-
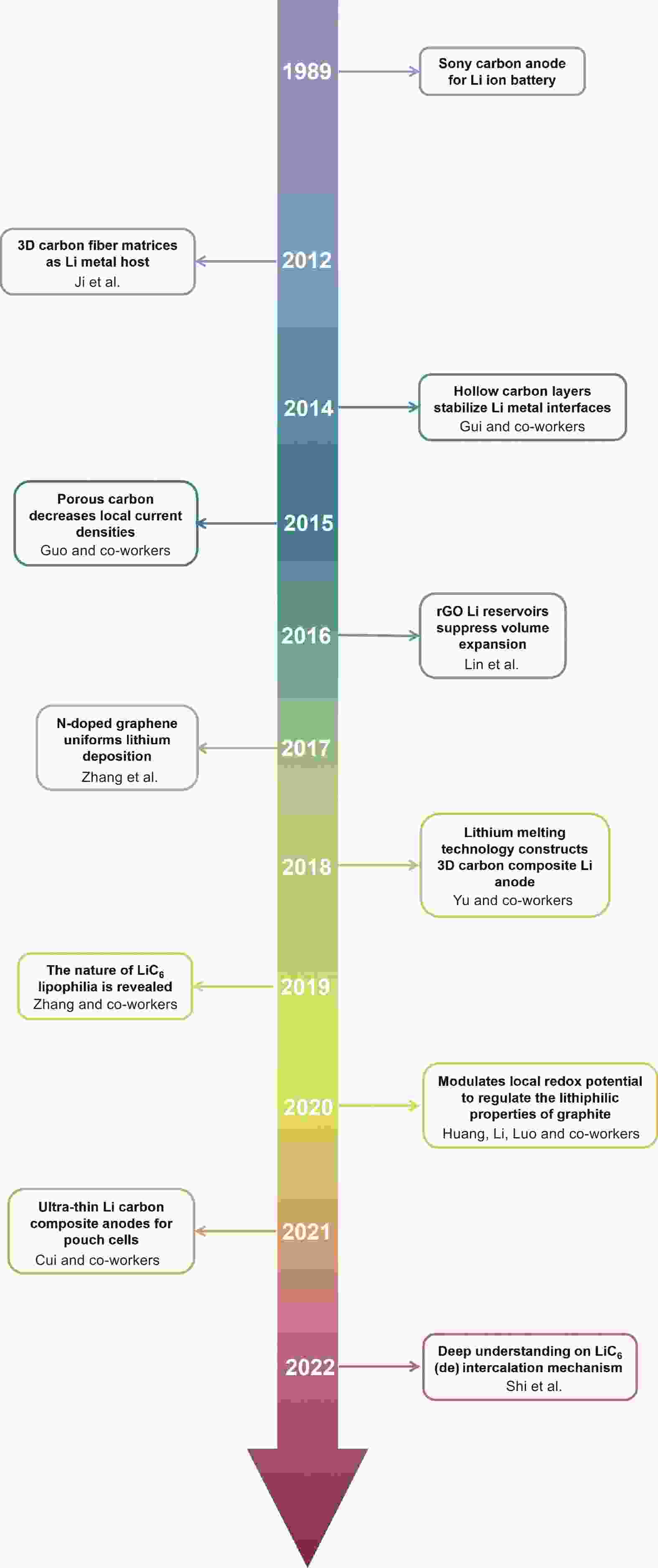
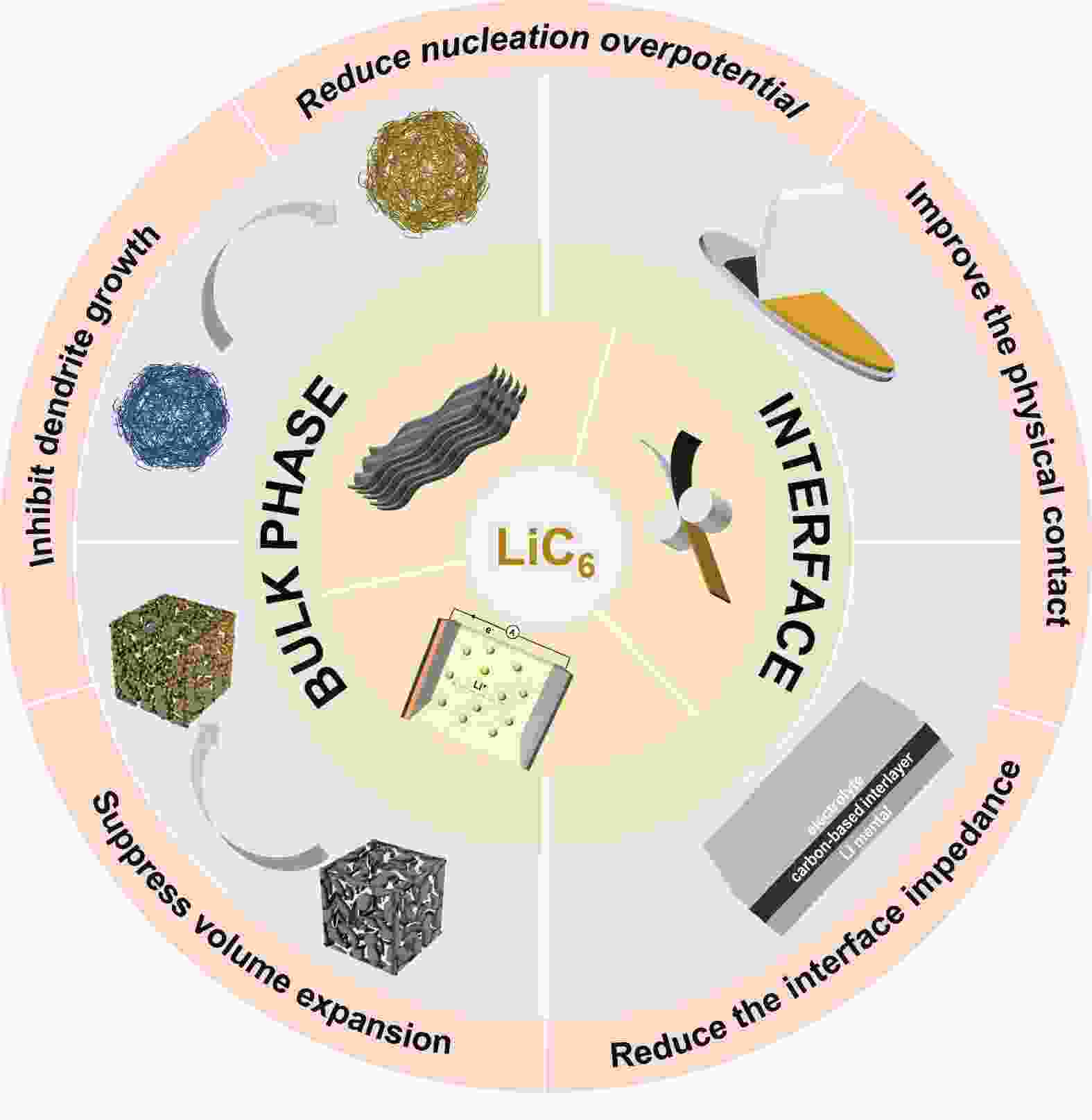
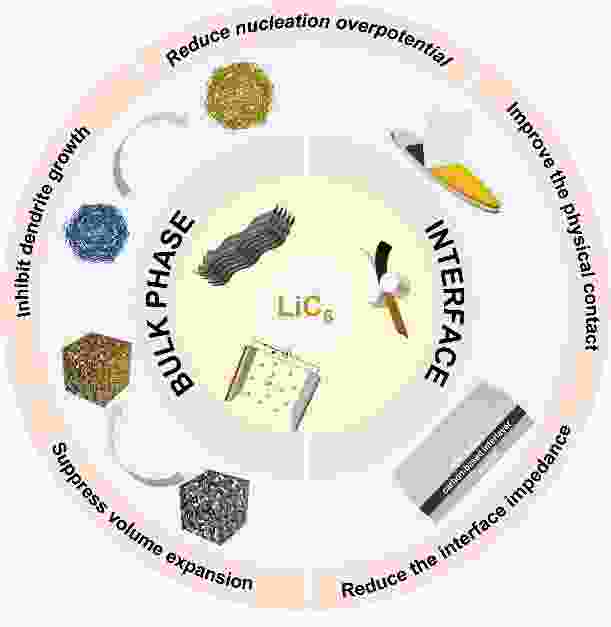
摘要: 锂金属电池(LMB)被认为是最有前景的下一代储能系统之一。然而,其实际应用存在极大的体积变化和锂枝晶的形成等重大挑战。为了应对这些挑战,引入炭材料的复合锂负极因其优异的化学和电化学稳定性以及在重复循环过程中的优异机械强度引起了极大关注。本文旨在全面概述锂化石墨(LiC6)的形成及其在锂沉积和剥离过程中在体相和界面区域的作用。在体相中,LiC6表现出优异的亲锂性能,降低了锂成核的过电位并促进锂的均匀沉积。当在电极-电解质界面引入LiC6时,它通过充当缓冲层来增强电极和电解质之间的接触,从而降低界面阻抗。最后,本文评述了锂碳复合负极发展的前景和挑战。
-
关键词:
- 锂-碳复合负极 /
- 锂化石墨(LiC6) /
- 亲锂化学 /
- 锂枝晶生长 /
- 锂沉积/剥离
Abstract: To solve the problems of large volume changes and the formation of lithium dendrites in lithium metal batteries, the incorporation of carbon into the lithium metal anodes has gained considerable attention due to its excellent chemical and electrochemical stability, as well as its exceptional mechanical strength that allows repeated cycling. This review provides a comprehensive overview of the formation of lithiated graphite (LiC6) and its role in both bulk and electrode-electrolyte interface regions during lithium plating and stripping. In bulk form, LiC6 has excellent lithiophilic properties, reducing the overpotential for lithium nucleation and promoting uniform lithium deposition. Additionally, when LiC6 is introduced at the electrode-electrolyte interfaces, it improves contact between the electrode and electrolyte by acting as a buffering layer, thereby reducing interfacial impedance. Finally, prospects and challenges for the development of Li/C composite anodes are discussed. -
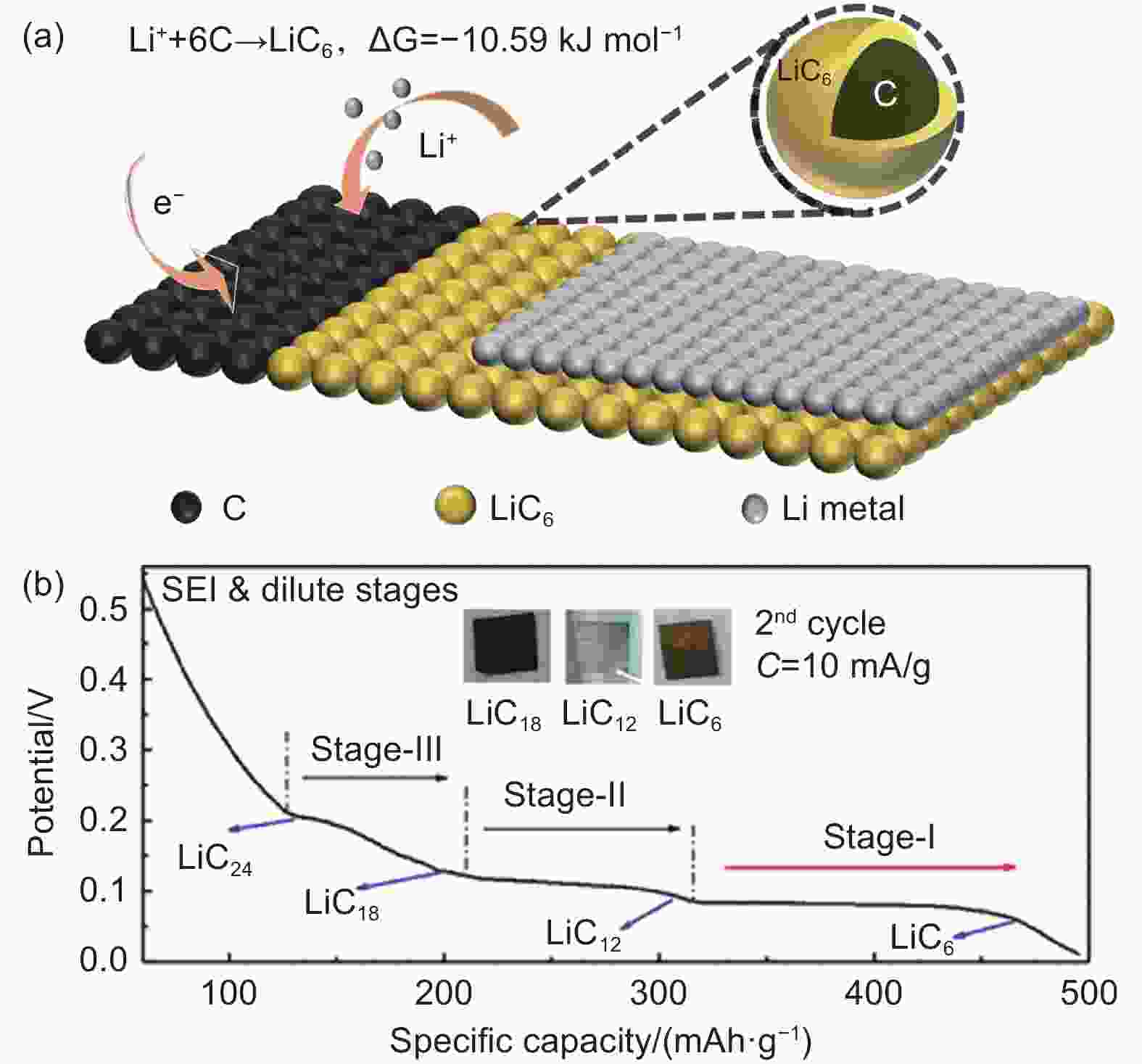
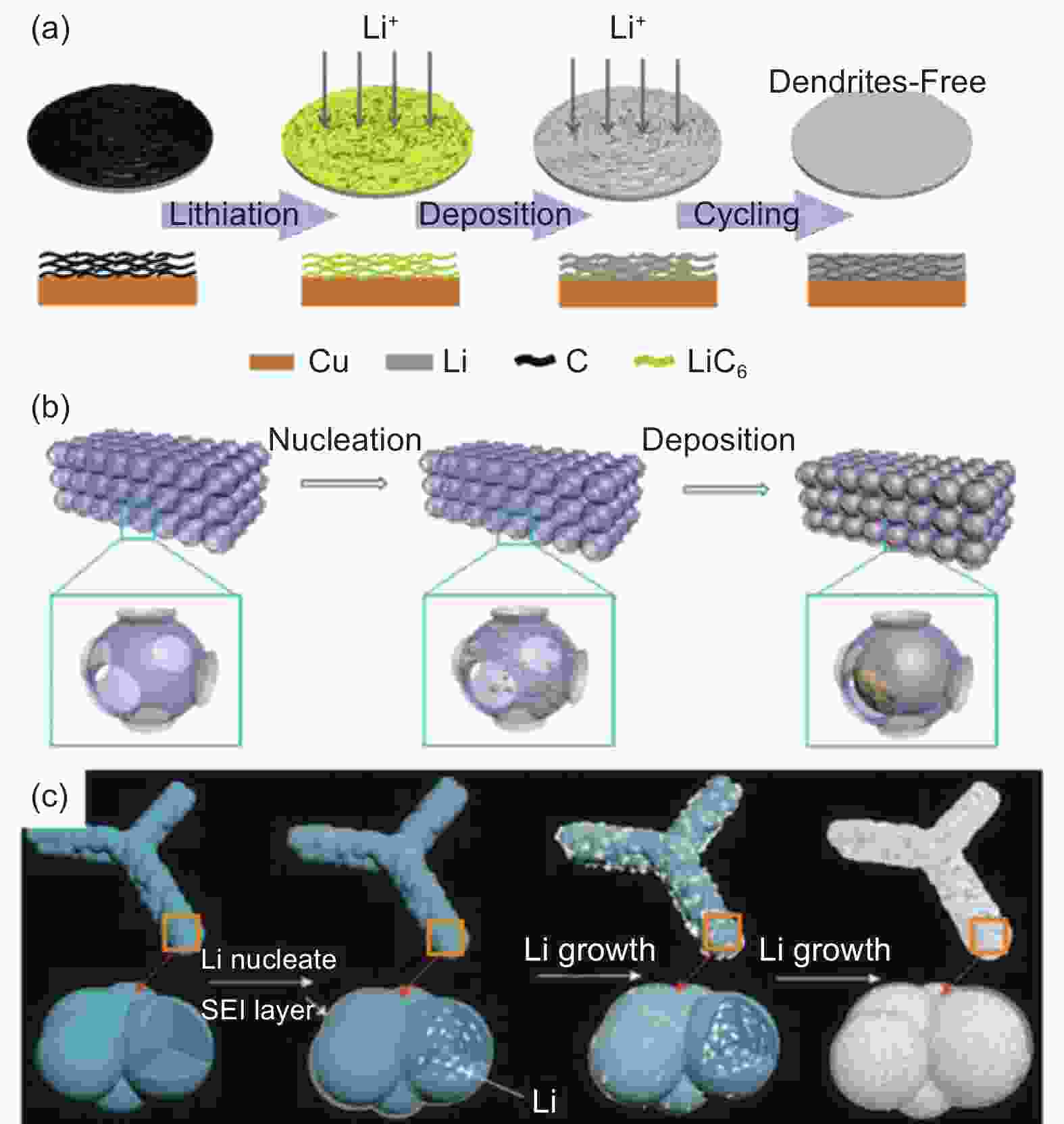
Figure 3. (a) Schematic of the mechanism of graphite reacted with Li metal to generate LiC6. (b) Different lithiation stages of a carbon-based anode during charging. Reproduced with permission[81]. Copyright 2018 American Chemical Society
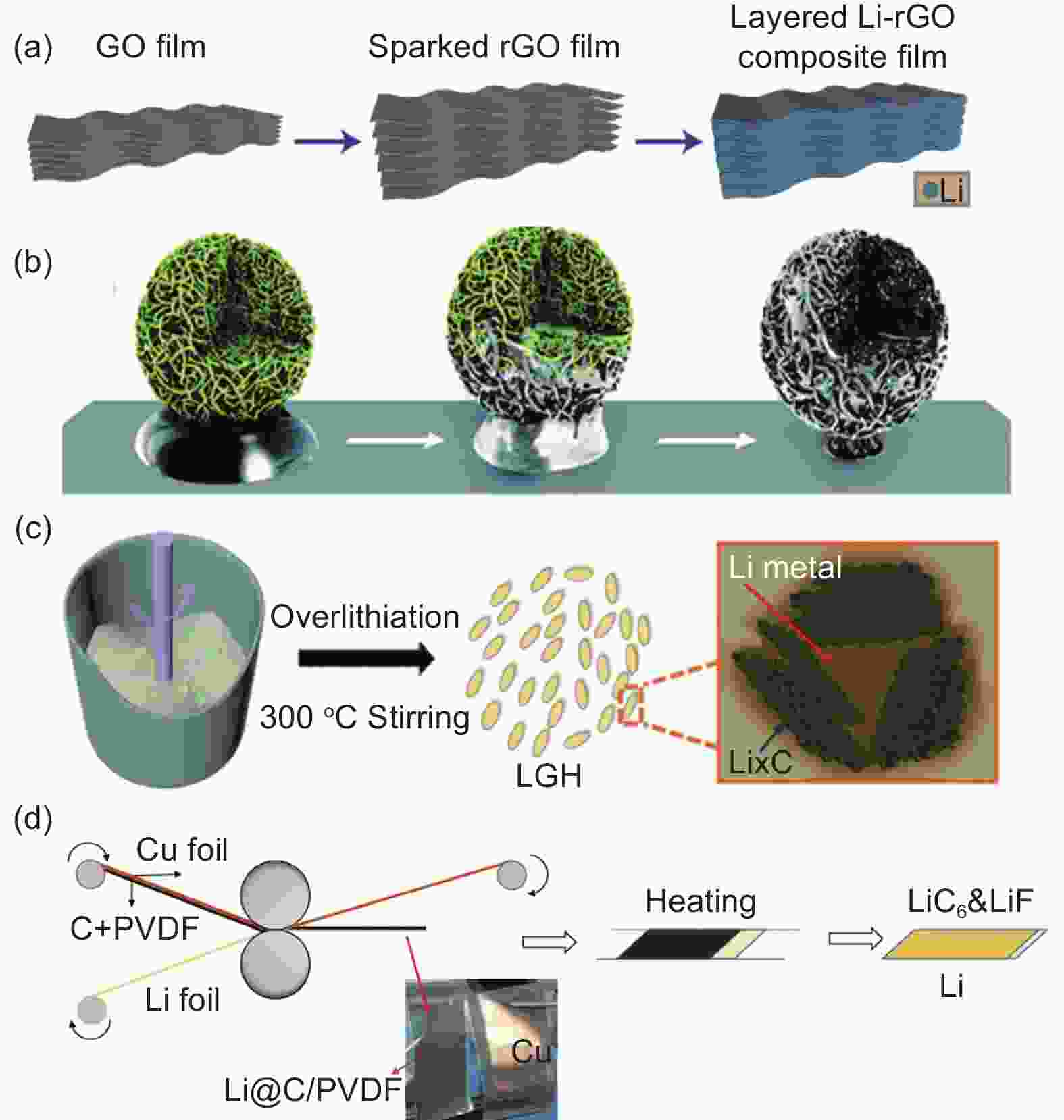
Figure 4. (a) Schematic illustration for the fabrication of the Li-rGO composite film by lithium metal melting. Reproduced with permission[65]. Copyright 2016, Springer Nature. (b) The construction of the Li-CNT composite anode. Reproduced with permission[95]. Copyright 2013, Royal Society of Chemistry. (c) The fabrication process of Li-graphite composite anode by the stirring and melting method. Reproduced with permission[99]. Copyright 2018, Elsevier. (d) Formation process of Li@LiC6&LiF-x composite anode materials. Reproduced with permission[104]. Copyright 2021 American Chemical Society
Figure 5. (a) Lithium process on a three-dimensional carbon modified electrode. Reproduced with permission[105]. Copyright 2019, Elsevier; (b) Schematic diagrams showing the Li nucleation and growth process on the 3D-GF. Reproduced with permission[106]. Copyright 2019, American Chemical Society. (c) Schematic diagrams of lithium plating process on the CMN. Reproduced with permission[107]. Copyright 2017, American Chemical Society
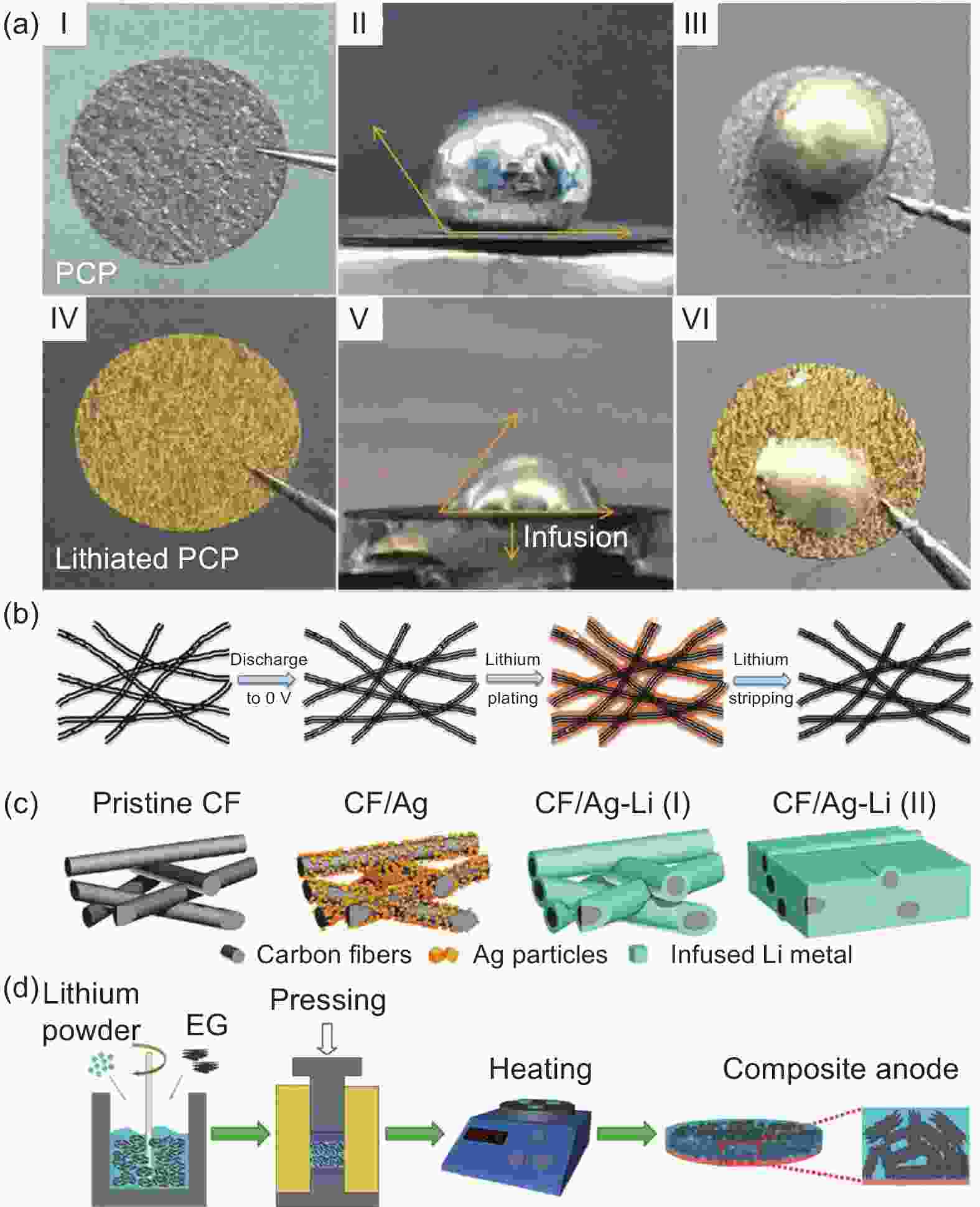
Figure 6. (a) Li wettability of PCP, lithiated PCP. ACA measurements of (I, II) PCP and (IV, V) lithiated PCP. Digital photos of a Li droplet on (III) PCP and (VI) lithiated PCP after cooling down to room temperature[68]. (b) Schematic diagrams of electrochemical plating/stripping process of lithium on the CNT sponges. Reproduced with permission[119]. Copyright 2019, American Chemical Society; (c) Schematic diagrams of coralloid layered composite electrode. Reproduced with permission[120]. Copyright 2018, Elsevier. (d) Schematic of the Li-EG composite anode fabrication process. Reproduced with permission[121]. Copyright 2019, Royal Society of Chemistry
Figure 7. Diagram of the overpotential and delithium mechanism in composite anode during initial and long cycles. Reproduced with permission[48]. Copyright 2022, American Chemical Society
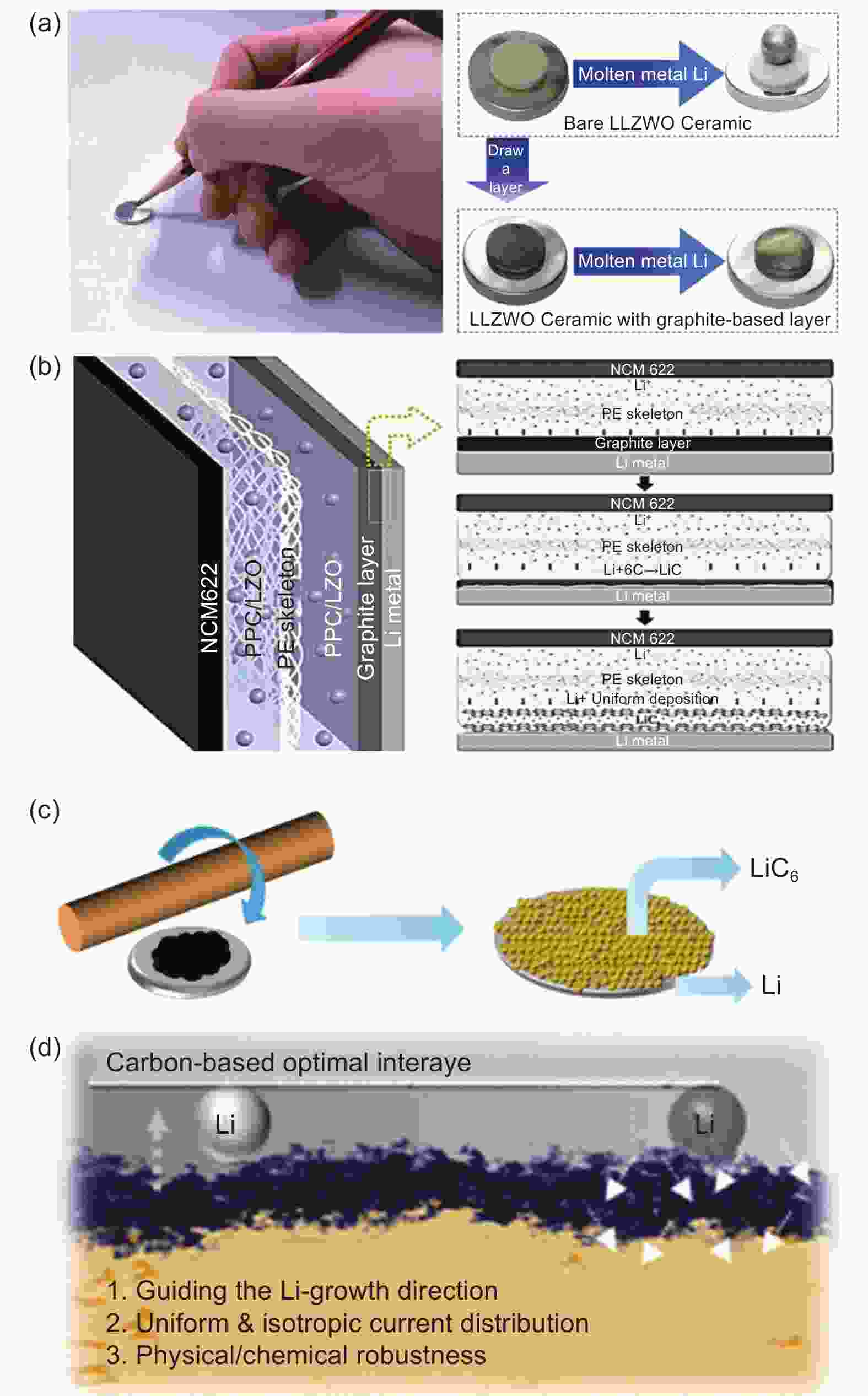
Figure 8. (a) A pencil sketch of the preparation of a graphite-based interfacial layer and a wetting behavior comparison between molten lithium metal on LALZWO ceramics with and without the interfacial layer. Reproduced with permission[122]. Copyright 2018, American Chemical Society. (b) Schematic diagram of the graphite-modified composite solid electrolyte membrane. Reproduced with permission[123]. Copyright 2020, American Chemical Society; (c) Schematic diagram of the preparation process of the heterogeneous interface layer[75]. (d) Schematic diagram of the mechanism of action of carbon-based optimal interlayer[124]
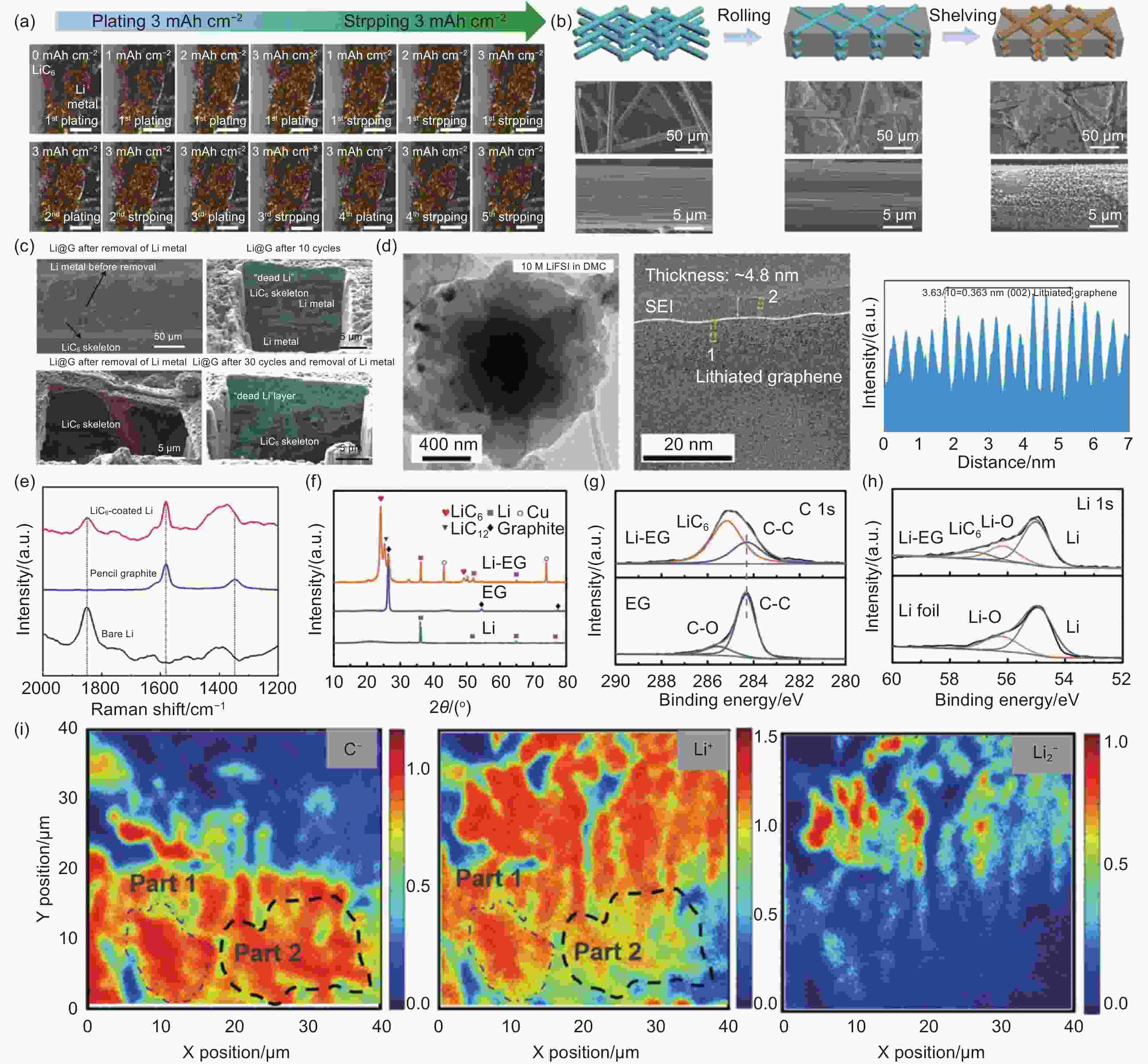
Figure 9. (a) Operado optical microscopic image of the Li@CP/LiC6 electrode-electrolyte interface. Reproduced with permission[127]. Copyright 2022, Elsevier. (b) Schematic illustration and high-resolution SEM images for pristine CF, initial Li/CF composite anode, and Li/CF composite anode shelving for 72 h with the formation of LiC6 layers. Reproduced with permission[49]. Copyright 2019, John Wiley and Sons; (c) Removal of cross-sectional SEM and FIB-SEM images of LiC6 and dead lithium backbones in the anterior and posterior Li@G anodes of lithium metal. Reproduced with permission[130]. Copyright 2021, Royal Society of Chemistry. (d) High-resolution cryo-EM image of lithium graphene shell. Reproduced with permission[77]. Copyright 2019, American Chemical Society. (e) Raman spectroscopy of bare lithium, pencil graphite and lithium coated with LiC6. (f) XRD spectra of Li, EG, Li-EG. (g) C 1s XPS spectra for EG and Li-EG. (h) Li and Li-EG for Li 1s XPS spectrum[121]. (i) the C−, Li+, and Li2− maps of the graphite electrode after 15 cycles by TOF-SIMS after depth sputtering[48]
-
[1] Armand M, Tarascon J M. Building better batteries[J]. Nature,2008,451(7179):652-657. doi: 10.1038/451652a [2] Palacín M R, de Guibert A. Why do batteries fail?[J]. Science,2016,351(6273):1253292. doi: 10.1126/science.1253292 [3] Yu W, Guo Y, Shang Z, et al. A review on comprehensive recycling of spent power lithium-ion battery in China[J]. eTransportation,2022,11:100155. doi: 10.1016/j.etran.2022.100155 [4] Yao N, Chen X, Fu Z H, et al. Applying classical, ab initio, and machine-learning molecular dynamics simulations to the liquid electrolyte for rechargeable batteries[J]. Chemical Reviews,2022,122(12):10970-11021. doi: 10.1021/acs.chemrev.1c00904 [5] Yuan H, Kong L, Li T, et al. A review of transition metal chalcogenide/graphene nanocomposites for energy storage and conversion[J]. Chinese Chemical Letters,2017,28(12):2180-2194. doi: 10.1016/j.cclet.2017.11.038 [6] Xu R, Sun Y, Wang Y, et al. Two-dimensional vermiculite separator for lithium sulfur batteries[J]. Chinese Chemical Letters,2017,28(12):2235-2238. doi: 10.1016/j.cclet.2017.09.065 [7] Yang X, Doyle-Davis K, Gao X, et al. Recent progress and perspectives on designing high-performance thick electrodes for all-solid-state lithium batteries[J]. eTransportation,2022,11:100152. doi: 10.1016/j.etran.2021.100152 [8] Cheng X B, Liu H, Yuan H, et al. A perspective on sustainable energy materials for lithium batteries[J]. Sus Mat,2021,1(1):38-50. [9] Yuan S, Kong T, Zhang Y, et al. Advanced electrolyte design for high-energy-density Li-metal batteries under practical conditions[J]. Angewandte Chemie International Edition,2021,60(49):25624-25638. doi: 10.1002/anie.202108397 [10] Zhang K, Hu Z, Chen J. Functional porous carbon-based composite electrode materials for lithium secondary batteries[J]. Journal of Energy Chemistry,2013,22(2):214-225. doi: 10.1016/S2095-4956(13)60027-3 [11] Zhang L, Zhu C, Yu S, et al. Status and challenges facing representative anode materials for rechargeable lithium batteries[J]. Journal of Energy Chemistry,2022,66:260-294. doi: 10.1016/j.jechem.2021.08.001 [12] Shen X, Zhang X Q, Ding F, et al. Advanced electrode materials in lithium batteries: Retrospect and prospect[J]. Energy Material Advances,2021,2021:1205324. [13] Kong L, Tang C, Peng H J, et al. Advanced energy materials for flexible batteries in energy storage: A review[J]. SmartMat,2020,1(1):e1007. [14] Ren Y, Cui Z, Bhargav A, et al. A self-healable sulfide/polymer composite electrolyte for long-life, low-lithium-excess lithium-metal batteries[J]. Advanced Functional Materials,2022,32(2):2106680. doi: 10.1002/adfm.202106680 [15] Jin C B, Shi P, Zhang X Q, et al. Advances in carbon materials for stable lithium metal batteries[J]. New Carbon Materials,2022,37(1):1-24. doi: 10.1016/S1872-5805(22)60573-0 [16] Li J, Kong Z, Liu X, et al. Strategies to anode protection in lithium metal battery: A review[J]. InfoMat,2021,3(12):1333-1363. doi: 10.1002/inf2.12189 [17] Huang Z H, Wei J S, Song T B, et al. Carbon dots crosslinked gel polymer electrolytes for dendrite-free and long-cycle lithium metal batteries[J]. SmartMat,2022,3(2):323-336. doi: 10.1002/smm2.1121 [18] Liu J, Yuan H, Tao X, et al. Recent progress on biomass-derived ecomaterials toward advanced rechargeable lithium batteries[J]. EcoMat,2020,2(1):e12019. [19] Lucero N, Vilcarino D, Datta D, et al. The roles of mxenes in developing advanced lithium metal anodes[J]. Journal of Energy Chemistry,2022,69:132-149. doi: 10.1016/j.jechem.2022.01.011 [20] Liu A, Liu T F, Yuan H D, et al. A review of biomass-derived carbon materials for lithium metal anodes[J]. New Carbon Materials,2022,37(4):658-674. doi: 10.1016/S1872-5805(22)60620-6 [21] Cheng X B, Zhang R, Zhao C Z, et al. A review of solid electrolyte interphases on lithium metal anode[J]. Advanced Science,2016,3(3):1500213. doi: 10.1002/advs.201500213 [22] Cheng X B, Zhang R, Zhao C Z, et al. Toward safe lithium metal anode in rechargeable batteries: A review[J]. Chemical Reviews,2017,117(15):10403-10473. doi: 10.1021/acs.chemrev.7b00115 [23] Huang Y, Duan J, Zheng X, et al. Lithium metal-based composite: An emerging material for next-generation batteries[J]. Matter,2020,3(4):1009-1030. doi: 10.1016/j.matt.2020.07.005 [24] Zheng X, Huang L, Ye X, et al. Critical effects of electrolyte recipes for Li and Na metal batteries[J]. Chem,2021,7(9):2312-2346. doi: 10.1016/j.chempr.2021.02.025 [25] Xu R, Cheng X B, Yan C, et al. Artificial interphases for highly stable lithium metal anode[J]. Matter,2019,1(2):317-344. doi: 10.1016/j.matt.2019.05.016 [26] Zhang R, Shen X, Zhang Y T, et al. Dead lithium formation in lithium metal batteries: A phase field model[J]. Journal of Energy Chemistry,2022,71:29-35. doi: 10.1016/j.jechem.2021.12.020 [27] Zhang X, Cheng X, Zhang Q. Nanostructured energy materials for electrochemical energy conversion and storage: A review[J]. Journal of Energy Chemistry,2016,25(6):967-984. doi: 10.1016/j.jechem.2016.11.003 [28] Zhao C Z, Chen P Y, Zhang R, et al. An ion redistributor for dendrite-free lithium metal anodes[J]. Science Advances,2018,4(11):eaat3446. doi: 10.1126/sciadv.aat3446 [29] Zhao C Z, Zhang Q. A leap towards stable Li-metal anode interphases[J]. Trends in Chemistry,2019,1(8):709-710. doi: 10.1016/j.trechm.2019.10.002 [30] Zhang H, Wang S, Wang Y, et al. Thiol-ene crosslinked cellulose-based gel polymer electrolyte with good structural integrity for high cycling performance lithium-metal battery[J]. Chinese Chemical Letters,2023,34(4):108031. doi: 10.1016/j.cclet.2022.108031 [31] Yang Z, Ruan Q, Xiong Y, et al. Highly stable lithium metal anode constructed by three-dimensional lithiophilic materials[J]. Batteries,2023,9(1):30. [32] Liu Y, Tao X, Wang Y, et al. Self-assembled monolayers direct a LiF-rich interphase toward long-life lithium metal batteries[J]. Science,2022,375(6582):739-745. doi: 10.1126/science.abn1818 [33] Xie Z, Zhou Y, Ling C, et al. “Series and parallel” design of ether linkage and imidazolium cation synergistically regulated four-armed polymerized ionic liquid for all-solid-state polymer electrolyte[J]. Chinese Chemical Letters,2022,33(3):1407-1411. doi: 10.1016/j.cclet.2021.08.031 [34] Chen S, Yu C, Wei C, et al. Revealing milling durations and sintering temperatures on conductivity and battery performances of Li2. 25Zr0. 75Fe025Cl6 electrolyte[J]. Chinese Chemical Letters,2023,34(5):107544. doi: 10.1016/j.cclet.2022.05.058 [35] Wang Z, Hou L P, Li Z, et al. Highly soluble organic nitrate additives for practical lithium metal batteries[J]. Carbon Energy,2022,5(1):e283. [36] Hou L P, Yao N, Xie J, et al. Modification of nitrate ion enables stable solid electrolyte interphase in lithium metal batteries[J]. Angewandte Chemie International Edition,2022,61(20):e202201406. [37] Zhao C Z, Duan H, Huang J Q, et al. Designing solid-state interfaces on lithium-metal anodes: A review[J]. Science China Chemistry,2019,62(10):1286-1299. doi: 10.1007/s11426-019-9519-9 [38] Liu H, Li T, Xu X, et al. Stable interfaces constructed by concentrated ether electrolytes to render robust lithium metal batteries[J]. Chinese Journal of Chemical Engineering,2021,37:152-158. doi: 10.1016/j.cjche.2021.03.021 [39] Hou L P, Yao L Y, Bi C X, et al. High-valence sulfur-containing species in solid electrolyte interphase stabilizes lithium metal anodes in lithium–sulfur batteries[J]. Journal of Energy Chemistry,2022,68:300-305. doi: 10.1016/j.jechem.2021.12.024 [40] Wang Z Y, Zhao C Z, Sun S et al. Achieving high-energy and high-safety lithium metal batteries with high-voltage-stable solid electrolytes[J]. Matter,2023,6(4):1096-1124. doi: 10.1016/j.matt.2023.02.012 [41] Lee Y G, Fujiki S, Jung C, et al. High-energy long-cycling all-solid-state lithium metal batteries enabled by silver-carbon composite anodes[J]. Nature Energy,2020,5(4):299-308. doi: 10.1038/s41560-020-0575-z [42] Zhang R, Shen X, Ju H T, et al. Driving lithium to deposit inside structured lithium metal anodes: A phase field model[J]. Journal of Energy Chemistry,2022,73:285-291. doi: 10.1016/j.jechem.2022.06.010 [43] Guo Y, Li H, Zhai T. Reviving lithium-metal anodes for next-generation high-energy batteries[J]. Advanced Materials,2017,29(29):1700007. doi: 10.1002/adma.201700007 [44] Wang C, Yang C, Zheng Z. Toward practical high-energy and high-power lithium battery anodes: Present and future[J]. Advanced Science,2022,9(9):2105213. doi: 10.1002/advs.202105213 [45] Shen X, Cheng X B, Shi P, et al. Lithium-matrix composite anode protected by a solid electrolyte layer for stable lithium metal batteries[J]. Journal of Energy Chemistry,2019,37:29-34. doi: 10.1016/j.jechem.2018.11.016 [46] Kong L, Zhang Q. Three-dimensional matrix for lithium metal anode for next-generation rechargeable batteries: Structure design and interface engineering[J]. Journal of Energy Chemistry, 2019: 167−168. [47] Li D, Luo L, Zhu J, et al. A hybrid lithium sulfonated polyoxadiazole derived single-ion conducting gel polymer electrolyte enabled effective suppression of dendritic lithium growth[J]. Chinese Chemical Letters,2022,33(2):1025-1031. doi: 10.1016/j.cclet.2021.07.021 [48] Shi P, Hou L P, Jin C B, et al. A successive conversion–deintercalation delithiation mechanism for practical composite lithium anodes[J]. Journal of the American Chemical Society,2022,144(1):212-218. doi: 10.1021/jacs.1c08606 [49] Shi P, Li T, Zhang R, et al. Lithiophilic LiC6 layers on carbon hosts enabling stable Li metal anode in working batteries[J]. Advanced Materials,2019,31(8):1807131. doi: 10.1002/adma.201807131 [50] Tang C, Titirici M M, Zhang Q. A review of nanocarbons in energy electrocatalysis: Multifunctional substrates and highly active sites[J]. Journal of Energy Chemistry,2017,26(6):1077-1093. doi: 10.1016/j.jechem.2017.08.008 [51] Jiang F N, Yang S J, Cheng X B, et al. Thermal safety of dendritic lithium against non-aqueous electrolyte in pouch-type lithium metal batteries[J]. Journal of Energy Chemistry,2022,72:158-165. doi: 10.1016/j.jechem.2022.05.005 [52] Wu Y, Wang S, Li H, et al. Progress in thermal stability of all-solid-state-Li-ion-batteries[J]. InfoMat,2021,3(8):827-853. doi: 10.1002/inf2.12224 [53] Jiang F N, Yang S J, Liu H, et al. Mechanism understanding for stripping electrochemistry of Li metal anode[J]. SusMat,2021,1(4):506-536. doi: 10.1002/sus2.37 [54] Xu X Q, Cheng X B, Jiang F N, et al. Dendrite-accelerated thermal runaway mechanisms of lithium metal pouch batteries[J]. SusMat,2022,2(4):435-444. doi: 10.1002/sus2.74 [55] Chen Y, Kang Y, Zhao Y, et al. A review of lithium-ion battery safety concerns: The issues, strategies, and testing standards[J]. Journal of Energy Chemistry,2021,59:83-99. doi: 10.1016/j.jechem.2020.10.017 [56] Yang S J, Yao N, Jiang F N, et al. Thermally stable polymer-rich solid electrolyte interphase for safe lithium metal pouch cells[J]. Angewandte Chemie International Edition,2022,61(51):e202214545. [57] Tang S, Guo W, Fu Y. Advances in composite polymer electrolytes for lithium batteries and beyond[J]. Advanced Energy Materials,2021,11(2):2000802. doi: 10.1002/aenm.202000802 [58] Ye S F, Chen X J, Zhang R et al. Revisiting the role of physical confinement and chemical regulation of 3D hosts for dendrite-free Li metal anode[J]. Nano-Micro Letters,2022,14(1):187. doi: 10.1007/s40820-022-00932-3 [59] Cai J, Song Y, Chen X, et al. Mof-derived conductive carbon nitrides for separator-modified Li-S batteries and flexible supercapacitors[J]. Journal of Materials Chemistry A,2020,8(4):1757-1766. doi: 10.1039/C9TA11958B [60] Xiao Y, Zhang Q, Yan J, et al. Compressible aligned carbon nanotube/MnO2 as high-rate electrode materials for supercapacitors[J]. Journal of Electroanalytical Chemistry,2012,684:32-37. doi: 10.1016/j.jelechem.2012.08.024 [61] Ahmad Y, Colin M, Gervillie-Mouravieff C, et al. Carbon in lithium-ion and post-lithium-ion batteries: Recent features[J]. Synthetic Metals,2021,280:116864. doi: 10.1016/j.synthmet.2021.116864 [62] Ji X, Liu D Y, Prendiville D G, et al. Spatially heterogeneous carbon-fiber papers as surface dendrite-free current collectors for lithium deposition[J]. Nano Today,2012,7(1):10-20. doi: 10.1016/j.nantod.2011.11.002 [63] Zheng G, Lee S W, Liang Z, et al. Interconnected hollow carbon nanospheres for stable lithium metal anodes[J]. Nature Nanotechnology,2014,9(8):618-623. doi: 10.1038/nnano.2014.152 [64] Yang C P, Yin Y X, Zhang S F, et al. Accommodating lithium into 3D current collectors with a submicron skeleton towards long-life lithium metal anodes[J]. Nature Communications,2015,6(1):8058. doi: 10.1038/ncomms9058 [65] Lin D, Liu Y, Liang Z, et al. Layered reduced graphene oxide with nanoscale interlayer gaps as a stable host for lithium metal anodes[J]. Nature Nanotechnology,2016,11(7):626-632. doi: 10.1038/nnano.2016.32 [66] Zhang R, Chen X R, Chen X, et al. Lithiophilic sites in doped graphene guide uniform lithium nucleation for dendrite-free lithium metal anodes[J]. Angewandte Chemie International Edition,2017,56(27):7764-7768. doi: 10.1002/anie.201702099 [67] Liu F, Xu R, Hu Z, et al. Regulating lithium nucleation via CNTs modifying carbon cloth film for stable Li metal anode[J]. Small,2019,15(5):1803734. doi: 10.1002/smll.201803734 [68] Duan J, Zheng Y, Luo W, et al. Is graphite lithiophobic or lithiophilic?[J]. National Science Review,2020,7(7):1208-1217. doi: 10.1093/nsr/nwz222 [69] Chen H, Yang Y, Boyle D T, et al. Free-standing ultrathin lithium metal–graphene oxide host foils with controllable thickness for lithium batteries[J]. Nature Energy,2021,6(8):790-798. doi: 10.1038/s41560-021-00833-6 [70] Chen X, Chen X R, Hou T Z, et al. Lithiophilicity chemistry of heteroatom-doped carbon to guide uniform lithium nucleation in lithium metal anodes[J]. Science Advances,2019,5(2):eaau7728. doi: 10.1126/sciadv.aau7728 [71] Tian R, Duan H, Guo Y, et al. High-coulombic-efficiency carbon/Li clusters composite anode without precycling or prelithiation[J]. Small,2018,14(33):1802226. doi: 10.1002/smll.201802226 [72] Wu X, Song B, Chien P H, et al. Structural evolution and transition dynamics in lithium ion battery under fast charging: An operando neutron diffraction investigation[J]. Advanced Science,2021,8(21):2102318. doi: 10.1002/advs.202102318 [73] Lin L D, Qin K, Hu Y S et al. A better choice to achieve high volumetric energy density: Anode-free lithium-metal batteries[J]. Advanced Materials,2022,34 (23):e2110323. doi: 10.1002/adma.202110323 [74] Li H Y, Yamaguchi T, Matsumoto S et al. Circumventing huge volume strain in alloy anodes of lithium batteries[J]. Nature Communications,2020,11(1):1584. doi: 10.1038/s41467-020-15452-0 [75] Zhang Z B, D W, Zhou X F, et al. LiC6 heterogeneous interface for stable lithium plating and stripping[J]. Acta Physico-Chimica Sinica,2021,37(2):2008092. [76] Shi J L, Tang C, Huang J Q, et al. Effective exposure of nitrogen heteroatoms in 3D porous graphene framework for oxygen reduction reaction and lithium-sulfur batteries[J]. Journal of Energy Chemistry,2018,27(1):167-175. doi: 10.1016/j.jechem.2017.09.014 [77] Wang H, Li Y, Li Y, et al. Wrinkled graphene cages as hosts for high-capacity Li metal anodes shown by cryogenic electron microscopy[J]. Nano Letters,2019,19(2):1326-1335. doi: 10.1021/acs.nanolett.8b04906 [78] Xu K. Li-ion battery electrolytes[J]. Nature Energy,2021,6(7):763-763. doi: 10.1038/s41560-021-00841-6 [79] Sun Y, Liu N, Cui Y. Promises and challenges of nanomaterials for lithium-based rechargeable batteries[J]. Nature Energy,2016,1(7):16071. doi: 10.1038/nenergy.2016.71 [80] Lu Y, Zhao C Z, Zhang R, et al. The carrier transition from Li atoms to Li vacancies in solid-state lithium alloy anodes[J]. Science Advances,2021,7(38):eabi5520. doi: 10.1126/sciadv.abi5520 [81] Liu Q, Li S, Wang S, et al. Kinetically determined phase transition from stage ii (LiC12) to stage i (LiC6) in a graphite anode for Li-ion batteries[J]. The Journal of Physical Chemistry Letters,2018,9(18):5567-5573. doi: 10.1021/acs.jpclett.8b02750 [82] Zinth V, von Lüders C, Wilhelm J, et al. Inhomogeneity and relaxation phenomena in the graphite anode of a lithium-ion battery probed by in situ neutron diffraction[J]. Journal of Power Sources,2017,361:54-60. doi: 10.1016/j.jpowsour.2017.06.060 [83] Son Y, Cha H, Jo C, et al. Reliable protocols for calculating the specific energy and energy density of Li-ion batteries[J]. Materials Today Energy,2021,21:100838. doi: 10.1016/j.mtener.2021.100838 [84] Liang Z, Lin D, Zhao J, et al. Composite lithium metal anode by melt infusion of lithium into a 3D conducting scaffold with lithiophilic coating[J]. Proceedings of the National Academy of Sciences,2016,113(11):2862-2867. doi: 10.1073/pnas.1518188113 [85] Yue X Y, Li X L, Bao J, et al. “Top-down” Li deposition pathway enabled by an asymmetric design for Li composite electrode[J]. Advanced Energy Materials,2019,9(35):1901491. doi: 10.1002/aenm.201901491 [86] Fu K, Gong Y, Liu B, et al. Toward garnet electrolyte–based Li metal batteries: An ultrathin, highly effective, artificial solid-state electrolyte/metallic Li interface[J]. Science Advances,2017,3(4):e1601659. doi: 10.1126/sciadv.1601659 [87] Liu Y, Lin D, Liang Z, et al. Lithium-coated polymeric matrix as a minimum volume-change and dendrite-free lithium metal anode[J]. Nature Communications,2016,7(1):10992. doi: 10.1038/ncomms10992 [88] Luo W, Gong Y, Zhu Y, et al. Transition from superlithiophobicity to superlithiophilicity of garnet solid-state electrolyte[J]. Journal of the American Chemical Society,2016,138(37):12258-12262. doi: 10.1021/jacs.6b06777 [89] Fu K, Gong Y, Fu Z, et al. Transient behavior of the metal interface in lithium metal–garnet batteries[J]. Angewandte Chemie International Edition,2017,56(47):14942-14947. doi: 10.1002/anie.201708637 [90] Han X, Gong Y, Fu K, et al. Negating interfacial impedance in garnet-based solid-state Li metal batteries[J]. Nature Materials,2017,16(5):572-579. doi: 10.1038/nmat4821 [91] Wang C, Gong Y, Liu B, et al. Conformal, nanoscale ZnO surface modification of garnet-based solid-state electrolyte for lithium metal anodes[J]. Nano Letters,2017,17(1):565-571. doi: 10.1021/acs.nanolett.6b04695 [92] Zhang Y, Luo W, Wang C, et al. High-capacity, low-tortuosity, and channel-guided lithium metal anode[J]. Proceedings of the National Academy of Sciences,2017,114(14):3584-3589. doi: 10.1073/pnas.1618871114 [93] Wang C, Xie H, Zhang L, et al. Universal soldering of lithium and sodium alloys on various substrates for batteries[J]. Advanced Energy Materials,2018,8(6):1701963. doi: 10.1002/aenm.201701963 [94] Wang S H, Yue J, Dong W, et al. Tuning wettability of molten lithium via a chemical strategy for lithium metal anodes[J]. Nature Communications,2019,10(1):4930. doi: 10.1038/s41467-019-12938-4 [95] Wang Y, Shen Y, Du Z, et al. A lithium–carbon nanotube composite for stable lithium anodes[J]. Journal of Materials Chemistry A,2017,5(45):23434-23439. doi: 10.1039/C7TA08531A [96] Yoshimatsu I, Hirai T, Yamaki J i. Lithium electrode morphology during cycling in lithium cells[J]. Journal of The Electrochemical Society,1988,135(10):2422. doi: 10.1149/1.2095351 [97] Xu W, Wang J, Ding F, et al. Lithium metal anodes for rechargeable batteries[J]. Energy & Environmental Science,2014,7(2):513-537. [98] Yin Y X, Yao H R, Guo Y G. Scientific and technological challenges toward application of lithium-sulfur batteries[J]. Chinese Physics B,2016,25(1):018801. doi: 10.1088/1674-1056/25/1/018801 [99] Liu S, Xia X, Deng S, et al. Large-scale synthesis of high-quality lithium-graphite hybrid anodes for mass-controllable and cycling-stable lithium metal batteries[J]. Energy Storage Materials,2018,15:31-36. doi: 10.1016/j.ensm.2018.03.012 [100] Li T, Liu H, Shi P, et al. Recent progress in carbon/lithium metal composite anode for safe lithium metal batteries[J]. Rare Metals,2018,37(6):449-458. doi: 10.1007/s12598-018-1049-3 [101] Zheng Z J, Ye H, Guo Z P. Recent progress in designing stable composite lithium anodes with improved wettability[J]. Advanced Science,2020,7(22):2002212. doi: 10.1002/advs.202002212 [102] Toyoura K, Koyama Y, Kuwabara A, et al. Effects of off-stoichiometry of LiC6 on the lithium diffusion mechanism and diffusivity by first principles calculations[J]. The Journal of Physical Chemistry C,2010,114(5):2375-2379. doi: 10.1021/jp910134u [103] Duan J, Wu W, Nolan A M, et al. Lithium–graphite paste: An interface compatible anode for solid-state batteries[J]. Advanced Materials,2019,31(10):1807243. doi: 10.1002/adma.201807243 [104] Deng Y, Wang M, Fan C, et al. Strategy to enhance the cycling stability of the metallic lithium anode in Li-metal batteries[J]. Nano Letters,2021,21(4):1896-1901. doi: 10.1021/acs.nanolett.1c00140 [105] Xu Y, Wang L, Jia W, et al. Three-dimensional carbon material as stable host for dendrite-free lithium metal anodes[J]. Electrochimica Acta,2019,301:251-257. doi: 10.1016/j.electacta.2019.01.114 [106] Pan L, Luo Z, Zhang Y, et al. Seed-free selective deposition of lithium metal into tough graphene framework for stable lithium metal anode[J]. ACS Applied Materials & Interfaces,2019,11(47):44383-44389. [107] Ye H, Xin S, Yin Y X, et al. Stable Li plating/stripping electrochemistry realized by a hybrid Li reservoir in spherical carbon granules with 3d conducting skeletons[J]. Journal of the American Chemical Society,2017,139(16):5916-5922. doi: 10.1021/jacs.7b01763 [108] Aurbach D, Zinigrad E, Cohen Y, et al. A short review of failure mechanisms of lithium metal and lithiated graphite anodes in liquid electrolyte solutions[J]. Solid State Ionics,2002,148(3):405-416. [109] Kim H, Jeong G, Kim Y U, et al. Metallic anodes for next generation secondary batteries[J]. Chemical Society Reviews,2013,42(23):9011-9034. doi: 10.1039/c3cs60177c [110] Cheng X B, Hou T Z, Zhang R, et al. Dendrite-free lithium deposition induced by uniformly distributed lithium ions for efficient lithium metal batteries[J]. Advanced Materials,2016,28(15):2888-2895. doi: 10.1002/adma.201506124 [111] Levi M D, Aurbach D. The mechanism of lithium intercalation in graphite film electrodes in aprotic media. Part 1. High resolution slow scan rate cyclic voltammetric studies and modeling[J]. Journal of Electroanalytical Chemistry,1997,421(1):79-88. [112] Levi M D, Aurbach D. Diffusion coefficients of lithium ions during intercalation into graphite derived from the simultaneous measurements and modeling of electrochemical impedance and potentiostatic intermittent titration characteristics of thin graphite electrodes[J]. The Journal of Physical Chemistry B,1997,101(23):4641-4647. doi: 10.1021/jp9701911 [113] Kganyago K R, Ngoepe P E. Structural and electronic properties of lithium intercalated graphite LiC6[J]. Physical Review B,2003,68(20):205111. doi: 10.1103/PhysRevB.68.205111 [114] Sethuraman V A, Hardwick L J, Srinivasan V, et al. Surface structural disordering in graphite upon lithium intercalation/deintercalation[J]. Journal of Power Sources,2010,195(11):3655-3660. doi: 10.1016/j.jpowsour.2009.12.034 [115] Schweidler S, Biasi L d, Schiele A, et al. Volume changes of graphite anodes revisited: A combined operando X-ray diffraction and in situ pressure analysis study[J]. Journal of Physical Chemistry C,2018,122:8829-8835. doi: 10.1021/acs.jpcc.8b01873 [116] Xu K, von Wald Cresce A. Li+-solvation/desolvation dictates interphasial processes on graphitic anode in Li ion cells[J]. Journal of Materials Research,2012,27(18):2327-2341. doi: 10.1557/jmr.2012.104 [117] Cheng H, Sun Q, Li L, et al. Emerging era of electrolyte solvation structure and interfacial model in batteries[J]. ACS Energy Letters,2022,7(1):490-513. doi: 10.1021/acsenergylett.1c02425 [118] Yan K, Lu Z, Lee H W, et al. Selective deposition and stable encapsulation of lithium through heterogeneous seeded growth[J]. Nature Energy,2016,1(3):16010. doi: 10.1038/nenergy.2016.10 [119] Yang G, Li Y, Tong Y, et al. Lithium plating and stripping on carbon nanotube sponge[J]. Nano Letters,2019,19(1):494-499. doi: 10.1021/acs.nanolett.8b04376 [120] Zhang R, Chen X, Shen X, et al. Coralloid carbon fiber-based composite lithium anode for robust lithium metal batteries[J]. Joule,2018,2(4):764-777. doi: 10.1016/j.joule.2018.02.001 [121] Zhao Q, Hao X, Su S, et al. Expanded-graphite embedded in lithium metal as dendrite-free anode of lithium metal batteries[J]. Journal of Materials Chemistry A,2019,7(26):15871-15879. doi: 10.1039/C9TA04240G [122] Shao Y, Wang H, Gong Z, et al. Drawing a soft interface: An effective interfacial modification strategy for garnet-type solid-state Li batteries[J]. ACS Energy Letters,2018,3(6):1212-1218. doi: 10.1021/acsenergylett.8b00453 [123] Zhang L K, Jing M X, Yang H, et al. Highly efficient interface modification between poly(propylene carbonate)-based solid electrolytes and a lithium anode by facile graphite coating[J]. ACS Sustainable Chemistry & Engineering,2020,8(46):17106-17115. [124] Kim S, Yoon G, Jung S K, et al. High-power hybrid solid-state lithium–metal batteries enabled by preferred directional lithium growth mechanism[J]. ACS Energy Letters,2023,8(1):9-20. doi: 10.1021/acsenergylett.2c02150 [125] Markevich E, Baranchugov V, Salitra G, et al. Behavior of graphite electrodes in solutions based on ionic liquids in in situ raman studies[J]. Journal of The Electrochemical Society,2008,155(2):A132-A137. doi: 10.1149/1.2811897 [126] Shadike Z, Zhao E, Zhou Y N, et al. Advanced characterization techniques for sodium-ion battery studies[J]. Advanced Energy Materials,2018,8(17):1702588. doi: 10.1002/aenm.201702588 [127] Tao L, Ma B, Luo F, et al. Gradient lithiation to load controllable, high utilization lithium in graphitic carbon host for high-energy batteries[J]. Nano Energy,2022,93:106808. doi: 10.1016/j.nanoen.2021.106808 [128] Zhou G, Zhao Y, Hu C, et al. Lithium deposition behavior in hard carbon hosts: Optical microscopy and scanning electron microscopy study[J]. Nano Research,2022,16:8638-8376. [129] Lu Y, Zhao C Z, Hu J K, et al. The void formation behaviors in working solid-state Li metal batteries[J]. Science Advances,2022,8(45):eadd0510. doi: 10.1126/sciadv.add0510 [130] He B, Deng W, Han Q, et al. Scalable fabrication of a large-area lithium/graphene anode towards a long-life 350 Wh kg−1 lithium metal pouch cell[J]. Journal of Materials Chemistry A,2021,9(45):25558-25566. doi: 10.1039/D1TA07660D [131] Hood Z D, Chen X, Sacci R L, et al. Elucidating interfacial stability between lithium metal anode and Li phosphorus oxynitride via in situ electron microscopy[J]. Nano Letters,2021,21(1):151-157. doi: 10.1021/acs.nanolett.0c03438 [132] Liang C, Zhang X, Xia S, et al. Unravelling the room-temperature atomic structure and growth kinetics of lithium metal[J]. Nature Communications,2020,11(1):5367. doi: 10.1038/s41467-020-19206-w [133] Zhu J, Wei P, Zeng Q, et al. MnS@N, S co-doped carbon core/shell nanocubes: Sulfur-bridged bonds enhanced Na-storage properties revealed by in situ raman spectroscopy and transmission electron microscopy[J]. Small,2020,16(45):2003001. doi: 10.1002/smll.202003001 [134] Du R, Jie Y, Chen Y, et al. Modulating lithium nucleation behavior through ultrathin interfacial layer for superior lithium metal batteries[J]. ACS Applied Energy Materials,2020,3(7):6692-6699. doi: 10.1021/acsaem.0c00842 [135] Ling M, Li F, Yi H, et al. Superior Na-storage performance of molten-state-blending-synthesized monoclinic NaVPO4F nanoplates for Na-ion batteries[J]. Journal of Materials Chemistry A,2018,6(47):24201-24209. doi: 10.1039/C8TA08842J [136] Wei P, Zhu J, Qiu Y, et al. One-dimensional core–shell motif nanowires with chemically-bonded transition metal sulfide-carbon heterostructures for efficient sodium-ion storage[J]. Chemical Science,2021,12(45):15054-15060. doi: 10.1039/D1SC04163K -





 下载:
下载: