Catalytic oxidation of NO at low concentrations by carbon nanofibers near room temperature
-
摘要: 采用聚丙烯腈 (PAN)作为静电纺丝前驱体,通过静电纺丝法制备了炭纳米纤维,经预氧化和炭化处理,得到了孔隙率高、比表面积大的PAN基炭纳米纤维 (PCNFs)。通过控制前驱体溶液的浓度,可以得到不同直径的PCNFs。制备的样品在室温 (20 °C)下能去除低浓度的NO (5×10−5)。结果表明,炭纳米纤维的微观结构可以影响其对NO的催化性能。CNFs直径越小,微孔越发达,比表面积越大,吸附和催化氧化效果越好。Abstract: Polyacrylonitrile (PAN) nanofibers obtained by electrospinning were used to prepare PAN-based carbon nanofibers (PCNFs) by pre-oxidation, carbonization and high-temperature treatment in NH3. The PCNFs were used for the removal of low concentrations of NO (5×10−5) near room temperature (20 °C) by catalytic oxidation. Results indicated that the PCNFs had high porosity and a large specific surface area, and their diameters could be regulated by changing the PAN concentration. The smaller the diameter of the PCNFs the more micropores were developed, and the larger the specific surface area the better the adsorption and catalytic oxidation performance.
-
Key words:
- Electrospinning /
- Porous carbon nanofibers /
- NO removal /
- Catalytic oxidation
-
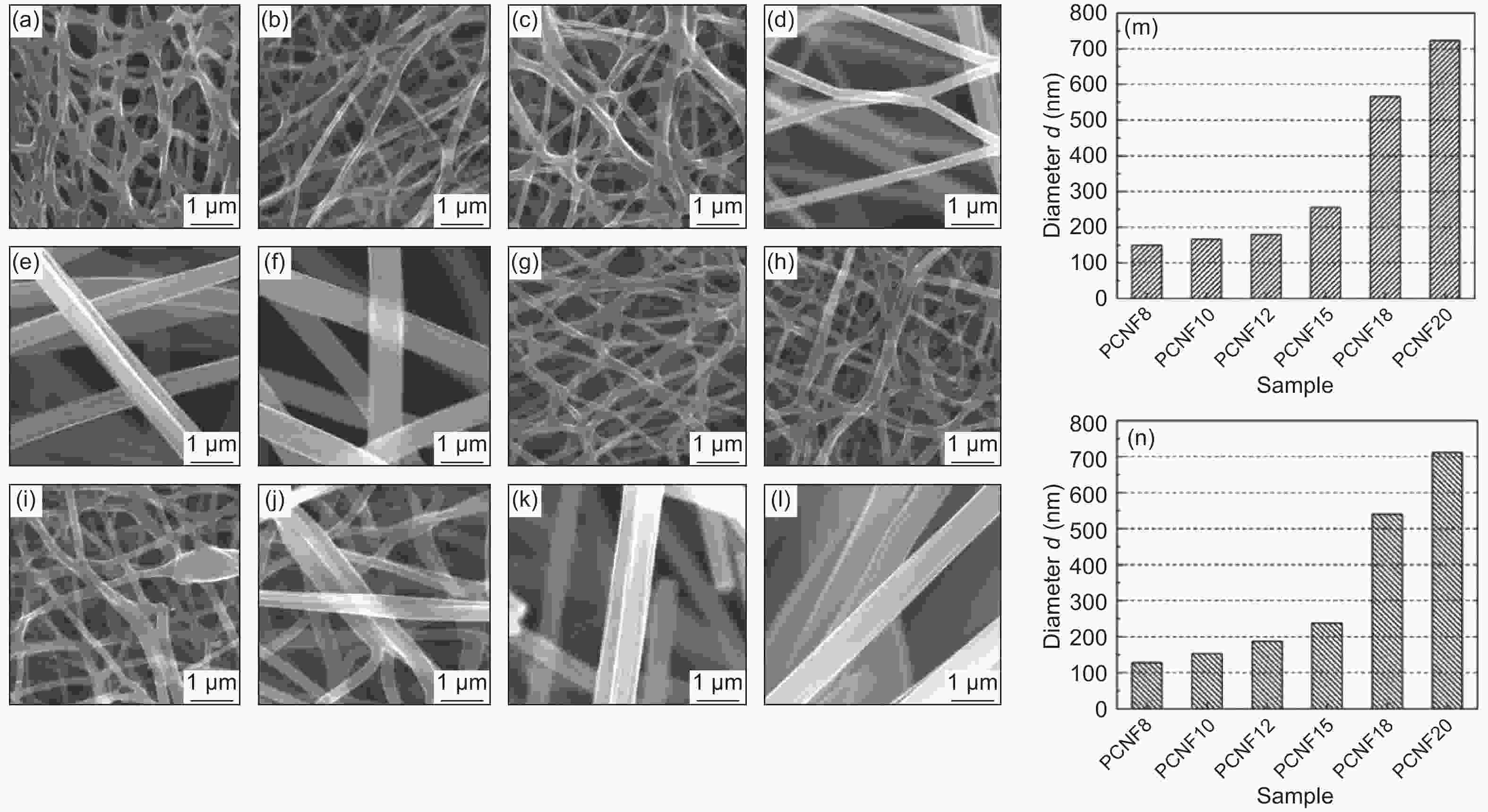
Figure 2. (a-f) SEM images of CNFs at different PAN concentrations at 800 °C: (a) 8 wt.%, (b) 10 wt.%, (c) 12 wt.%, (d) 15 wt%, (e) 18 wt.% and (f) 20 wt.%, and (g-l) SEM images of NH3 activated CNFs at 800 °C with different concentrations of PAN: (g) 8 wt.%, (h) 10 wt.%, (i) 12 wt.%, (j) 15 wt.%, (k) 18 wt.% and (l) 20 wt.%, and (m) average diameters of carbonized CNFs and (n) average diameters of the activated CNFs.
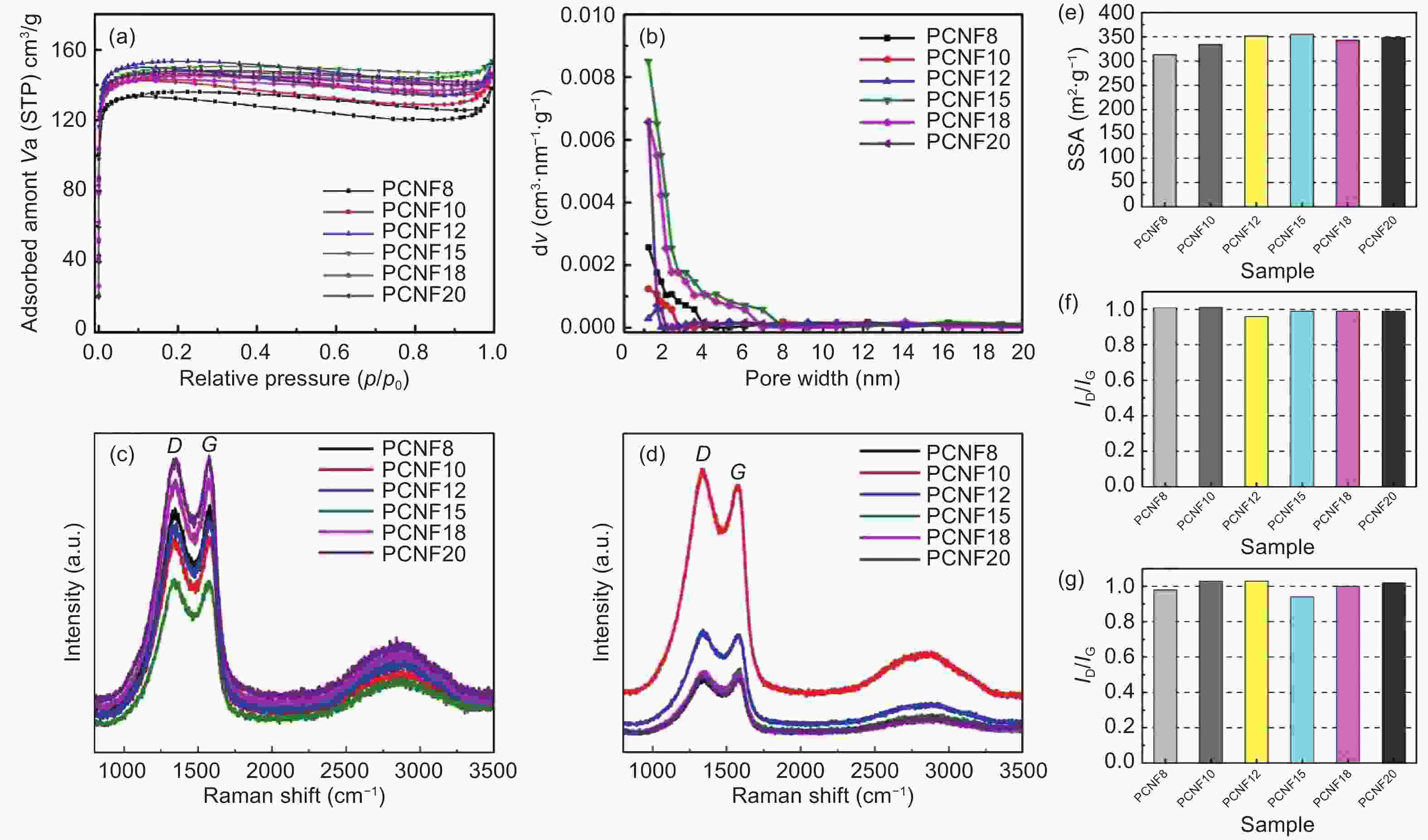
Figure 3. (a) Adsorption and desorption curves of CNFs activated by NH3 at 800 °C, (b) pore size distributions of CNFs at 800 °C, (c) Raman spectra of CNFs at 800 °C, (d) Raman spectra of NH3 activated CNFs at 800 °C, (e) SSA of NH3-activated CNFs at 800 °C, (f) ID/IG values of carbonized CNFs at 800 °C and (g) ID/IG values of NH3-activated CNFs at 800 °C.
Figure 4. (a-f) Removal of NO by CNFs at different concentrations of PAN at the same carbonization temperature: (a) 8 wt.%, (b) 10 wt.%, (c) 12 wt.%, (d) 15 wt.%, (e)18 wt.%, (f) 20 wt.%, (g−l) Removal of NO by PCNFs at different PAN concentrations obtained at the same activation temperature: (g) 8 wt.%, (h) 10 wt.%, (i) 12 wt.%, (j) 15 wt.%, (k) 18 wt.%, (l) 20 wt.% and conversion of NO to NO2 by (m) CNFs and (n) PCNFs.
Table 1. Pore parameters of PCNFs activated at 800 °C.
PCNFs Burn-off(wt%) SSA
(cm3 g−1)Pore volume
(cm3 g−1)Micropore
volume
ratio (%)BET TPV Vmicro Vmeso PCNF-N8 54.28 313.09 0.2095 0.1957 0.0138 93.4 PCNF-N10 55.30 333.61 0.2222 0.2085 0.0137 93.8 PCNF-N12 58.75 351.51 0.2312 0.2193 0.0227 94.9 PCNF-N15 59.00 354.80 0.2357 0.2263 0.0094 96.0 PCNF-N18 58.75 342.75 0.2207 0.2197 0.0010 99.5 PCNF-N20 56.15 348.47 0.2235 0.2218 0.0017 99.2 -
[1] Hu M M, Zhang Z, Atkinson J D, et al. Porous materials for steady-state NO conversion: Comparisons of activated carbon fiber cloths, zeolites and metal-organic frameworks[J]. Chemical Engineering Journal,2019,360:89-96. doi: 10.1016/j.cej.2018.11.102 [2] Gong X L, Mengqi Peng, Dong Wang. Experimental study on NO removal by surface activatedbamboo charcoal[J]. Atmospheric Pollution Research,2019,10:474-479. doi: 10.1016/j.apr.2018.09.007 [3] Fu-Tian You, GW Y, Zhen-Jiao Xing, et al. Enhancement of NO catalytic oxidation on activated carbon at room temperature[J]. Applied Surface Science,2019,471:633-644. doi: 10.1016/j.apsusc.2018.12.066 [4] Wang Y, Li H, Wang S, et al. Investigation of sulphated CuCl2/TiO2 catalyst for simultaneous removal of HgO and NO in SCR process[J]. Fuel Processing Technology,2019,188:179-189. doi: 10.1016/j.fuproc.2019.02.009 [5] Locci C, Vervisch L, Farcy B, et al. Selective Non-catalytic reduction (SNCR) of nitrogen oxide emissions: A perspective from numerical modeling[J]. Flow, Turbulence and Combustion,2017,100(2):301-340. [6] Xu T T, Liu Y Y, Jiang Z M, et al. Enhancing the NO sensing properties of the SnO2 nanowires sensors by Ar–O2 plasma modification[J]. Journal of Materials Science: Materials in Electronics,2018,29(16):13897-13902. doi: 10.1007/s10854-018-9522-1 [7] Zhu G, Hojamberdiev M, Zhang S, et al. Enhancing visible-light-induced photocatalytic activity of BiOI microspheres for NO removal by synchronous coupling with Bi metal and graphene[J]. Applied Surface Science,2019,467-468:968-978. doi: 10.1016/j.apsusc.2018.10.246 [8] Lin Y, Li Y, Xu Z, et al. Transformation of functional groups in the reduction of NO with NH3 over nitrogen-enriched activated carbons[J]. Fuel,2018,223:312-323. doi: 10.1016/j.fuel.2018.01.092 [9] Wang Z, Jin H, Wang K, et al. A two-step method for the integrated removal of HCl, SO2 and NO at low temperature using viscose-based activated carbon fibers modified by nitric acid[J]. Fuel,2019,239:272-281. doi: 10.1016/j.fuel.2018.11.002 [10] Silas K, Ghani W A, Choong T S Y, et al. Breakthrough studies of Co3O4 supported activated carbon monolith for simultaneous SO2/NO removal from flue gas[J]. Fuel Processing Technology,2018,180:155-165. doi: 10.1016/j.fuproc.2018.08.018 [11] Abdulrasheed A A, Jalil A A, Triwahyono S, et al. Surface modification of activated carbon for adsorption of SO2 and NOX: A review of existing and emerging technologies[J]. Renewable and Sustainable Energy Reviews,2018,94:1067-1085. doi: 10.1016/j.rser.2018.07.011 [12] Silas K, Ghani W A W A K, Choong T S Y, et al. Carbonaceous materials modified catalysts for simultaneous SO2/NOx removal from flue gas: A review[J]. Catalysis Reviews,2018,61(1):134-161. [13] Yang L, Jiang W, Yao L, et al. Suitability of pyrolusite as additive to activated coke for low‐temperature NO removal[J]. Journal of Chemical Technology & Biotechnology,2017,93(3):690-697. [14] Zhang K, He Y, Wang Z, et al. Multi-stage semi-coke activation for the removal of SO2 and NO[J]. Fuel,2017,210:738-747. doi: 10.1016/j.fuel.2017.08.107 [15] Hou M, Cen W, Zhang H, et al. Adsorption and oxidation of NO on graphene oxides: A dispersion corrected density functional theory investigation[J]. Applied Surface Science,2015,339:55-61. doi: 10.1016/j.apsusc.2015.02.158 [16] Ijaz S, Ehsan M F, Ashiq M N, et al. Preparation of CdS@CeO2 core/shell composite for photocatalytic reduction of CO2 under visible-light irradiation[J]. Applied Surface Science,2016,390:550-559. doi: 10.1016/j.apsusc.2016.08.098 [17] Mondal K, Ali M A, Srivastava S, et al. Electrospun functional micro/nanochannels embedded in porous carbon electrodes for microfluidic biosensing[J]. Sensors and Actuators B: Chemical,2016,229:82-91. doi: 10.1016/j.snb.2015.12.108 [18] Juan Yang, J X, Xiangyang Zhou, et al. Functionalized N-doped porous carbon nanofiber webs for a lithium–sulfur battery with high capacity and rate performance[J]. The Journal of Physical Chemistry C,2014,118(4):1800-1807. doi: 10.1021/jp410385s [19] Lee J K Y, Chen N, Peng S, et al. Polymer-based composites by electrospinning: Preparation & functionalization with nanocarbons[J]. Progress in Polymer Science,2018,86:40-84. doi: 10.1016/j.progpolymsci.2018.07.002 [20] Mercante L A, Facure M H M, Locilento D A, et al. Solution blow spun PMMA nanofibers wrapped with reduced graphene oxide as an efficient dye adsorbent[J]. New Journal of Chemistry,2017,41(17):9087-9094. doi: 10.1039/C7NJ01703K [21] Liang Q, Ye L, Xu Q, et al. Graphitic carbon nitride nanosheet-assisted preparation of N-enriched mesoporous carbon nanofibers with improved capacitive performance[J]. Carbon,2015,94:342-348. doi: 10.1016/j.carbon.2015.07.001 [22] Van Caneghem J, De Greef J, Block C, et al. NOx reduction in waste incinerators by selective catalytic reduction (SCR) instead of selective non catalytic reduction (SNCR) compared from a life cycle perspective: a case study[J]. Journal of Cleaner Production,2016,112:4452-4460. doi: 10.1016/j.jclepro.2015.08.068 [23] Lee J Y, Hsu C H, Su C I, et al. A study on activated carbon nanofibrous adsorbents prepared by technology for electrospun composite yarn[J]. Fibers and Polymers,2015,16(11):2437-2444. doi: 10.1007/s12221-015-5494-4 [24] Zhang Y, Ma D, Wu J, et al. One–step preparation of CNTs/InVO4 hollow nanofibers by electrospinning and its photocatalytic performance under visible light[J]. Applied Surface Science,2015,353:1260-1268. doi: 10.1016/j.apsusc.2015.06.143 [25] Liao G, You Q, Xia H, et al. Preparation and properties of novel epoxy composites containing electrospun PA6/F-MWNTs fibers[J]. Polymer Engineering & Science,2016,56(11):1259-1266. [26] Youm J S, Kim J H, Kim C, et al. Densifying and strengthening of electrospun polyacrylonitrile-based nanofibers by uniaxial two-step stretching[J]. Journal of Applied Polymer Science, 2016, 133(37). [27] Hwang S, Kim S, Cho Gb, et al. Carbon nanotubes radially anchored on carbon fibers formed by polyacrylonitrile[J]. Materials Research Bulletin,2018,97:49-55. doi: 10.1016/j.materresbull.2017.08.039 [28] Lee Y J, Kim H B, Jeun J P, et al. Preparation and study on nickel oxide reduction of polyacrylonitrile-based carbon nanofibers by thermal treatment[J]. J Nanosci Nanotechnol,2015,15(8):6028-31. doi: 10.1166/jnn.2015.10435 [29] Shen Y, Ge X, Chen M. Catalytic oxidation of nitric oxide (NO) with carbonaceous materials[J]. RSC Advances,2016,6(10):8469-8482. doi: 10.1039/C5RA24148K [30] Yin D, Li J, Wang J, et al. Low-temperature selective catalytic reduction of nox with urea supported on carbon xerogels[J]. Industrial & Engineering Chemistry Research,2018,57(20):6842-6852. [31] Fu Y, Zhang Y, Li G, et al. NO removal activity and surface characterization of activated carbon with oxidation modification[J]. Journal of the Energy Institute,2017,90(5):813-823. doi: 10.1016/j.joei.2016.06.002 [32] Porta A, Pellegrinelli T, Castoldi L, et al. Low temperature NOx adsorption study on Pd-promoted zeolites[J]. Topics in Catalysis,2018,61(18-19):2021-2034. doi: 10.1007/s11244-018-1045-8 [33] Busolo T, Ura D P, Kim S K, et al. Surface potential tailoring of PMMA fibers by electrospinning for enhanced triboelectric performance[J]. Nano Energy,2019,57:500-506. doi: 10.1016/j.nanoen.2018.12.037 -





 下载:
下载: