Recent advances in MXene-based nanomaterials for high-performance lithium metal anodes
-
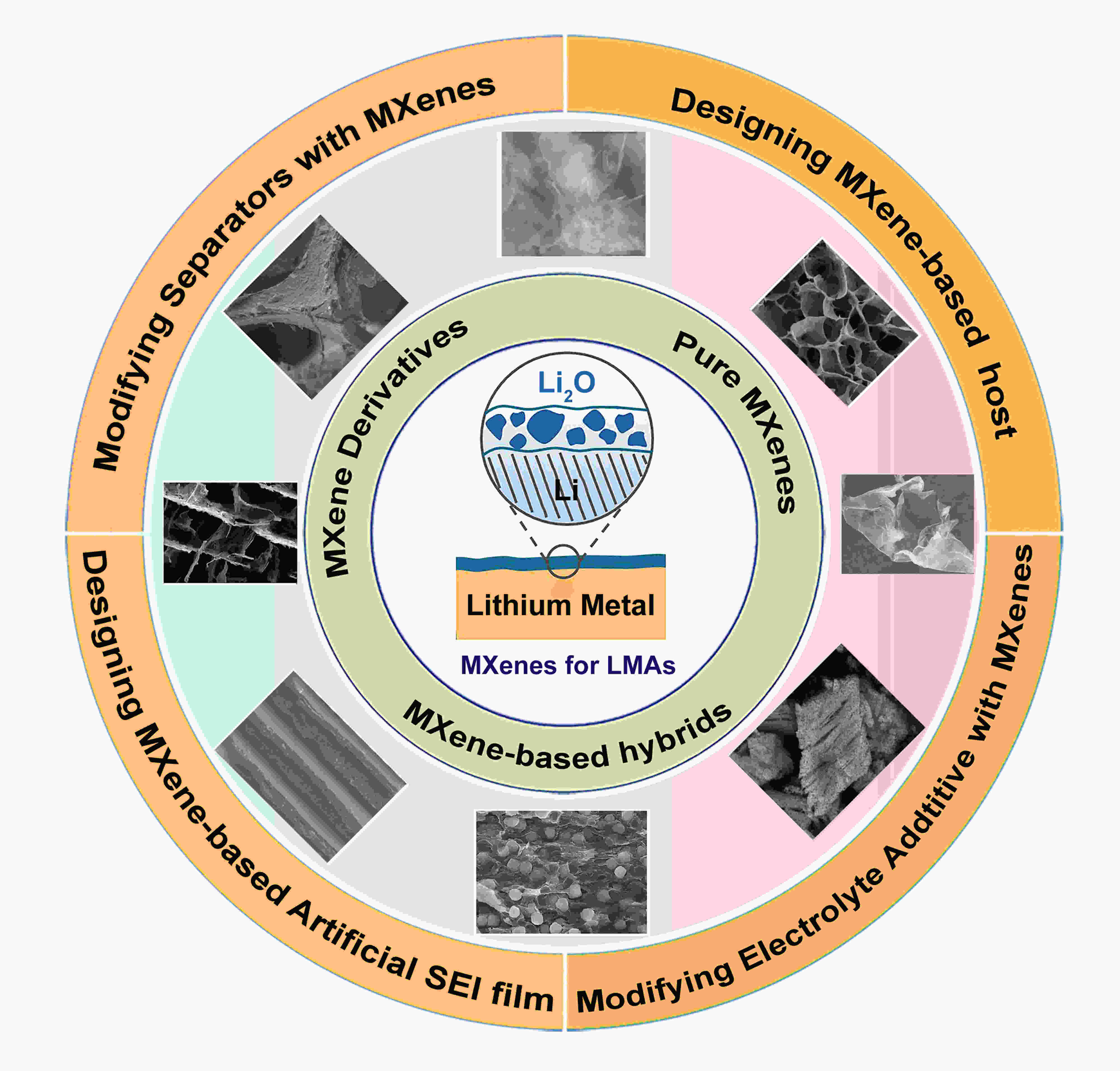
摘要: 锂金属负极由于其具有高的理论容量和低的电化学电势,被认为是高能量密度可充电电池中最具吸引力和前景的负极。然而,其在镀/脱锂过程中,锂枝晶的不可控生长导致了电极的快速退化和严重的安全问题,严重阻碍了其实际应用。为了解决这些问题,使用具有高电导率、优异机械性能和丰富表面官能团的二维过渡金属碳化物/氮化物(MXenes)来诱导锂均匀成核并缓解体积变化,最终抑制锂枝晶的形成。本文综述了用于金属锂负极的MXene基纳米材料的最新进展。首先介绍了金属锂负极的技术挑战。然后从三个方面总结了MXene基纳米材料用于抑制锂枝晶生长和构建稳定金属锂负极的设计和使用:(1)使用结构化负极,如MXene/锂负极、MXene/金属/锂负极、MXene/碳/锂负极、MXene/氧化物/锂负极等;(2)构建人工SEI膜;(3)修饰电解液成分。最后,简要讨论了MXene基纳米材料在下一代锂金属电池应用中的挑战和前景。Abstract: To tackle the issues of rapid electrode degradation and severe safety issues caused by the uncontrollable growth of lithium dendrites in Li metal anodes (LMAs), two-dimensional transition metal carbides/nitrides (MXenes) with a high electrical conductivity, excellent mechanical properties, and abundant surface functional groups have been used as hosts to induce uniform Li nucleation and alleviate the volume changes, eventually inhibiting the formation of Li dendrites. Recent advances in the use of MXene-based nanomaterials in LMAs are summarized. The problems with using LMAs are first considered, and the ways of using MXene-based nanomaterials for suppressing Li dendrite growth and constructing stable LMAs are then summarized. These include the use of MXenes, MXene-metal hybrids, MXene-carbon hybrids, and MXene derivatives as hosts for the anodes and as additives to modify the electrolyte compositions to increase ionic conductivity and inhibit polymer crystallization. Finally, the challenges and prospects for using MXene-based nanomaterials in next-generation LMAs are briefly discussed.
-
Key words:
- Lithium metal anodes /
- MXene /
- Dendrites /
- Lithium metal batteries
-
Figure 2. (a) Schematic illustration of the synthesis of Ti3C2 MXene (graphene, BN)-lithium films[66]; (b) SEM image of the Ti3C2-Li composite anode[66]; (c) Cross-sectional SEM image of the Ti3C2-Li composite anode[66]; (d) Schematic showing the sufficient transport pathways for electron and Li-ion in Ti3C2-Li composite anode[66]; (e) Schematic illustration of lithium plating on bare lithium and parallelly aligned MXene (PA-MXene) layers[67]; (f) Typical SEM images of PA-MXene layers on lithium. Inset in (f) is the top view of PA-MXene[67]; (g) Two-side press of MXene stacks onto a thin Li host[69]; (h) SEM images of the ILC-Li electrode upon plating at 3 mA cm−2 and 3 mAh cm−2[69]; (i) SEM images of the ILC-Li electrode upon stripping at 3 mA cm−2 and 3 mAh cm−2[69]. Reprinted with permission
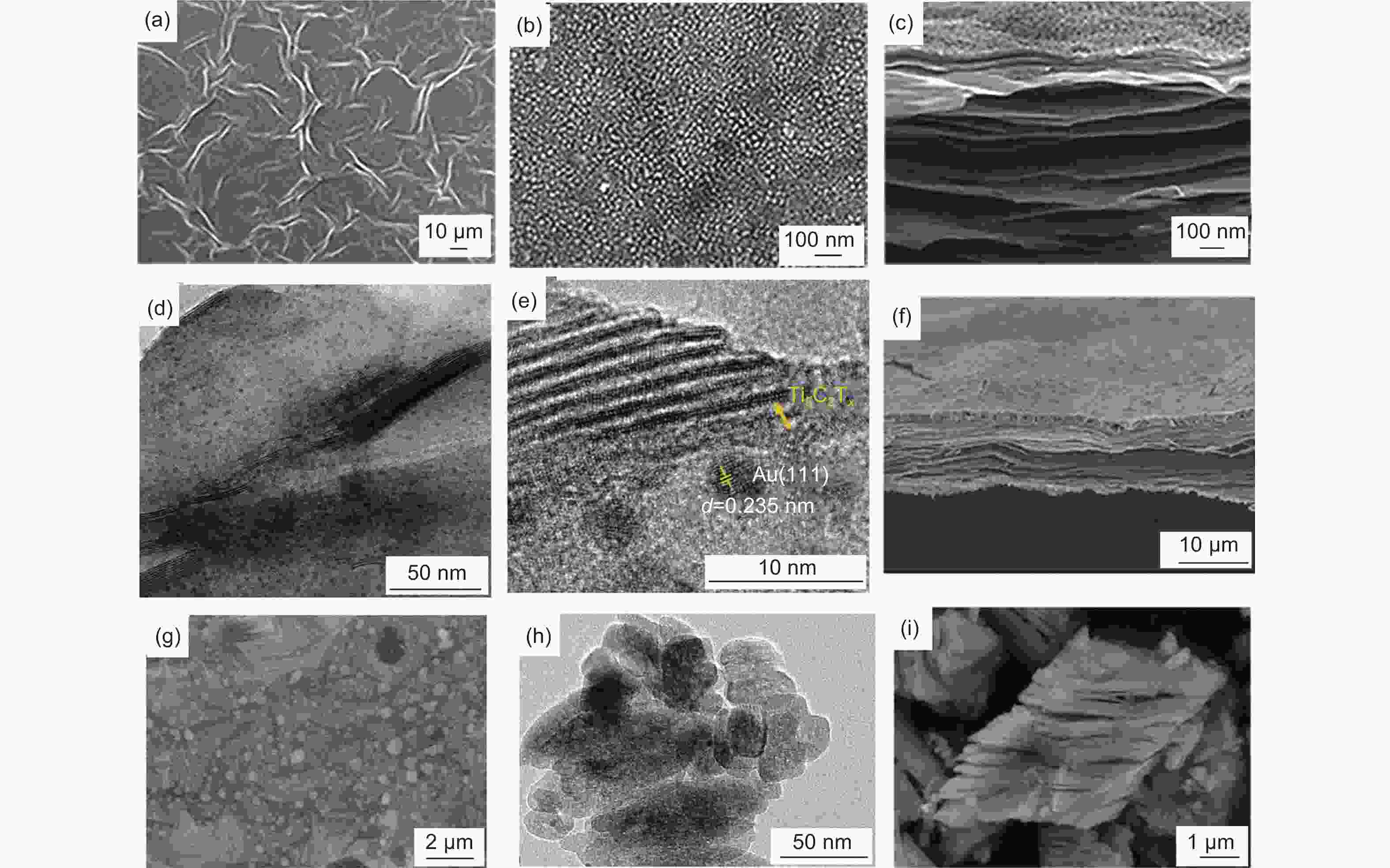
Figure 3. (a) The synthesis schematic diagram of v-Ti3C2Tx electrodes[70]; (b-c) Top view SEM image of v-Ti3C2Tx electrodes[70]; (d) Cross-section SEM image of v-Ti3C2Tx electrodes[70]; (e) Coulombic efficiencies of v-Ti3C2Tx and h-Ti3C2Tx electrodes at 1.0 mA cm−2 for 1.0 mAh cm−2[70]; (f) Schematic illustration of the stripping and plating states of perpendicular MXene-Li and rGO-Li arrays. The left bottom in a) is the SEM images of as-prepared perpendicular MXene-Li arrays. The middle bottom in panel (a) is the SEM image of perpendicular MXene-Li arrays after lithium stripping for 20 mAh cm−271; (g) Top view SEM images of perpendicular MXene-Li arrays[71]; (h) Top view SEM images of rGO-Li[71]; (i) Scheme of the fabrication of Ti3C2Tx MXene and a high-concentration MXene ink (~300 mg mL−1)[75]; (j) Scheme of 3D printing MXene arrays and lattices to guide the nucleation and growth of lithium[75]. Reprinted with permission
Figure 4. (a) The Top view SEM images of MXene paper[81]; (b) The Top view SEM images of MXene@Au paper[81]; (c) Cross-sectional SEM image of MXene@Au paper[81]; (d) TEM image of Ti3C2Tx@Au paper[81]; (e) HRTEM image of Ti3C2Tx@Au paper[81]; (f) Cross-sectional SEM image a of Ti3C2Tx MXene@Zn paper[83]; (g) Top view SEM images of MXene@RP paper[85]; (h) TEM images of MXene@RP paper[85]; (i) SEM image of NS-Nb2C[86]. Reprinted with permission
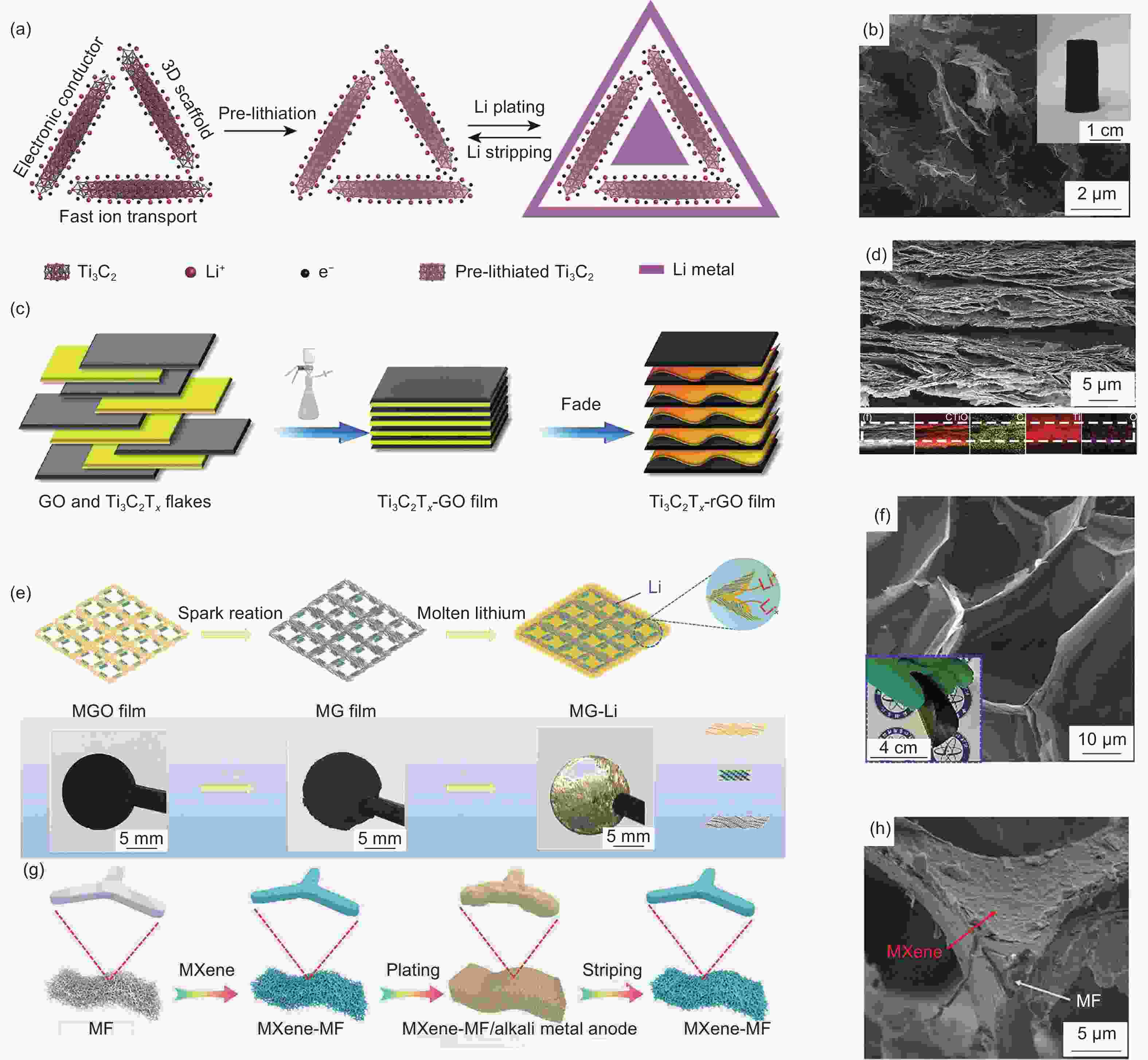
Figure 5. (a) Schematic illustration of Li deposition in the lamellar-structured CNT/MXene/SnO2 composite host based on lithiophilic gradient[93]; (b) Cross-sectional SEM image of the CNT/MXene/SnO2 host[93]; (c) Schematic illustration of lithium plating on MXene@CNF film[94]; (d) Magnified SEM image of MXene@CNF film[94]; (e) Schematic illustration of the C-MXene/AgNW scaffold fabrication[95]; (f) Magnified SEM image showing a joint of the C0.5-MXene/AgNW scaffold[95]; (g) The schematic diagram for the Li stripping-plating process of Ti3C2-LiB-Li hybrid and LiB-Li[96]; (h) The corresponding cross-section image of the Ti3C2-LiB-Li[96]. Reprinted with permission
Figure 6. (a) The Ti3C2 MXene aerogel scaffolds for Li metal anodes[98]; (b) SEM image of the MXene/rGO aerogel. Inset: a photograph of the aerogel[98]; (c) Schematic diagram of Li-Ti3C2Tx-rGO preparation[99]; (d) SEM cross-section images of Ti3C2Tx-rGO[99]; (e) Schematic illustration of the fabrication process of the 3D MG-Li anode and corresponding photographs of the MGO film, MG film, and MG-Li anode[100]; (f) SEM image of the MG film. Inset is a photograph of the bent MG film[100]; (g) Schematic illustration of the fabrication process of the 3D MXene-MF for the alkali-metal anode[101]; (h) SEM image of MXene-MF[101]. Reprinted with permission
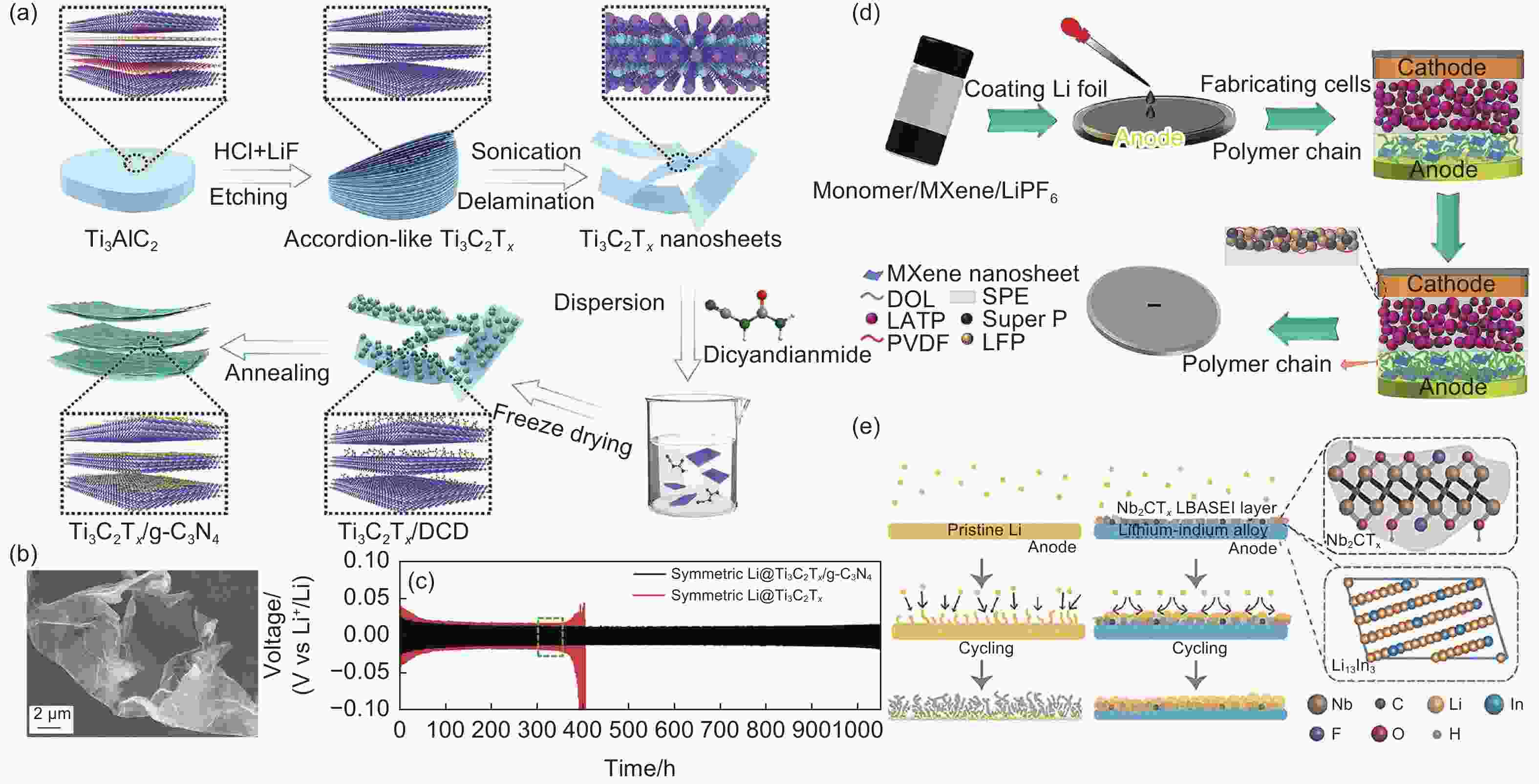
Figure 7. (a) Schematic illustration of the fabrication process of Ti3C2Tx/g-C3N4 hybrid[104]; (b) SEM image of Ti3C2Tx/g-C3N4[104]; (c) Voltage-time profiles of Li plating/stripping in different symmetric cells with the capacity of 0.5 mAh cm−2 at 0.5 mA cm−2 and Detailed voltage profiles for selective cycles in panel[104]; (d) Schematic diagram of the in-situ generation process of MA-SEI in the Li metal anode interface[105]; (e) Schematic illustration for the Li deposition and migration processes occurring at the Nb2CTX Li-In alloy anode[106]. Reprinted with permission
Table 1. Summary of the MXene-based nanomaterials for Li-metal anodes
Electrodes Current density/(mA cm−2) Capacity/(mAh cm−2) Electrolyte Overpotential/mV Cycle life/h Ref. Ti3C2 MXene
(graphene, BN)1 mA cm−2 1 mAh cm−2 1 M LiPF6 in EC/DMC
(1∶1, v/v)32 mV 400 h [66] ILC-Li 3 mA cm−2 3 mAh cm−2 1 M LiPF6 in EC/EMC
(3∶7, weight/weight) with 2wt% VC15-20 mV 2250 h [69] 3DP-MXene arrays 1 mA cm−2 1 mAh cm−2 1 M LiTFSI in DOL/DME
(1∶1, v/v) with 1wt% LiNO310 mV 1200 h [75] MXene@Au 1 mA cm−2 1 mAh cm−2 1 M LiTFSI in DOL/DME
(1∶1, v/v) with 2wt% LiNO3/ 650 h [81] Zn-MXene 1 mA cm−2 1 mAh cm−2 1 M LiTFSI in DOL/DME
(1∶1, v/v).16 mV 1200 h [82] MXene/ZnO 1 mA cm−2 1 mAh cm−2 2 M LiTFSI in DME
with 1wt% FEC37±6 mV 250 h [84] CNT/MXene/SnO2 40 mA cm−2 1 mAh cm−2 1 M LiPF6 in EC/EMC
(1∶1, v/v) with 15wt% FEC85 mV 500 cycles [93] MXene@CNF 0.5 mA cm−2 1 mAh cm−2 1 M LiTFSI in DOL/DME
(1∶1, v/v) with 1wt% LiNO347 mV 1300 h [94] Ti3C2-LiB-Li 1 mA cm−2 1 mAh cm−2 1 M LiTFSI in DOL/DME
(1∶1, v/v) with 1wt% LiNO3/ 1000 h [96] Ti3C2Tx-rGO 1 mA cm−2 1 mAh cm−2 1M LiPF6 in EC/DMC/EMC
(1∶1:1, v/v/v) with 5.0% FEC36 mV 1400 h [99] 3D MG 5 mA cm−2 1 mAh cm−2 1 M LiTFSI in DOL/DME
(1∶1, v/v) with 1wt% LiNO325 mV 400 h [100] MXene-MF 5 mA cm−2 5 mAh cm−2 1 M LiTFSI in DOL/DME
(1∶1, v/v) with 1wt% LiNO3.13 mV 700 h [101] Note: M: mol L−1 -
[1] Tarascon J M, Armand M. Building better batteries[J]. Nature,2008,451(7179):652-657. doi: 10.1038/451652a [2] Liu Y M, Zhang Y J, Cheng K, et al. Inside cover: Selective electrochemical reduction of carbon dioxide to ethanol on a boron- and nitrogen-co-doped nanodiamond[J]. Angewandte Chemie,2017,56(49):15474-15474. doi: 10.1002/anie.201710883 [3] Etacheri V, Marom R, Elazari R, et al. Challenges in the development of advanced Li-ion batteries: A review[J]. Energy & Environmental Science,2011,4(9):3243-3262. [4] Lin D C, Liu Y Y, Cui Y. Reviving the lithium metal anode for high-energy batteries[J]. Nature Nanotechnology,2017,12(3):194-206. doi: 10.1038/nnano.2017.16 [5] Xu W, Wang J L, Ding F, et al. Lithium metal anodes for rechargeable batteries[J]. Energy & Environmental Science,2014,7(2):513-537. [6] Tarascon J M, Armand M. Issues and challenges facing rechargeable lithium batteries[J]. Nature,2001,414(6861):359-367. doi: 10.1038/35104644 [7] Li N W, Yin Y X, Yang C P, et al. An artificial solid electrolyte interphase layer for stable lithium metal anodes[J]. Advanced Materials,2016,28(9):1853-1858. doi: 10.1002/adma.201504526 [8] Wang L P, Wang Q J, Jia W S, et al. Li metal coated with amorphous Li3PO4 via magnetron sputtering for stable and long-cycle life lithium metal batteries[J]. Journal of Power Sources,2017,342:175-182. doi: 10.1016/j.jpowsour.2016.11.097 [9] Baloch M, Shanmukaraj D, Bondarchuk O, et al. Variations on Li3N protective coating using ex-situ and in-situ techniques for Li degree in sulphur batteries[J]. Energy Storage Materials,2017,9:141-149. doi: 10.1016/j.ensm.2017.06.016 [10] Yan C, Yao Y X, Chen X, et al. Lithium nitrate solvation chemistry in carbonate electrolyte sustains high-voltage lithium metal batteries[J]. Angewandte Chemie,2018,57(43):14292-14292. doi: 10.1002/anie.201811031 [11] Patra J, Huang H T, Xue W J, et al. Moderately concentrated electrolyte improves solid-electrolyte interphase and sodium storage performance of hard carbon[J]. Energy Storage Materials,2019,16:146-154. doi: 10.1016/j.ensm.2018.04.022 [12] Wang M Q, Peng Z, Luo W W, et al. Tailoring lithium deposition via an SEI-functionalized membrane derived from LiF decorated layered carbon structure[J]. Advanced Energy Materials,2019,9(12):1802912. doi: 10.1002/aenm.201802912 [13] Liu Y J, Tao X Y, Wang Y, et al. Self-assembled monolayers direct a LiF-rich interphase toward long-life lithium metal batteries[J]. Science,2022,375(6582):739-745. doi: 10.1126/science.abn1818 [14] Li Y T, Xu B Y, Xu H H, et al. Hybrid polymer/garnet electrolyte with a small interfacial resistance for lithium-ion batteries[J]. Angewandte Chemie,2017,129(3):771-774. doi: 10.1002/ange.201608924 [15] Sheng O W, Jin C B, Ju Z J, et al. Stabilizing Li4SnS4 electrolyte from interface to bulk phase with a gradient lithium iodide/polymer layer in lithium metal batteries[J]. Nano Letters,2022,22(20):8346-8354. doi: 10.1021/acs.nanolett.2c03291 [16] Wan J Y, Xie J, Kong X, et al. Ultrathin, flexible, solid polymer composite electrolyte enabled with aligned nanoporous host for lithium batteries[J]. Nature Nanotechnology,2019,14(7):705-711. doi: 10.1038/s41565-019-0465-3 [17] Lin D C, Yuen P Y, Liu Y Y, et al. A silica-aerogel-reinforced composite polymer electrolyte with high ionic conductivity and high modulus[J]. Advanced Materials,2018,30(32):1802661. doi: 10.1002/adma.201802661 [18] Chi S S, Liu Y C, Zhao N, et al. Solid polymer electrolyte soft interface layer with 3D lithium anode for all-solid-state lithium batteries[J]. Energy Storage Materials,2019,17:309-316. doi: 10.1016/j.ensm.2018.07.004 [19] Sun C Z, Li Y P, Jin J, et al. ZnO nanoarray-modified nickel foam as a lithiophilic skeleton to regulate lithium deposition for lithium-metal batteries[J]. Journal of Materials Chemistry A,2019,7(13):7752-7759. doi: 10.1039/C9TA00862D [20] Deng W, Zhou X F, Fang Q, et al. Microscale lithium metal stored inside cellular graphene scaffold toward advanced metallic lithium anodes[J]. Advanced Energy Materials,2018,8(12):1703152. doi: 10.1002/aenm.201703152 [21] Naguib M, Kurtoglu M, Presser V, et al. Two-dimensional nanocrystals: Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2[J]. Advanced Materials,2011,23(37):4207-4207. doi: 10.1002/adma.201190147 [22] Luo J M, Zheng J H, Nai J W, et al. Atomic sulfur covalently engineered interlayers of Ti3C2 MXene for ultra-fast sodium-ion storage by enhanced pseudocapacitance[J]. Advanced Functional Materials,2019,29(10):1808107. doi: 10.1002/adfm.201808107 [23] Naguib M, Mochalin V N, Barsoum M W, et al. 25th anniversary article: MXenes: A new family of two-dimensional materials[J]. Advanced Materials,2014,26(7):992-1005. doi: 10.1002/adma.201304138 [24] Sarycheva A, Gogotsi Y. Raman spectroscopy analysis of the structure and surface chemistry of Ti3C2Tx MXene[J]. Chemistry of Materials,2020,32(8):3480-3488. doi: 10.1021/acs.chemmater.0c00359 [25] Urbankowski P, Anasori B, Makaryan T, et al. Synthesis of two-dimensional titanium nitride Ti4N3 (MXene)[J]. Nanoscale,2016,8(22):11385-11391. doi: 10.1039/C6NR02253G [26] Halim J, Kota S, Lukatskaya M R, et al. Synthesis and characterization of 2D molybdenum carbide (MXene)[J]. Advanced Functional Materials,2016,26(18):3118-3127. doi: 10.1002/adfm.201505328 [27] Nan J X, Guo X, Xiao J, et al. Nanoengineering of 2D MXene-based materials for energy storage applications[J]. Small,2021,17(9):1902085. doi: 10.1002/smll.201902085 [28] Sun W, Shah S A, Chen Y, et al. Electrochemical etching of Ti2AlC to Ti2CTx (MXene) in low-concentration hydrochloric acid solution[J]. Journal of Materials Chemistry A,2017,5(41):21663-21668. doi: 10.1039/C7TA05574A [29] Yang S, Zhang P P, Wang F X, et al. Fluoride-free synthesis of two-dimensional titanium carbide (MXene) using a binary aqueous system[J]. Angewandte Chemie,2018,57(47):15491-15495. doi: 10.1002/anie.201809662 [30] Ding R, Lyu Y, Wu Z, et al. Effective piezo-phototronic enhancement of flexible photodetectors based on 2D hybrid perovskite ferroelectric single-crystalline thin-films[J]. Advanced Materials,2021,33(32):2170252. doi: 10.1002/adma.202170252 [31] Li T F, Yao L L, Liu Q L, et al. Fluorine-free synthesis of high-purity Ti3C2Tx (T=OH, O) via alkali treatment[J]. Angewandte Chemie,2018,57(21):6115-6119. doi: 10.1002/anie.201800887 [32] Natu V, Pai R, Sokol M, et al. 2D Ti3C2Tz MXene synthesized by water-free etching of Ti3AlC2 in polar organic solvents[J]. Chem,2020,6(3):616-630. doi: 10.1016/j.chempr.2020.01.019 [33] Li Y B, Shao H, Lin Z F, et al. A general lewis acidic etching route for preparing MXenes with enhanced electrochemical performance in non-aqueous electrolyte[J]. Nature Materials,2020,19(8):894-899. doi: 10.1038/s41563-020-0657-0 [34] Xiao X, Yu H M, Jin H Y, et al. Salt-templated synthesis of 2D metallic mon and other nitrides[J]. ACS Nano,2017,11(2):2180-2186. doi: 10.1021/acsnano.6b08534 [35] Mei J, Ayoko G A, Hu C, et al. Thermal reduction of sulfur-containing MAX phase for MXene production[J]. Chemical engineering journal,2020,395:125111. doi: 10.1016/j.cej.2020.125111 [36] Xu C, Wang L B, Liu Z B, et al. Large-area high-quality 2D ultrathin Mo2C superconducting crystals[J]. Nature Materials,2015,14(11):1135-1141. doi: 10.1038/nmat4374 [37] Luo J M, Matios E, Wang H, et al. Interfacial structure design of MXene-basednanomaterials for electrochemical energy storage and conversion[J]. InfoMat,2020,2(6):1057-1076. doi: 10.1002/inf2.12118 [38] Luo J M, Fang C, Jin C B, et al. Tunable pseudocapacitance storage of MXene by cation pillaring for high performance sodium-ion capacitors[J]. Journal of Materials Chemistry A,2018,6(17):7794-7806. doi: 10.1039/C8TA02068J [39] Cho K T, Ridgway P, Weber A Z, et al. Erratum: High performance hydrogen/bromine redox flow battery for grid-scale energy storage[J]. Journal of the Electrochemical Society,2013,160(8):X9-X9. doi: 10.1149/2.006308jes [40] Albertus P, Girishkumar G, McCloskey B, et al. Identifying capacity limitations in the li/oxygen battery using experiments and modeling[J]. Journal of the Electrochemical Society,2011,158(3):A343-A351. doi: 10.1149/1.3527055 [41] Shen X, Liu H, Cheng X B, et al. Beyond lithium ion batteries: Higher energy density battery systems based on lithium metal anodes[J]. Energy Storage Materials,2018,12:161-175. doi: 10.1016/j.ensm.2017.12.002 [42] Zhang T, Zhang L, Hou Y L. MXenes: Synthesis strategies and lithium-sulfur battery applications[J]. eScience,2022,2(2):164-182. doi: 10.1016/j.esci.2022.02.010 [43] Wang Q, Liu B, Shen Y, et al. Confronting the challenges in lithium anodes for lithium metal batteries[J]. Advanced Science,2021,8(17):2101111. doi: 10.1002/advs.202101111 [44] Howlett P C, MacFarlane D R, Hollenkamp A F. A sealed optical cell for the study of lithium-electrode| electrolyte interfaces[J]. Journal of Power Sources,2003,114(2):277-284. doi: 10.1016/S0378-7753(02)00603-1 [45] Albertus P, Babinec S, Litzelman S, et al. Status and challenges in enabling the lithium metal electrode for high-energy and low-cost rechargeable batteries[J]. Nature Energy,2018,3(1):16-21. [46] Zhang X Y, Wang A X, Liu X J, et al. Dendrites in lithium metal anodes: Suppression, regulation, and elimination[J]. Accounts of Chemical Research,2019,52(11):3223-3232. doi: 10.1021/acs.accounts.9b00437 [47] Chazalviel J N. Electrochemical aspects of the generation of ramified metallic electrodeposits[J]. Physical Review,1990,42(12):7355-7367. doi: 10.1103/PhysRevA.42.7355 [48] Fleury V, Chazalviel J N, Rosso M, et al. The role of the anions in the growth speed of fractal electrodeposits[J]. Journal of Electroanalytical Chemistry and Interfacial Electrochemistry,1990,290(1):249-255. [49] Liang Y R, Xiao Y, Yan C, et al. A bifunctional ethylene-vinyl acetate copolymer protective layer for dendrites-free lithium metal anodes[J]. Journal of Energy Chemistry,2020,48:203-207. doi: 10.1016/j.jechem.2020.01.027 [50] Yamaki J, Tobishima S, Hayashi K, et al. A consideration of the morphology of electrochemically deposited lithium in an organic electrolyte[J]. Journal of Power Sources,1998,74(2):219-227. doi: 10.1016/S0378-7753(98)00067-6 [51] Wang X, Zeng W, Hong L, et al. Stress-driven lithium dendrite growth mechanism and dendrite mitigation by electroplating on soft substrates[J]. Nature Energy,2018,3(3):227-235. doi: 10.1038/s41560-018-0104-5 [52] Peled E. The electrochemical-behavior of alkali and alkaline-earth metals in non-aqueous battery systems-the solid electrolyte interphase model[J]. Journal of the Electrochemical Society,1979,126(12):2047-2051. doi: 10.1149/1.2128859 [53] Peled E, Menkin S. Review-SEI: Past, present and future[J]. Journal of the Electrochemical Society,2017,164(7):A1703-A1719. doi: 10.1149/2.1441707jes [54] Thevenin J G, Muller R H. Impedance of lithium electrodes in a propylene carbonate electrolyte[J]. Journal of the Electrochemical Society,1987,134(2):273-280. doi: 10.1149/1.2100445 [55] Verma P, Maire P, Novák P. A review of the features and analyses of the solid electrolyte interphase in Li-ion batteries[J]. Electrochimica Acta,2010,55(22):6332-6341. doi: 10.1016/j.electacta.2010.05.072 [56] Aurbach D, Chusid O. In situ FTIR spectroelectrochemical studies of surface films formed on Li and nonactive electrodes at low potentials in Li salt solutions containing CO2[J]. Journal of the Electrochemical Society,1993,140(11):L155-L157. doi: 10.1149/1.2221034 [57] Odziemkowski M, Irish D E. An electrochemical study of the reactivity at the lithium electrolyte/bare lithium metal interface I. Purified electrolytes[J]. Journal of the Electrochemical Society,1992,139(11):3063-3074. doi: 10.1149/1.2069033 [58] Kanamura K, Shiraishi S, Takehara Z. Electrochemical deposition of very smooth lithium using nonaqueous electrolytes containing HF[J]. Journal of the Electrochemical Society,1996,143(7):2187-2197. doi: 10.1149/1.1836979 [59] Ghazi Z A, Sun Z, Sun C, et al. Key aspects of lithium metal anodes for lithium metal batteries[J]. Small,2019,15(32):1900687. doi: 10.1002/smll.201900687 [60] Shi P, Li T, Zhang R, et al. Lithiophilic LiC6 layers on carbon hosts enabling stable li metal anode in working batteries[J]. Advanced Materials,2019,31(8):1807131. doi: 10.1002/adma.201807131 [61] Hu W, Zheng M, Xu B, et al. Design of hollow carbon-based materials derived from metal-organic frameworks for electrocatalysis and electrochemical energy storage[J]. Journal of Materials Chemistry A,2021,9(7):388-3917. [62] Zhu Z, Liu Y, Ju Z, et al. Synthesis of diverse green carbon nanomaterials through fully utilizing biomass carbon source assisted by KOH[J]. ACS Applied Materials & Interfaces,2019,11(27):24205-24211. [63] Chi S S, Liu Y, Song W L, et al. Prestoring lithium into stable 3D nickel foam host as dendrite-free lithium metal anode[J]. Advanced Functional Materials,2017,27(24):1700348. doi: 10.1002/adfm.201700348 [64] Fan W, Li N W, Zhang X, et al. A dual-salt gel polymer electrolyte with 3D cross-linked polymer network for dendrite-free lithium metal batteries[J]. Advanced Science,2018,5(9):1800559. doi: 10.1002/advs.201800559 [65] Lucero N, Vilcarino D, Datta D, et al. The roles of MXenes in developing advanced lithium metal anodes[J]. Journal of Energy Chemistry,2022,69:132-149. doi: 10.1016/j.jechem.2022.01.011 [66] Li B, Zhang D, Liu Y, et al. Flexible Ti3C2 MXene-lithium film with lamellar structure for ultrastable metallic lithium anodes[J]. Nano Energy,2017,39:654-661. doi: 10.1016/j.nanoen.2017.07.023 [67] Zhang D, Wang S, Li B, et al. Horizontal growth of lithium on parallelly aligned MXene layers towards dendrite-free metallic lithium anodes[J]. Advanced Materials,2019,31(33):1901820. doi: 10.1002/adma.201901820 [68] Gu J, Chen H, Shi Y, et al. Eliminating lightning-rod effect of lithium anodes via sine-wave analogous MXene layers[J]. Advanced Energy Materials,2022,12(36):2201181. doi: 10.1002/aenm.202201181 [69] Chen X, Shang M, Niu J. Inter-layer-calated thin li metal electrode with improved battery capacity retention and dendrite suppression[J]. Nano Letters,2020,20(4):2639-2646. doi: 10.1021/acs.nanolett.0c00201 [70] Chen Q, Wei Y, Zhang X, et al. Vertically aligned MXene nanosheet arrays for high-rate lithium metal anodes[J]. Advanced Energy Materials,2022,12(18):2200072. doi: 10.1002/aenm.202200072 [71] Cao Z, Zhu Q, Wang S, et al. Perpendicular MXene arrays with periodic interspaces toward dendrite-free lithium metal anodes with high-rate capabilities[J]. Advanced Functional Materials,2020,30(5):1908075. doi: 10.1002/adfm.201908075 [72] Tian X, Jin J, Yuan S, et al. Emerging 3D-printed electrochemical energy storage devices: A critical review[J]. Advanced Energy Materials,2017,7(17):1700127. doi: 10.1002/aenm.201700127 [73] Zhang C, McKeon L, Kremer M P, et al. Additive-free MXene inks and direct printing of micro-supercapacitors[J]. Nature Communications,2019,10(1):1795-1795. doi: 10.1038/s41467-019-09398-1 [74] Li K, Liang M, Wang H, et al. 3D MXene architectures for efficient energy storage and conversion[J]. Advanced Functional Materials,2020,30(47):2000842. doi: 10.1002/adfm.202000842 [75] Shen K, Li B, Yang S. 3D printing dendrite-free lithium anodes based on the nucleated MXene arrays[J]. Energy Storage Materials,2020,24:670-675. doi: 10.1016/j.ensm.2019.08.015 [76] Luo J M, Zhang W K, Yuan H D, et al. Pillared structure design of MXene with ultralarge interlayer spacing for high-performance lithium-ion capacitors[J]. ACS Nano,2017,11(3):2459-2469. doi: 10.1021/acsnano.6b07668 [77] Luo J M, Wang C L, Wang H, et al. Pillared MXene with ultralarge interlayer spacing as a stable matrix for high performance sodium metal anodes[J]. Advanced Functional Materials,2019,29(3):1805946. doi: 10.1002/adfm.201805946 [78] Tang J, Peng X, Lin T, et al. Confining ultrafine tin monophosphide in Ti3C2Tx interlayers for rapid and stable sodium ion storage[J]. eScience,2021,1(2):203-211. doi: 10.1016/j.esci.2021.12.004 [79] Pu J, Li J, Shen Z, et al. Interlayer lithium plating in Au nanoparticles pillared reduced graphene oxide for lithium metal anodes[J]. Advanced Functional Materials,2018,28(41):1804133. doi: 10.1002/adfm.201804133 [80] Yan K, Lu Z, Lee H W, et al. Selective deposition and stable encapsulation of lithium through heterogeneous seeded growth[J]. Nature Energy,2016,1(3):16010. doi: 10.1038/nenergy.2016.10 [81] Qian Y, Wei C, Tian Y, et al. Constructing ultrafine lithiophilic layer on MXene paper by sputtering for stable and flexible 3D lithium metal anode[J]. Chemical Engineering Journal,2021,421:129685. doi: 10.1016/j.cej.2021.129685 [82] Gu J, Zhu Q, Shi Y, et al. Single zinc atoms immobilized on MXene (Ti3C2Clx) layers toward dendrite-free lithium metal anodes[J]. ACS Nano,2020,14(1):891-898. doi: 10.1021/acsnano.9b08141 [83] Tian Y, An Y, Wei C, et al. Flexible and free-standing Ti3C2Tx MXene@Zn paper for dendrite-free aqueous zinc metal batteries and nonaqueous lithium metal batteries[J]. ACS Nano,2019,13(10):11676-11685. doi: 10.1021/acsnano.9b05599 [84] Shen Y, Pu Z, Zhang Y, et al. MXene/ZnO flexible freestanding film as a dendrite-free support in lithium metal batteries[J]. Journal of Materials Chemistry A, 2022, 1(33): 17199-1727. [85] Na Z, Li W, Li L, et al. Conductive iodine-doped red phosphorus enabled dendrite-free lithium deposition on MXene matrix[J]. Small,2022,18(48):2204341. doi: 10.1002/smll.202204341 [86] Zhang W, Jin H, Du Y, et al. Sulfur and nitrogen codoped Nb2C MXene for dendrite-free lithium metal battery[J]. Electrochimica Acta,2021,390:138812. doi: 10.1016/j.electacta.2021.138812 [87] Wei C, Fei H, Tian Y, et al. Isotropic Li nucleation and growth achieved by an amorphous liquid metal nucleation seed on MXene framework for dendrite-free Li metal anode[J]. Energy Storage Materials,2020,26:223-233. doi: 10.1016/j.ensm.2020.01.005 [88] Zhu M, Xu K, Li D, et al. Guiding smooth Li plating and stripping by a spherical island model for lithium metal anodes[J]. ACS Applied Materials & Interfaces,2020,12(34):38098-38105. [89] Oyakhire S T, Huang W, Wang H, et al. Revealing and elucidating ALD-derived control of lithium plating microstructure[J]. Advanced Energy Materials,2020,10(44):2002736. doi: 10.1002/aenm.202002736 [90] Luan J, Zhang Q, Yuan H, et al. Sn layer decorated copper mesh with superior lithiophilicity for stable lithium metal anode[J]. Chemical Engineering Journal,2020,395:124922. doi: 10.1016/j.cej.2020.124922 [91] Luo J M, Tao X Y, Zhang J, et al. Sn4+ ion decorated highly conductive Ti3C2 MXene: Promising lithium-ion anodes with enhanced volumetric capacity and cyclic performance[J]. ACS Nano,2016,10(2):2491-2499. doi: 10.1021/acsnano.5b07333 [92] Luo J M, Lu X, Matios E, et al. Tunable MXene-derived 1D/2D hybrid nanoarchitectures as a stable matrix for dendrite-free and ultrahigh capacity sodium metal anode[J]. Nano Letters,2020,20(10):7700-7708. doi: 10.1021/acs.nanolett.0c03215 [93] Liu Y, Sun C, Lu Y, et al. Lamellar-structured anodes based on lithiophilic gradient enable dendrite-free lithium metal batteries with high capacity loading and fast-charging capability[J]. Chemical Engineering Journal,2023,451:138570. doi: 10.1016/j.cej.2022.138570 [94] Wang C Y, Zheng Z J, Feng Y Q, et al. Topological design of ultrastrong MXene paper hosted Li enables ultrathin and fully flexible lithium metal batteries[J]. Nano Energy,2020,74:104817. doi: 10.1016/j.nanoen.2020.104817 [95] Qian X, Fan X, Peng Y, et al. Polysiloxane cross-linked mechanically stable MXene-based lithium host for ultrastable lithium metal anodes with ultrahigh current densities and capacities[J]. Advanced Functional Materials,2021,31(6):2008044. doi: 10.1002/adfm.202008044 [96] Zhao Z, Li B. Multi-storey corridor structured host for a large area capacity and high rate metallic lithium anode[J]. Electrochimica Acta,2021,365:137341. doi: 10.1016/j.electacta.2020.137341 [97] Shao J J, Lv W; Yang Q H. Graphene: Self-assembly of graphene oxide at interfaces[J]. Advanced Materials,2014,26(32):5732-5732. doi: 10.1002/adma.201470222 [98] Zhang X, Lv R, Wang A, et al. MXene aerogel scaffolds for high-rate lithium metal anodes[J]. Angewandte Chemie,2018,57(46):15028-15033. doi: 10.1002/anie.201808714 [99] Fang Y, Zhang Y, Zhu K, et al. Lithiophilic three -dimensional porous Ti3C2Tx-rGO membrane as a stable scaffold for safe alkali metal (Li or Na) anodes[J]. ACS Nano,2019,13(12):14319-14328. doi: 10.1021/acsnano.9b07729 [100] Shi H, Zhang C J, Lu P, et al. Conducting and lithiophilic MXene/graphene framework for high-capacity, dendrite-free lithium–metal anodes[J]. ACS Nano,2019,13(12):14308-14318. doi: 10.1021/acsnano.9b07710 [101] Shi H, Yue M, Zhang C J, et al. 3D flexible, conductive, and recyclable Ti3C2Tx MXene-melamine foam for high-areal-capacity and long-lifetime alkali-metal anode[J]. ACS Nano,2020,14(7):8678-8688. doi: 10.1021/acsnano.0c03042 [102] Guo D, Ming F, Shinde D B, et al. Covalent assembly of two-dimensional COF-on-MXene heterostructures enables fast charging lithium hosts[J]. Advanced Functional Materials,2021,31(25):2101194-n/a. doi: 10.1002/adfm.202101194 [103] Wang J, Yang M, Zou G, et al. Lithiation MXene derivative skeletons for wide-temperature lithium metal anodes[J]. Advanced Functional Materials,2021,31(21):2101180. doi: 10.1002/adfm.202101180 [104] Zhao F, Zhai P, Wei Y, et al. Constructing artificial SEI layer on lithiophilic MXene surface for high-performance lithium metal anodes[J]. Advanced Science,2022,9(6):2103930. doi: 10.1002/advs.202103930 [105] Huang T, Xiong W, Ye X, et al. Constructing robust polymer/two-dimensional Ti3C2Tx solid-state electrolyte interphase via in-situ polymerization for high-capacity long-life and dendrite-free lithium metal anodes[J]. Journal of Colloid and Interface Science,2022,628:583-594. doi: 10.1016/j.jcis.2022.08.101 [106] Lee S H, Kim M S, Lee J-H, et al. A Li-In alloy anode and Nb2CTx artificial solid-electrolyte interphase for practical li metal batteries[J]. Journal of materials chemistry A,2022,10(8):4157-4169. doi: 10.1039/D1TA09366E [107] Lagadec M F, Zahn R; Wood V. Characterization and performance evaluation of lithium-ion battery separators[J]. Nature Energy,2019,4(1):16-25. [108] Zhang Y, Yang G, Lehmann M L, et al. Separator effect on zinc electrodeposition behavior and its implication for zinc battery lifetime[J]. Nano Letters,2021,21(24):10446-10452. doi: 10.1021/acs.nanolett.1c03792 [109] Hao H, Hutter T, Boyce B L, et al. Review of multifunctional separators: Stabilizing the cathode and the anode for alkali (Li, Na, and K) metal–sulfur and selenium batteries[J]. Chemical Reviews,2022,122(9):8053-8125. doi: 10.1021/acs.chemrev.1c00838 [110] Li Y, Gao T, Ni D, et al. Two birds with one stone: Interfacial engineering of multifunctional janus separator for lithium–sulfur batteries[J]. Advanced Materials,2022,34(5):2107638. doi: 10.1002/adma.202107638 [111] Zhou W, Chen M, Tian Q, et al. Cotton-derived cellulose film as a dendrite-inhibiting separator to stabilize the zinc metal anode of aqueous zinc ion batteries[J]. Energy Storage Matarials,2022,44:57-65. doi: 10.1016/j.ensm.2021.10.002 [112] Zhao Q, Zhou R, Wang C, et al. Anion immobilization enabled by cation-selective separators for dendrite-free lithium metal batteries[J]. Advanced Functional Materials,2022,32(23):2112711. doi: 10.1002/adfm.202112711 [113] Han X, Chen J, Chen M, et al. Induction of planar Li growth with designed interphases for dendrite-free Li metal anodes[J]. Energy Storage Materials,2021,39:250-258. doi: 10.1016/j.ensm.2021.12.033 [114] Wen J, Huang L, Huang Y, et al. A lithium-MXene composite anode with high specific capacity and low interfacial resistance for solid-state batteries[J]. Energy Storage Materials,2022,45:934-940. [115] Kumar B, Scanlon L G, Spry R J. On the origin of conductivity enhancement in polymer-ceramic composite electrolytes[J]. Journal of Power Sources,2001,96(2):337-342. doi: 10.1016/S0378-7753(00)00665-0 [116] Pan Q, Zheng Y, Kota S, et al. 2D MXene-containing polymer electrolytes for all-solid-state lithium metal batteries[J]. Nanoscale Advances,2019,1(1):395-402. doi: 10.1039/C8NA00206A -





 下载:
下载: