800884 [90] Liu F, Xu R, Hu Z, et al. Regulating lithium nucleation via CNTs modifying carbon cloth film for stable Li metal anode[J]. Small,2019,15(5):e1803734. doi: 10.1002/smll.201803734 [91] Zhang H, Liao X, Guan Y, et al. Lithiophilic-lithiophobic gradient interfacial layer for a highly stable lithium metal anode[J]. Nature Communications,2018,9(1):3729. doi: 10.1038/s41467-018-06126-z [92] Wang X, Pan Z, Yang J, et al. Stretchable fiber-shaped lithium metal anode[J]. Energy Storage Materials,2019,22:179-184. doi: 10.1016/j.ensm.2019.01.013 [93] Guo F, Wang Y, Kang T, et al. A Li-dual carbon composite as stable anode material for Li batteries[J]. Energy Storage Materials,2018,15:116-123. doi: 10.1016/j.ensm.2018.03.018 [94] Kang T, Wang Y, Guo F, et al. Self-assembled monolayer enables slurry-coating of Li Anode[J]. ACS Central Science,2019,5(3):468-476. doi: 10.1021/acscentsci.8b00845 [95] Zhan Y X, Shi P, Jin C B, et al. Regulating the two-stage accumulation mechanism of inactive lithium for practical composite lithium metal anodes[J]. Advanced Functional Materials,2022,32(43):2206834. doi: 10.1002/adfm.202206834 [96] Zuo T T, Wu X W, Yang C P, et al. Graphitized carbon fibers as multifunctional 3D current collectors for high areal capacity Li anodes[J]. Advanced Materials,2017,29(29):1700389. doi: 10.1002/adma.201700389 [97] Chen Y, Elangovan A, Zeng D, et al. Vertically aligned carbon nanofibers on Cu foil as a 3D current collector for reversible Li plating/stripping toward high-performance Li-S batteries[J]. Advanced Functional Materials,2019,30(4):1906444. [98] Lin K, Qin X, Liu M, et al. Ultrafine titanium nitride sheath decorated carbon nanofiber network enabling stable lithium metal anodes[J]. Advanced Functional Materials,2019,29(46):1903229. doi: 10.1002/adfm.201903229 [99] Shi P, Li T, Zhang R, et al. Lithiophilic LiC(6) layers on carbon hosts enabling stable Li metal anode in working batteries[J]. Advanced Materials,2019,31(8):e1807131. doi: 10.1002/adma.201807131 [100] Niu C, Pan H, Xu W, et al. Self-smoothing anode for achieving high-energy lithium metal batteries under realistic conditions[J]. Nature Nanotechnology,2019,14(6):594-601. doi: 10.1038/s41565-019-0427-9 [101] Tao L, Hu A, Yang Z, et al. A surface chemistry approach to tailoring the hydrophilicity and lithiophilicity of carbon films for hosting high-performance lithium metal anodes[J]. Advanced Functional Materials,2020,30(31):2000585. doi: 10.1002/adfm.202000585 [102] Fang Y, Zeng Y, Jin Q, et al. Nitrogen-doped amorphous Zn-carbon multichannel fibers for stable lithium metal anodes[J]. Angewandte Chemie International Edtion,2021,60(15):8515-8520. doi: 10.1002/anie.202100471 [103] Wang J, Zhang J, Duan S, et al. Lithium atom surface diffusion and delocalized deposition propelled by atomic metal catalyst toward ultrahigh-capacity dendrite-free lithium anode[J]. Nano Letters,2022,22(19):8008-8017. doi: 10.1021/acs.nanolett.2c02611 [104] Zheng G, Lee S W, Liang Z, et al. Interconnected hollow carbon nanospheres for stable lithium metal anodes[J]. Nature Nanotechnology,2014,9(8):618-623. doi: 10.1038/nnano.2014.152 [105] Yan K, Lu Z, Lee H W, et al. Selective deposition and stable encapsulation of lithium through heterogeneous seeded growth[J]. Nature Energy,2016,1(3):16010. doi: 10.1038/nenergy.2016.10 [106] Zhang T, Lu H, Yang J, et al. Stable lithium metal anode enabled by a lithiophilic and electron/ion conductive framework[J]. ACS Nano,2020,14(5):5618-5627. doi: 10.1021/acsnano.9b10083 [107] Ye W, Pei F, Lan X, et al. Stable nano-encapsulation of lithium through seed-Free selective deposition for high-performance Li battery anodes[J]. Advanced Energy Materials,2020,10(7):1902956. doi: 10.1002/aenm.201902956 [108] Ye W, Wang L, Yin Y, et al. Lithium storage in bowl-like carbon: The effect of surface curvature and space geometry on Li metal deposition[J]. ACS Energy Letters,2021,6(6):2145-2152. doi: 10.1021/acsenergylett.1c00456 [109] Wang T S, Liu X, Zhao X, et al. Regulating uniform Li plating/stripping via dual-conductive metal-organic frameworks for high-rate lithium metal batteries[J]. Advanced Functional Materials,2020,30(16):2000786. doi: 10.1002/adfm.202000786 [110] Kim J, Lee J, Yun J, et al. Functionality of dual-phase lithium storage in a porous carbon host for lithium-metal anode[J]. Advanced Functional Materials,2020,30(15):1910538. doi: 10.1002/adfm.201910538 [111] Huang M, Yao Z, Yang Q, et al. Consecutive nucleation and confinement modulation towards Li plating in seeded capsules for durable Li-metal batteries[J]. Angewandte Chemie International Edtion,2021,60(25):14040-14050. doi: 10.1002/anie.202102552 [112] Zhou S, Chen W, Shi J, et al. Efficient diffusion of superdense lithium via atomic channels for dendrite-free lithium-metal batteries[J]. Energy & Environmental Science,2022,15(1):196-205. [113] Shen X, Li Y, Qian T, et al. Lithium anode stable in air for low-cost fabrication of a dendrite-free lithium battery[J]. Nature Communications,2019,10(1):900. doi: 10.1038/s41467-019-08767-0 [114] Cui C, Yang C, Eidson N, et al. A highly reversible, dendrite-free lithium metal anode enabled by a lithium-fluoride-enriched interphase[J]. Advanced Materials,2020,32(12):e1906427. doi: 10.1002/adma.201906427 [115] Pathak R, Chen K, Gurung A, et al. Ultrathin bilayer of graphite/SiO2 as solid interface for reviving Li metal anode[J]. Advanced Energy Materials,2019,9(36):1901486. doi: 10.1002/aenm.201901486 [116] Jin C, Sheng O, Zhang W, et al. Sustainable, inexpensive, naturally multi-functionalized biomass carbon for both Li metal anode and sulfur cathode[J]. Energy Storage Materials,2018,15:218-225. doi: 10.1016/j.ensm.2018.04.001 [117] Jin C, Sheng O, Lu Y, et al. Metal oxide nanoparticles induced step-edge nucleation of stable Li metal anode working under an ultrahigh current density of 15 mA cm−2[J]. Nano Energy,2018,45:203-209. doi: 10.1016/j.nanoen.2017.12.055 [118] Ye L, Liao M, Cheng X, et al. Lithium-metal anodes Working at 60 mA cm-2 and 60 mAh cm-2 through nanoscale lithium-ion adsorbing[J]. Angewandte Chemie International Edtion,2021,60(32):17419-17425. doi: 10.1002/anie.202106047 [119] Jiang Z, Zeng Z, Yang C, et al. Nitrofullerene, a C60-based bifunctional additive with smoothing and protecting effects for stable lithium metal anode[J]. Nano Letters,2019,19(12):8780-8786. doi: 10.1021/acs.nanolett.9b03562 -





 下载:
下载: