Research progress on nanoporous carbons produced by the carbonization of metal organic frameworks
-
摘要: 纳米多孔炭材料具有高的比表面积、良好的热稳定性和化学稳定性等优点,广泛应用于气体吸附、催化和电化学等领域。尽管目前已做了大量的工作,但是以自模板策略制备纳米多孔炭材料仍存在挑战。结构多样可裁的金属有机骨架(MOF)材料具有规则可调的孔径、高的孔隙率和比表面积等优点,已被证明是制备功能化纳米多孔炭材料的理想前驱体。本文综述了近年来MOF自模板炭化制备纳米多孔炭材料的研究进展,重点介绍以炭化不同的MOF-客体类型为途径获得的多孔炭材料。这将有助于进一步定向开发功能化的新型炭材料,以优化其在更广泛应用领域的性能。Abstract: Nanoporous carbons (NPCs) are widely used in gas adsorption, catalysis and electrochemistry because of their high specific surface area, good thermal and chemical stability, etc. Although a lot of work has been done, there are still great challenges in the fabrication of NPCs. Metal organic frameworks (MOFs) with tailorable structures have the advantages of a regular and adjustable pore size, high porosity and high specific surface area, and have proved to be ideal precursors for the preparation of NPCs. To better grasp the state of the art in the preparation of NPCs, we review recent research progress on the fabrication of NPCs by the carbonization of MOFs, with a focus on the carbonization of different combinations of MOFs and guest precursors, both to tune the resulting pore sizes/textures and surface chemical structures/species and to improve the electrical conductivity and structural stability of the product in different applications.
-
Key words:
- Metal organic framework /
- Self-template /
- Carbonization /
- Nanoporous carbon
-
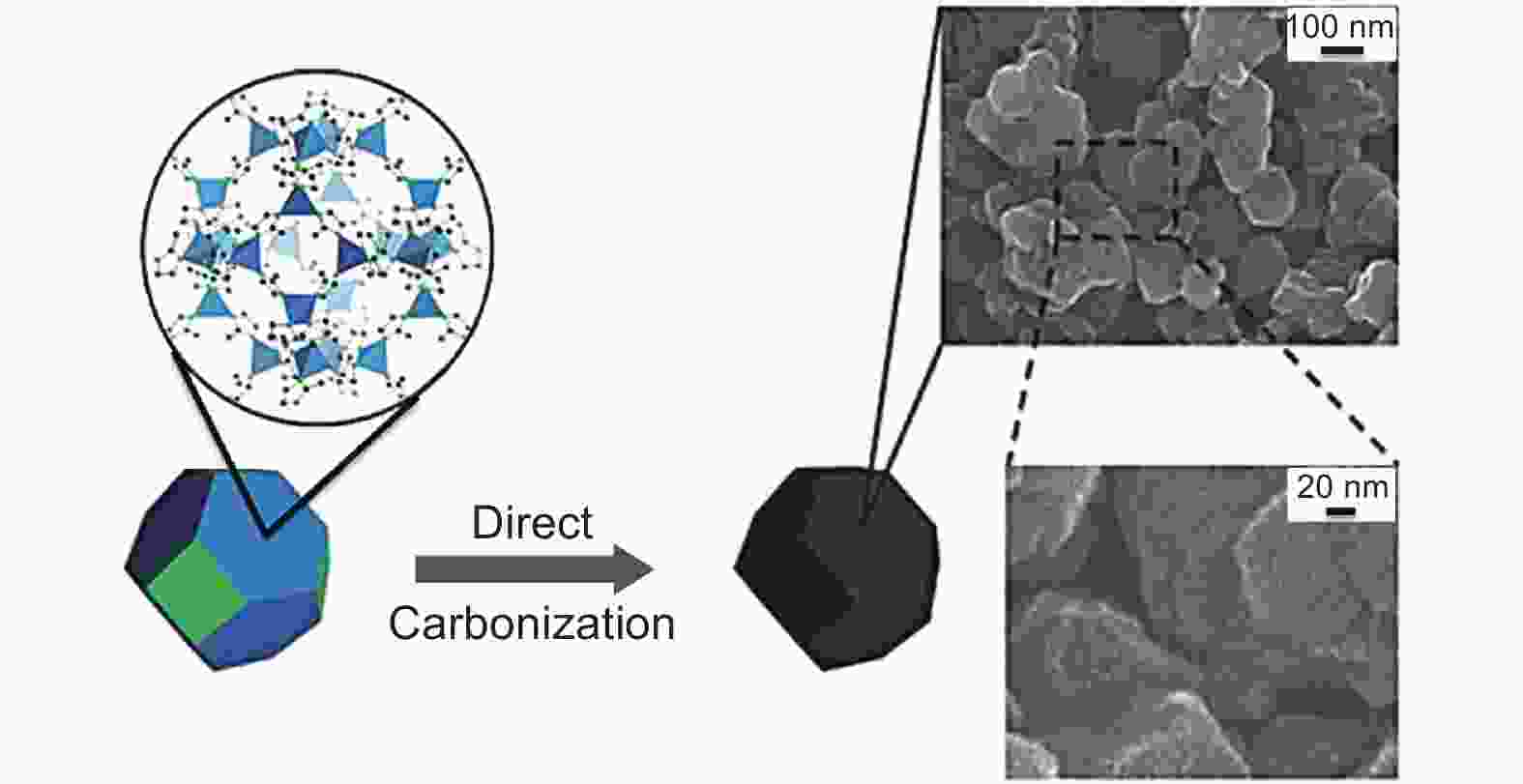
Figure 1. Scheme of the synthesis of NPC via direct carbonization of ZIF-8[40]. Reprinted with permission.
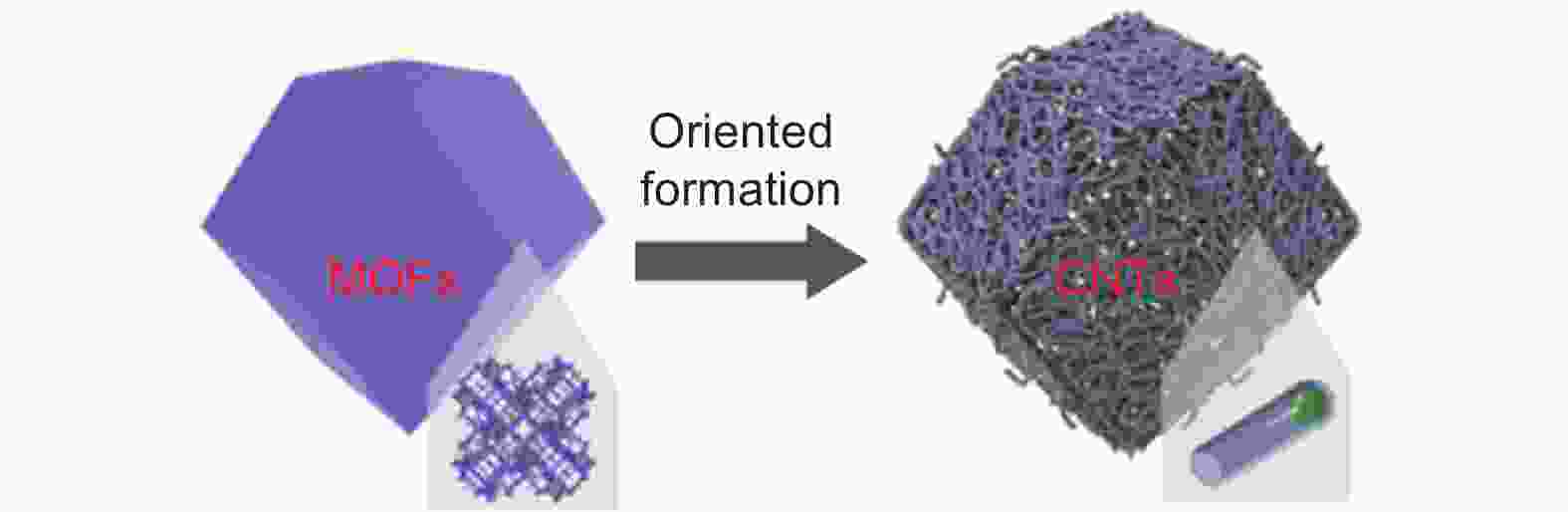
Figure 2. Scheme of the synthesis of N-doped CNT-assembled hollow dodecahedra from ZIF-67[42]. Reprinted with permission.
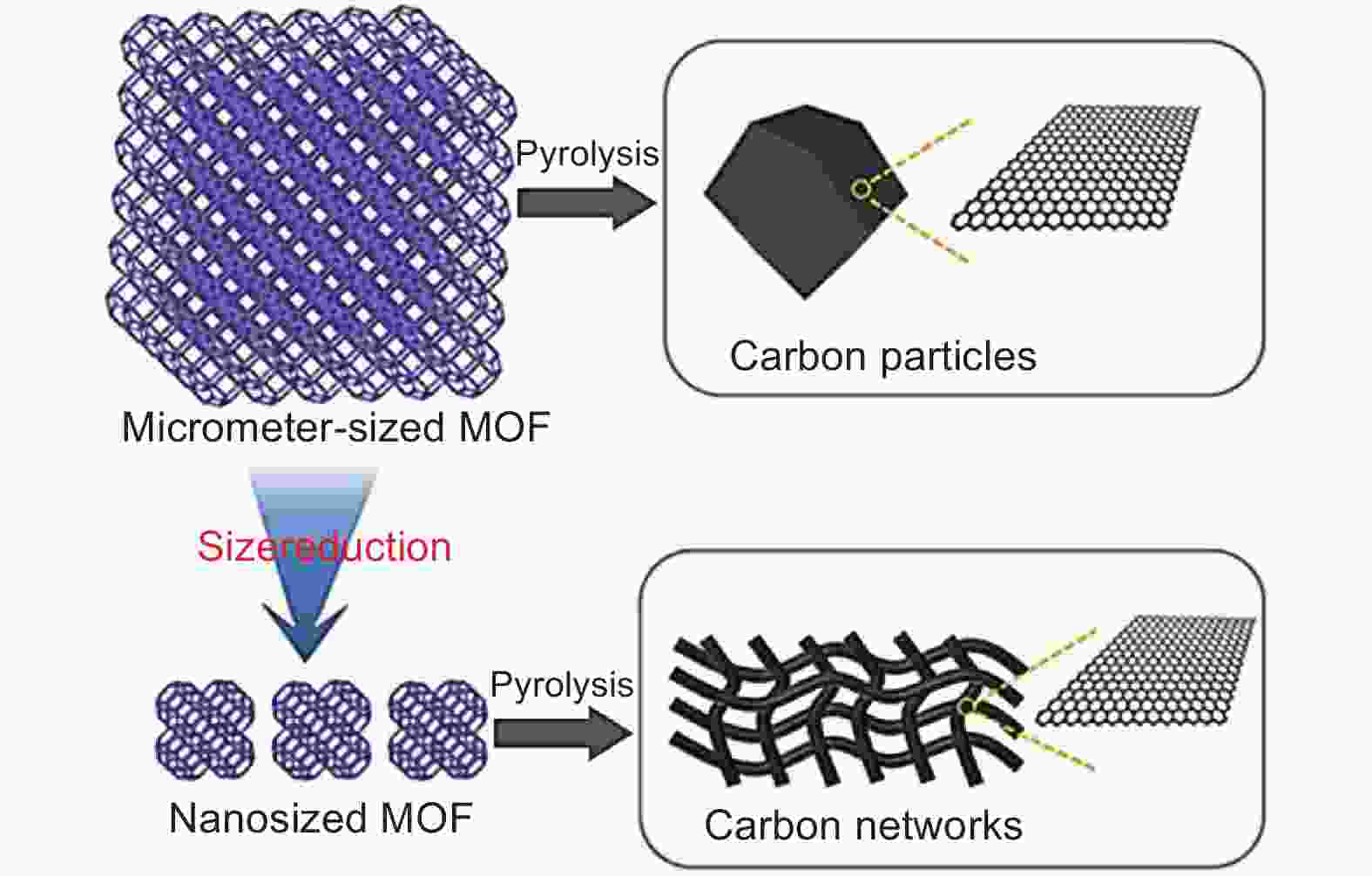
Figure 3. Scheme of the synthesis of graphitic carbon networks through size-reduction of ZIF-67 crystals[43]. Reprinted with permission.
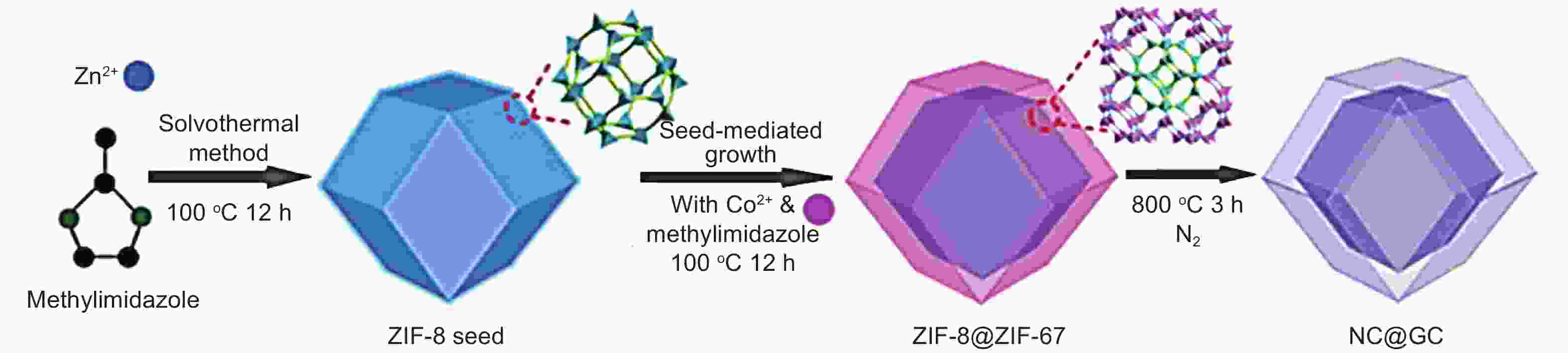
Figure 4. Scheme of the synthesis of core-shell ZIF-8@ZIF-67 nanocrystal and NC@GC[46]. Reprinted with permission.
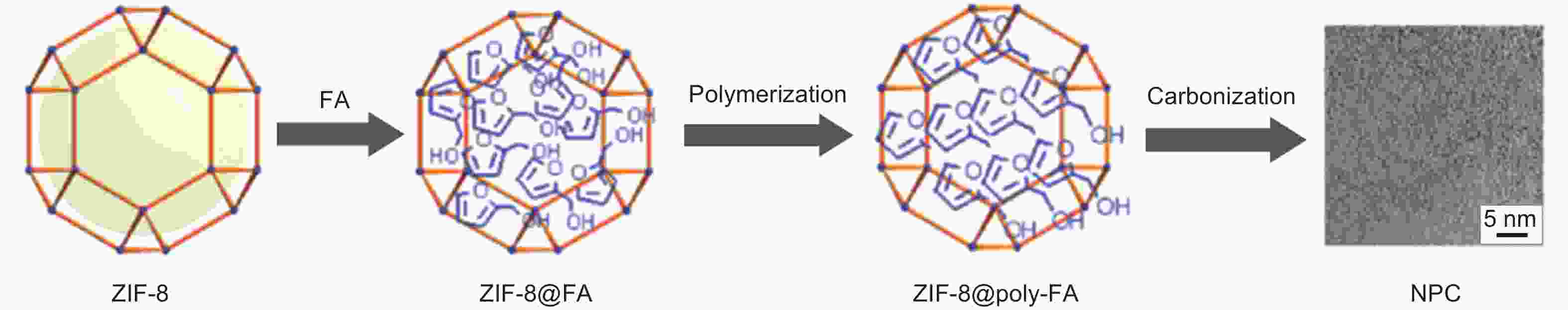
Figure 5. Scheme of the synthesis process for NPC[47]. Reprinted with permission.
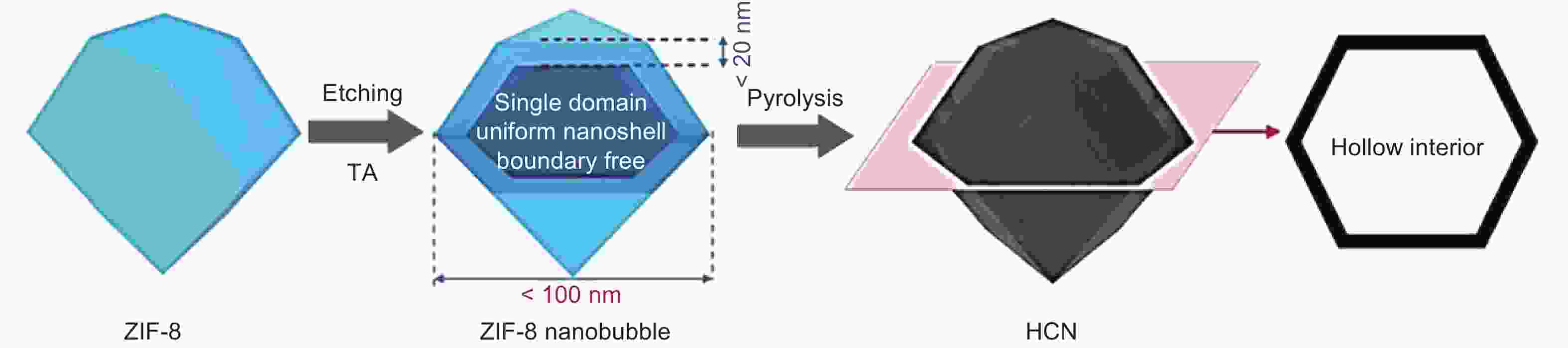
Figure 6. Scheme of the synthesis of hollow carbon nanobubble[56]. Reprinted with permission.
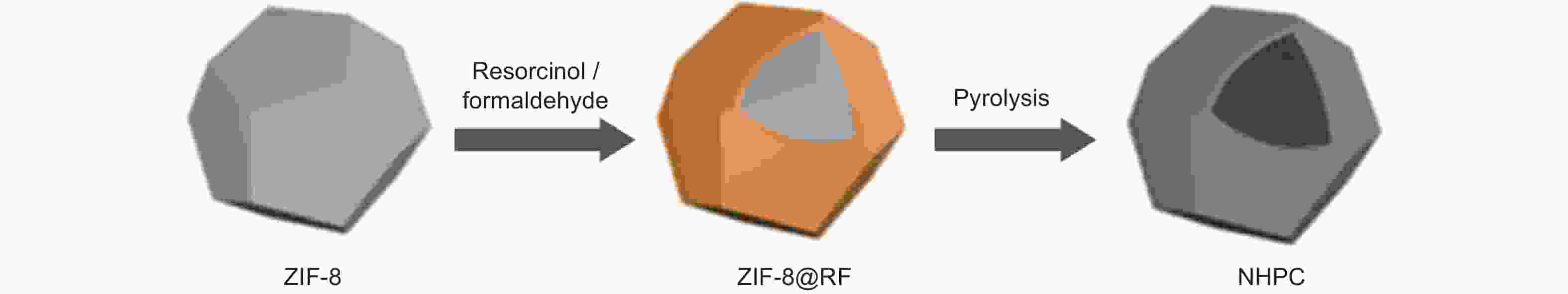
Figure 7. Scheme of the synthesis of N-doped hollow porous carbon[36]. Reprinted with permission.
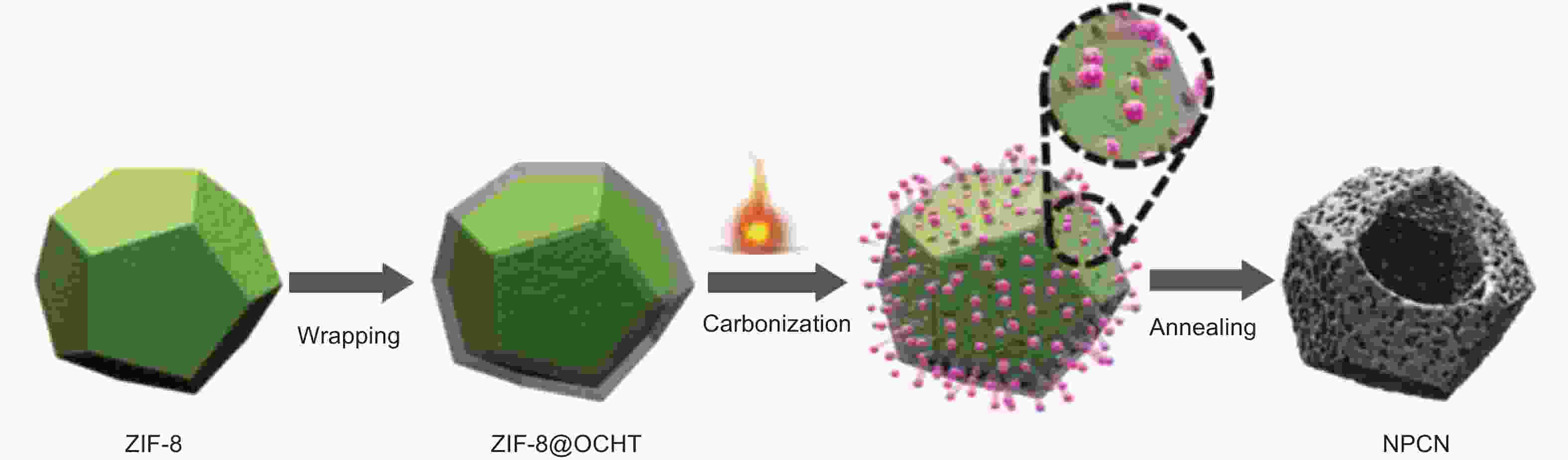
Figure 8. Scheme of the synthesis of N,P co-doped carbon nanocage[59]. Reprinted with permission.
Figure 9. Scheme of the synthesis of NPC fibers[62]. Reprinted with permission.
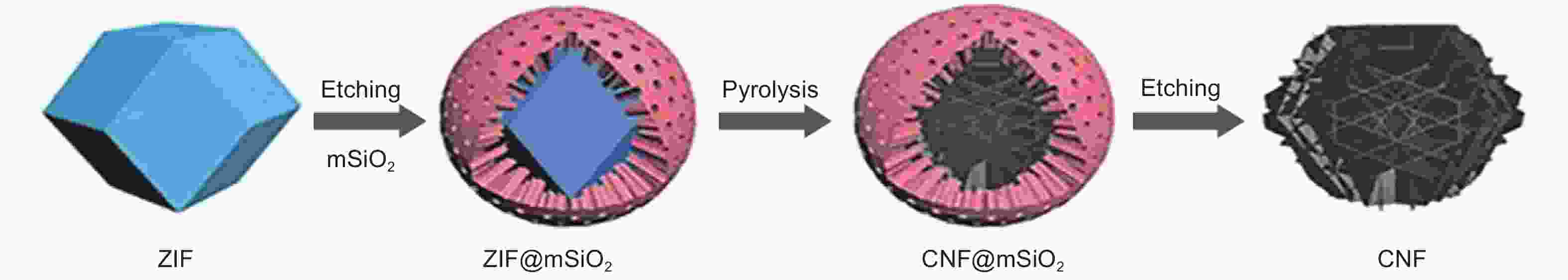
Figure 10. Scheme of the synthesis of carbon nano framework[35]. Reprinted with permission.
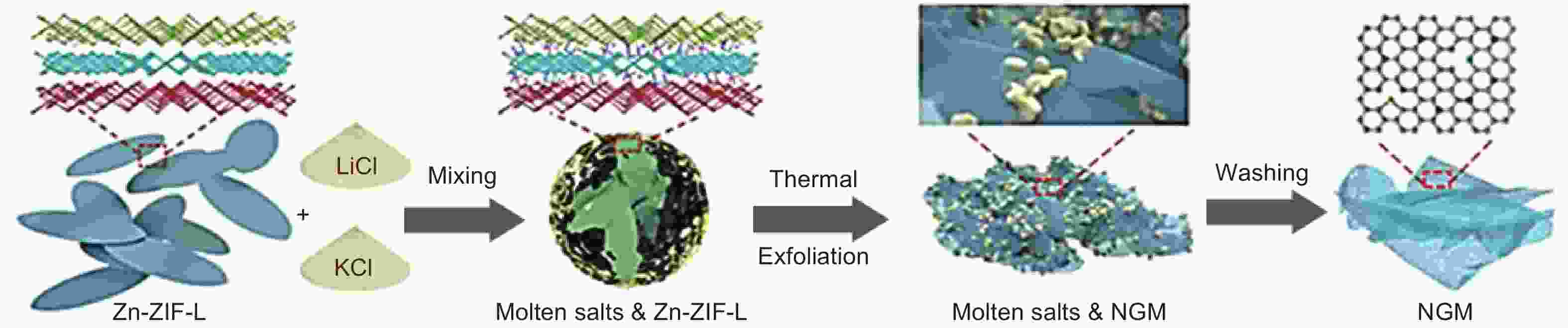
Figure 11. Scheme of thermal exfoliation of Zn-ZIF-L to produce N-doped graphene nanomesh[80]. Reprinted with permission.
-
[1] Zhang S, Mandai T, Ueno K, et al. Hydrogen-bonding supramolecular protic salt as an “all-in-one” precursor for nitrogen-doped mesoporous carbons for CO2 adsorption[J]. Nano Energy,2015,13:376-386. doi: 10.1016/j.nanoen.2015.03.006 [2] Chen Y, Ji S, Wang Y, et al. Isolated single iron atoms anchored on N-doped porous carbon as an efficient electrocatalyst for the oxygen reduction reaction[J]. Angewandte Chemie International Edition,2017,56(24):6937-6941. doi: 10.1002/anie.201702473 [3] Lin T, Chen I W, Liu F, et al. Nitrogen-doped mesoporous carbon of extraordinary capacitance for electrochemical energy storage[J]. Science,2015,350(6267):1508-1513. doi: 10.1126/science.aab3798 [4] Hu X, Sun X, Yoo S J, et al. Nitrogen-rich hierarchically porous carbon as a high-rate anode material with ultra-stable cyclability and high capacity for capacitive sodium-ion batteries[J]. Nano Energy,2019,56:828-839. doi: 10.1016/j.nanoen.2018.11.081 [5] Yarlagadda V, Carpenter M K, Moylan T E, et al. Boosting fuel cell performance with accessible carbon mesopores[J]. ACS Energy Letters,2018,3(3):618-621. doi: 10.1021/acsenergylett.8b00186 [6] Lin J, Peng Z, Liu Y, et al. Laser-induced porous graphene films from commercial polymers[J]. Nature Communications,2014,5(1):1-8. [7] Fagan J A, Hároz E H, Ihly R, et al. Isolation of > 1 nm diameter single-wall carbon nanotube species using aqueous two-phase extraction[J]. ACS Nano,2015,9(5):5377-5390. doi: 10.1021/acsnano.5b01123 [8] Luo W, Zhao T, Li Y, et al. A micelle fusion-aggregation assembly approach to mesoporous carbon materials with rich active sites for ultrasensitive ammonia sensing[J]. Journal of the American Chemical Society,2016,138(38):12586-12595. doi: 10.1021/jacs.6b07355 [9] Li M, Zhang Y, Wang X, et al. Gas pickering emulsion templated hollow carbon for high rate performance lithium sulfur batteries[J]. Advanced Functional Materials,2016,26(46):8408-8417. doi: 10.1002/adfm.201603241 [10] Lakhi K S, Park D H, Al Bahily K, et al. Mesoporous carbon nitrides: Synthesis, functionalization and applications[J]. Chemical Society Reviews,2017,46(1):72-101. doi: 10.1039/C6CS00532B [11] Sua J, Fang C, Yang M, et al. A controllable soft-templating approach to synthesize mesoporous carbon microspheres derived from d-xylose via hydrothermal method[J]. Journal of Materials Science & Technology,2020,38(3):183-188. [12] Liang T, Wei R, Shen P, et al. Hierarchical glucose-based carbons prepared by soft templating and sol-gel process for CO2 capture[J]. Journal of Power Sources,2017,24(6):1637-1645. [13] Xue X, Yang H, Yang T, et al. N, P-coordinated fullerene-like carbon nanostructures with dual active centers toward highly-efficient multi-functional electrocatalysis for CO2RR, ORR and Zn-air battery[J]. Journal of Materials Chemistry A,2017,7(25):15271-15277. [14] He Y, Zhuang X, Lei C, et al. Porous carbon nanosheets: Synthetic strategies and electrochemical energy related applications[J]. Nano Today,2019,24:103-119. doi: 10.1016/j.nantod.2018.12.004 [15] Osmieri L, Escudero-Cid R, Armandi M, et al. Fe-N/C catalysts for oxygen reduction reaction supported on different carbonaceous materials. Performance in acidic and alkaline direct alcohol fuel cells[J]. Applied Catalysis B: Environmental,2017,205:637-653. doi: 10.1016/j.apcatb.2017.01.003 [16] Yao Y, Chen Z, Zhang A, et al. Surface-coating synthesis of nitrogen-doped inverse opal carbon materials with ultrathin micro/mesoporous graphene-like walls for oxygen reduction and supercapacitors[J]. Journal of Materials Chemistry A,2017,5(48):25237-25248. doi: 10.1039/C7TA08354H [17] Ng W, Yang Y, Veen K V D, et al. Enhancing the performance of 3D porous N-doped carbon in oxygen reduction reaction and supercapacitor via boosting the mesomacropore interconnectivity using the “exsolved” dual-template[J]. Carbon,2018,129:293-300. doi: 10.1016/j.carbon.2017.12.019 [18] Zeng H, Wang W, Li J, et al. In situ generated dual-template method for Fe/N/S co-doped hierarchically porous honeycomb carbon for high-performance oxygen reduction[J]. ACS Applied Materials & Interfaces,2018,10(10):8721-8729. [19] Moghadam P Z, Li A, Wiggin S B, et al. Development of a cambridge structural database subset: A collection of metal-organic frameworks for past, present and future[J]. Chemistry of Materials,2017,29(7):2618-2625. doi: 10.1021/acs.chemmater.7b00441 [20] Huang Y B, Liang J, Wang X S, et al. Multifunctional metal-organic framework catalysts: Synergistic catalysis and tandem reactions[J]. Chemical Society Reviews,2017,46(1):126-157. doi: 10.1039/C6CS00250A [21] Wang Z S, Li M, Peng Y L, et al. An ultrastable metal azolate framework with binding pockets for optimal carbon dioxide capture[J]. Angewandte Chemie International Edition,2019,58(45):16071-16076. doi: 10.1002/anie.201909046 [22] Li L, Lin R B, Krishna R, et al. Ethane/ethylene separation in a metal-organic framework with iron-peroxo sites[J]. Science,2018,362(6413):443-446. doi: 10.1126/science.aat0586 [23] Yang Q, Xu Q, Jiang H L. Metal-organic frameworks meet metal nanoparticles: Synergistic effect for enhanced catalysis[J]. Chemical Society Reviews,2017,46(15):4774-4808. doi: 10.1039/C6CS00724D [24] Mingabudinova L R, Vinogradov V V, Milichko V A, et al. Metal-organic frameworks as competitive materials for non-linear optics[J]. Chemical Society Reviews,2016,45(19):5408-5431. doi: 10.1039/C6CS00395H [25] Sheberla D, Bachman J C, Elias J S, et al. Conductive MOF electrodes for stable supercapacitors with high areal capacitance[J]. Nature Materials,2017,16(2):220-224. doi: 10.1038/nmat4766 [26] Minguez Espallargas G, Coronado E. Magnetic functionalities in MOFs: From the framework to the pore[J]. Chemical Society Reviews,2018,47(2):533-557. doi: 10.1039/C7CS00653E [27] Wu M X, Yang Y W. Metal-organic framework (MOF)-based drug/cargo delivery and cancer therapy[J]. Advanced Materials,2017,29(23):1606134. doi: 10.1002/adma.201606134 [28] Dolgopolova E A, Rice A M, Martin C R, et al. Photochemistry and photophysics of MOFs: Steps towards MOF-based sensing enhancements[J]. Chemical Society Reviews,2018,47(13):4710-4728. doi: 10.1039/C7CS00861A [29] Zhou H C, Long J R, Yaghi O M. Introduction to metal-organic frameworks[J]. Chemical Reviews,2012,112(2):673-674. doi: 10.1021/cr300014x [30] Sherry B D, Fürstner A. The promise and challenge of iron-catalyzed cross coupling[J]. Accounts of Chemical Research,2008,41(11):1500-1511. doi: 10.1021/ar800039x [31] Sun J K, Xu Q. Functional materials derived from open framework templates/precursors: Synthesis and applications[J]. Energy & Environmental Science,2014,7(7):2071-2100. [32] Yang L, Zeng X, Wang W, et al. Recent progress in MOF-derived, heteroatom-doped porous carbons as highly efficient electrocatalysts for oxygen reduction reaction in fuel cells[J]. Advanced Functional Materials,2017,28(7):1704537. [33] Ren Q, Wang H, Lu X F, et al. Recent progress on MOF-derived heteroatom-doped carbon-based electrocatalysts for oxygen reduction reaction[J]. Advanced Science,2018,5(3):1700515. doi: 10.1002/advs.201700515 [34] Lai Q, Zhao Y, Liang Y, et al. In situ confinement pyrolysis transformation of ZIF-8 to nitrogen-enriched meso-microporous carbon frameworks for oxygen reduction[J]. Advanced Functional Materials,2016,26(45):8334-8344. doi: 10.1002/adfm.201603607 [35] Shang L, Yu H, Huang X, et al. Well-dispersed ZIF-derived Co, N-co-doped carbon nanoframes through mesoporous-silica-protected carbonization as efficient oxygen reduction electrocatalysts[J]. Advanced Materials,2016,28(8):1668-1674. doi: 10.1002/adma.201505045 [36] Yang H, Bradley S J, Chan A, et al. Catalytically active bimetallic nanoparticles supported on porous carbon capsules derived from metal-organic framework composites[J]. Journal of the American Chemical Society,2016,138(36):11872-11881. doi: 10.1021/jacs.6b06736 [37] Yang S J, Kim T, Im J H, et al. MOF-derived hierarchically porous carbon with exceptional porosity and hydrogen storage capacity[J]. Chemistry of Materials,2012,24(3):464-470. doi: 10.1021/cm202554j [38] Hu M, Reboul J, Furukawa S, et al. Direct carbonization of Al-based porous coordination polymer for synthesis of nanoporous carbon[J]. Journal of the American Chemical Society,2012,134(6):2864-2867. doi: 10.1021/ja208940u [39] Lim S, Suh K, Kim Y, et al. Porous carbon materials with a controllable surface area synthesized from metal-organic frameworks[J]. Chemical Communications,2012,48(60):7447-7449. doi: 10.1039/c2cc33439a [40] Chaikittisilp W, Hu M, Wang H, et al. Nanoporous carbons through direct carbonization of a zeolitic imidazolate framework for supercapacitor electrodes[J]. Chemical Communications,2012,48(58):7259-7261. doi: 10.1039/c2cc33433j [41] Xia B Y, Yan Y, Li N, et al. A metal-organic framework-derived bifunctional oxygen electrocatalyst[J]. Nature Energy,2016,1(1):1-8. [42] Meng J, Niu C, Xu L, et al. General oriented formation of carbon nanotubes from metal-organic frameworks[J]. Journal of the American Chemical Society,2017,139(24):8212-8221. doi: 10.1021/jacs.7b01942 [43] Zhang W, Jiang X, Wang X, et al. Spontaneous weaving of graphitic carbon networks synthesized by pyrolysis of ZIF-67 crystals[J]. Angewandte Chemie International Edition,2017,56(29):8435-8440. doi: 10.1002/anie.201701252 [44] Pachfule P, Shinde D, Majumder M, et al. Fabrication of carbon nanorods and graphene nanoribbons from a metal-organic framework[J]. Nature Chemistry,2016,8(7):718-724. doi: 10.1038/nchem.2515 [45] Tang J, Salunkhe R R, Zhang H, et al. Bimetallic metal-organic frameworks for controlled catalytic graphitization of nanoporous carbons[J]. Scientific Reports,2016,6:30295. doi: 10.1038/srep30295 [46] Tang J, Salunkhe R R, Liu J, et al. Thermal conversion of core-shell metal-organic frameworks: A new method for selectively functionalized nanoporous hybrid carbon[J]. Journal of the American Chemical Society,2015,137(4):1572-1580. doi: 10.1021/ja511539a [47] Liu B, Shioyama H, Akita T, et al. Metal-organic framework as a template for porous carbon synthesis[J]. Journal of the American Chemical Society,2008,130:5390-5391. doi: 10.1021/ja7106146 [48] Liu B, Shioyama H, Jiang H, et al. Metal-organic framework (MOF) as a template for syntheses of nanoporous carbons as electrode materials for supercapacitor[J]. Carbon,2010,48(2):456-463. doi: 10.1016/j.carbon.2009.09.061 [49] Jiang H L, Liu B, Lan Y Q, et al. From metal-organic framework to nanoporous carbon: Toward a very high surface area and hydrogen uptake[J]. Journal of the American Chemical Society,2011,133(31):11854-11857. doi: 10.1021/ja203184k [50] Zhang P, Sun F, Shen Z, et al. ZIF-derived porous carbon: A promising supercapacitor electrode material[J]. Journal of Materials Chemistry A,2014,2(32):12873-12880. doi: 10.1039/C4TA00475B [51] Hu J, Wang H, Gao Q, et al. Porous carbons prepared by using metal-organic framework as the precursor for supercapacitors[J]. Carbon,2010,48(12):3599-3606. doi: 10.1016/j.carbon.2010.06.008 [52] Li J, Chen Y, Tang Y, et al. Metal-organic framework templated nitrogen and sulfur co-doped porous carbons as highly efficient metal-free electrocatalysts for oxygen reduction reactions[J]. Journal of Materials Chemistry A,2014,2(18):6316-6319. doi: 10.1039/C3TA15335E [53] Aijaz A, Fujiwara N, Xu Q. From metal-organic framework to nitrogen-decorated nanoporous carbons: High CO2 uptake and efficient catalytic oxygen reduction[J]. Journal of the American Chemical Society,2014,136(19):6790-6793. doi: 10.1021/ja5003907 [54] Li J S, Li S L, Tang Y J, et al. Heteroatoms ternary-doped porous carbons derived from MOFs as metal-free electrocatalysts for oxygen reduction reaction[J]. Scientific Reports,2014,4:5130. [55] Lai Y, Gan Y, Zhang Z, et al. Metal-organic frameworks-derived mesoporous carbon for high performance lithium–selenium battery[J]. Electrochimica Acta,2014,146:134-141. doi: 10.1016/j.electacta.2014.09.045 [56] Zhang W, Jiang X, Zhao Y, et al. Hollow carbon nanobubbles: monocrystalline MOF nanobubbles and their pyrolysis[J]. Chemical Science,2017,8(5):3538-3546. doi: 10.1039/C6SC04903F [57] Wang J, Luo X, Young C, et al. A glucose-assisted hydrothermal reaction for directly transforming metal-organic frameworks into hollow carbonaceous materials[J]. Chemistry of Materials,2018,30(13):4401-4408. doi: 10.1021/acs.chemmater.8b01792 [58] Wang X, Na Z, Yin D, et al. Phytic acid-assisted formation of hierarchical porous CoP/C nanoboxes for enhanced lithium storage and hydrogen generation[J]. ACS Nano,2018,12(12):12238-12246. doi: 10.1021/acsnano.8b06039 [59] Kale V S, Hwang M, Chang H, et al. Microporosity-controlled synthesis of heteroatom codoped carbon nanocages by Wrap-Bake-sublime approach for flexible all-solid-state-supercapacitors[J]. Advanced Functional Materials,2018,28(37):1803786. doi: 10.1002/adfm.201803786 [60] Yang D H, Kong L, Zhong M, et al. Metal-organic gel-derived FexOy/Nitrogen-doped carbon films for enhanced lithium storage[J]. Small,2019,15(3):1804058. doi: 10.1002/smll.201804058 [61] Xue J, Wu T, Dai Y, et al. Electrospinning and electrospun nanofibers: methods, materials, and applications[J]. Chemical Reviews,2019,119(8):5298-5415. doi: 10.1021/acs.chemrev.8b00593 [62] Wang C, Liu C, Li J, et al. Electrospun metal-organic framework derived hierarchical carbon nanofibers with high performance for supercapacitors[J]. Chemical Communications,2017,53(10):1751-1754. doi: 10.1039/C6CC09832K [63] Chen L F, Lu Y, Yu L, et al. Designed formation of hollow particle-based nitrogen-doped carbon nanofibers for high-performance supercapacitors[J]. Energy & Environmental Science,2017,10(8):1777-1783. [64] Chen Y, Li X, Park K, et al. Nitrogen-doped carbon for sodium-ion battery anode by self-etching and graphitization of bimetallic MOF-based composite[J]. Chem,2017,3(1):152-163. doi: 10.1016/j.chempr.2017.05.021 [65] He Y, Hwang S, Cullen D A, et al. Highly active atomically dispersed CoN4 fuel cell cathode catalysts derived from surfactant-assisted MOFs: Carbon-shell confinement strategy[J]. Energy & Environmental Science,2019,12(1):250-260. [66] Meng Y, Wang G H, Bernt S, et al. Crystal-like microporous hybrid solid nanocast from Cr-MIL-101[J]. Chemical Communications,2011,47(37):10479-10481. doi: 10.1039/c1cc13699b [67] Deng X, Li J, Zhu S, et al. Boosting the capacitive storage performance of MOF-derived carbon frameworks via structural modulation for supercapacitors[J]. Energy Storage Materials,2019,23:491-498. doi: 10.1016/j.ensm.2019.04.015 [68] Ni D, Jiang D, Ehlerding E B, et al. Radiolabeling silica-based nanoparticles via coordination chemistry: Basic principles, strategies and applications[J]. Accounts of Chemical Research,2018,51(3):778-788. doi: 10.1021/acs.accounts.7b00635 [69] Croissant J G, Fatieiev Y, Khashab N M. Degradability and clearance of silicon, organosilica, silsesquioxane, silica mixed oxide, and mesoporous silica nanoparticles[J]. Advanced Materials,2017,29(9):1604634. doi: 10.1002/adma.201604634 [70] Li Z, Zeng H C. Armored MOFs: Enforcing soft microporous MOF nanocrystals with hard mesoporous silica[J]. Journal of the American Chemical Society,2014,136(15):5631-5639. doi: 10.1021/ja409675j [71] Yoon S B, Sohn K, Kim J Y, et al. Fabrication of carbon capsules with hollow macroporous core/mesoporous shell structures[J]. Advanced Materials,2002,14(1):19-21. doi: 10.1002/1521-4095(20020104)14:1<19::AID-ADMA19>3.0.CO;2-X [72] Liu C, Huang X, Wang J, et al. Hollow mesoporous carbon nanocubes: Rigid-Interface-Induced outward contraction of metal-organic frameworks[J]. Advanced Functional Materials,2018,28(6):1705253. doi: 10.1002/adfm.201705253 [73] Zhu Y, Murali S, Stoller M D, et al. Carbon-based supercapacitors produced by activation of graphene[J]. Science,2011,332(6037):1537-1541. doi: 10.1126/science.1200770 [74] Tai Z, Zhang Q, Liu Y, et al. Activated carbon from the graphite with increased rate capability for the potassium ion battery[J]. Carbon,2017,123:54-61. doi: 10.1016/j.carbon.2017.07.041 [75] Zou K, Deng Y, Chen J, et al. Hierarchically porous nitrogen-doped carbon derived from the activation of agriculture waste by potassium hydroxide and urea for high-performance supercapacitors[J]. Journal of Power Sources,2018,378:579-588. doi: 10.1016/j.jpowsour.2017.12.081 [76] Almasoudi A, Mokaya R. Preparation and hydrogen storage capacity of templated and activated carbons nanocast from commercially available zeolitic imidazolate framework[J]. Journal of Materials Chemistry,2012,22(1):146-152. doi: 10.1039/C1JM13314D [77] Wang T, Kim H K, Liu Y, et al. Bottom-up formation of carbon-based structures with multilevel hierarchy from MOF-guest polyhedra[J]. Journal of the American Chemical Society,2018,140(19):6130-6136. doi: 10.1021/jacs.8b02411 [78] Qian Y, An T, Birgersson K E, et al. Web-like interconnected carbon networks from NaCl-assisted pyrolysis of ZIF-8 for highly efficient oxygen reduction catalysis[J]. Small,2018,14(16):1704169. doi: 10.1002/smll.201704169 [79] Niu W, Yang Y. Amorphous MOF introduced N-doped graphene: an efficient and versatile electrocatalyst for zinc-air battery and water splitting[J]. ACS Applied Energy Materials,2018,1(6):2440-2445. doi: 10.1021/acsaem.8b00594 [80] Xia W, Tang J, Li J, et al. Defect-rich graphene nanomesh produced by thermal exfoliation of metal-organic frameworks for the oxygen reduction reaction[J]. Angewandte Chemie International Edition,2019,58(38):13354-13359. doi: 10.1002/anie.201906870 [81] Zhong S, Zhan C, Cao D. Zeolitic imidazolate framework-derived nitrogen-doped porous carbons as high performance supercapacitor electrode materials[J]. Carbon,2015,85:51-59. doi: 10.1016/j.carbon.2014.12.064 [82] Wan L, Shamsaei E, Easton C D, et al. ZIF-8 derived nitrogen-doped porous carbon/carbon nanotube composite for high-performance supercapacitor[J]. Carbon,2017,121:330-336. doi: 10.1016/j.carbon.2017.06.017 [83] Wei J, Hu Y, Liang Y, et al. Graphene oxide/core-shell structured metal-organic framework nano-sandwiches and their derived cobalt/N-doped carbon nanosheets for oxygen reduction reactions[J]. Journal of Materials Chemistry A,2017,5(21):10182-10189. doi: 10.1039/C7TA00276A [84] Li C, Hu C, Zhao Y, et al. Decoration of graphene network with metal–organic frameworks for enhanced electrochemical capacitive behavior[J]. Carbon,2014,78:231-242. doi: 10.1016/j.carbon.2014.06.076 [85] Secchi E, Marbach S, Nigues A, et al. Massive radius-dependent flow slippage in carbon nanotubes[J]. Nature,2016,537(7619):210-213. doi: 10.1038/nature19315 [86] Liu Y, Shen Y, Sun L, et al. Elemental superdoping of graphene and carbon nanotubes[J]. Nature Communications,2016,7(1):1-9. [87] He X, Gao W, Xie L, et al. Wafer-scale monodomain films of spontaneously aligned single-walled carbon nanotubes[J]. Nature Nanotechnology,2016,11(7):633-638. doi: 10.1038/nnano.2016.44 [88] Voiry D, Yang J, Kupferberg J, et al. High-quality graphene via microwave reduction of solution-exfoliated graphene oxide[J]. Science,2016,353(6306):1413-1416. doi: 10.1126/science.aah3398 [89] Abraham J, Vasu K S, Williams C D, et al. Tunable sieving of ions using graphene oxide membranes[J]. Nature Nanotechnology,2017,12(6):546-550. doi: 10.1038/nnano.2017.21 [90] Cao Y, Fatemi V, Fang S, et al. Unconventional superconductivity in magic-angle graphene superlattices[J]. Nature,2018,556(7699):43-50. doi: 10.1038/nature26160 [91] Zhao Y, Liu J, Hu Y, et al. Highly compression-tolerant supercapacitor based on polypyrrole-mediated graphene foam electrodes[J]. Advanced Materials,2013,25(4):591-595. doi: 10.1002/adma.201203578 [92] Deng X, Zhu S, Li J, et al. Ball-in-cage nanocomposites of metal-organic frameworks and three-dimensional carbon networks: synthesis and capacitive performance[J]. Nanoscale,2017,9(19):6478-6485. doi: 10.1039/C7NR01548H [93] Zhu S, Li J, He C, et al. Soluble salt self-assembly-assisted synthesis of three-dimensional hierarchical porous carbon networks for supercapacitors[J]. Journal of Materials Chemistry A,2015,3(44):22266-22273. doi: 10.1039/C5TA04646G [94] Deng X, Zhu S, Li J, et al. Bio-inspired three-dimensional carbon network with enhanced mass-transfer ability for supercapacitors[J]. Carbon,2019,143:728-735. doi: 10.1016/j.carbon.2018.11.055 -





 下载:
下载: