Reduced graphene oxide encapsulated MnO microspheres as an anode for high-rate lithium ion capacitors
-
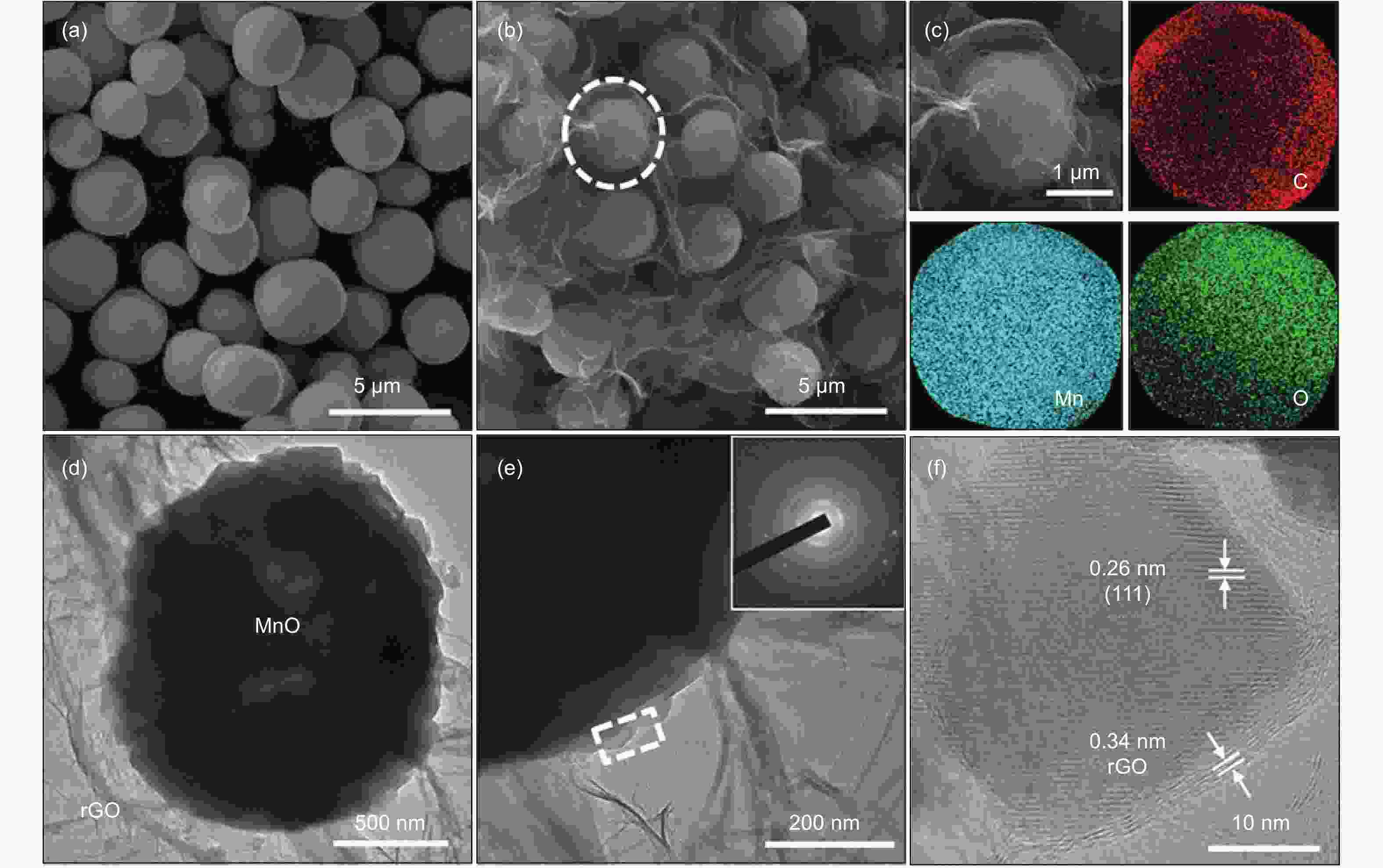
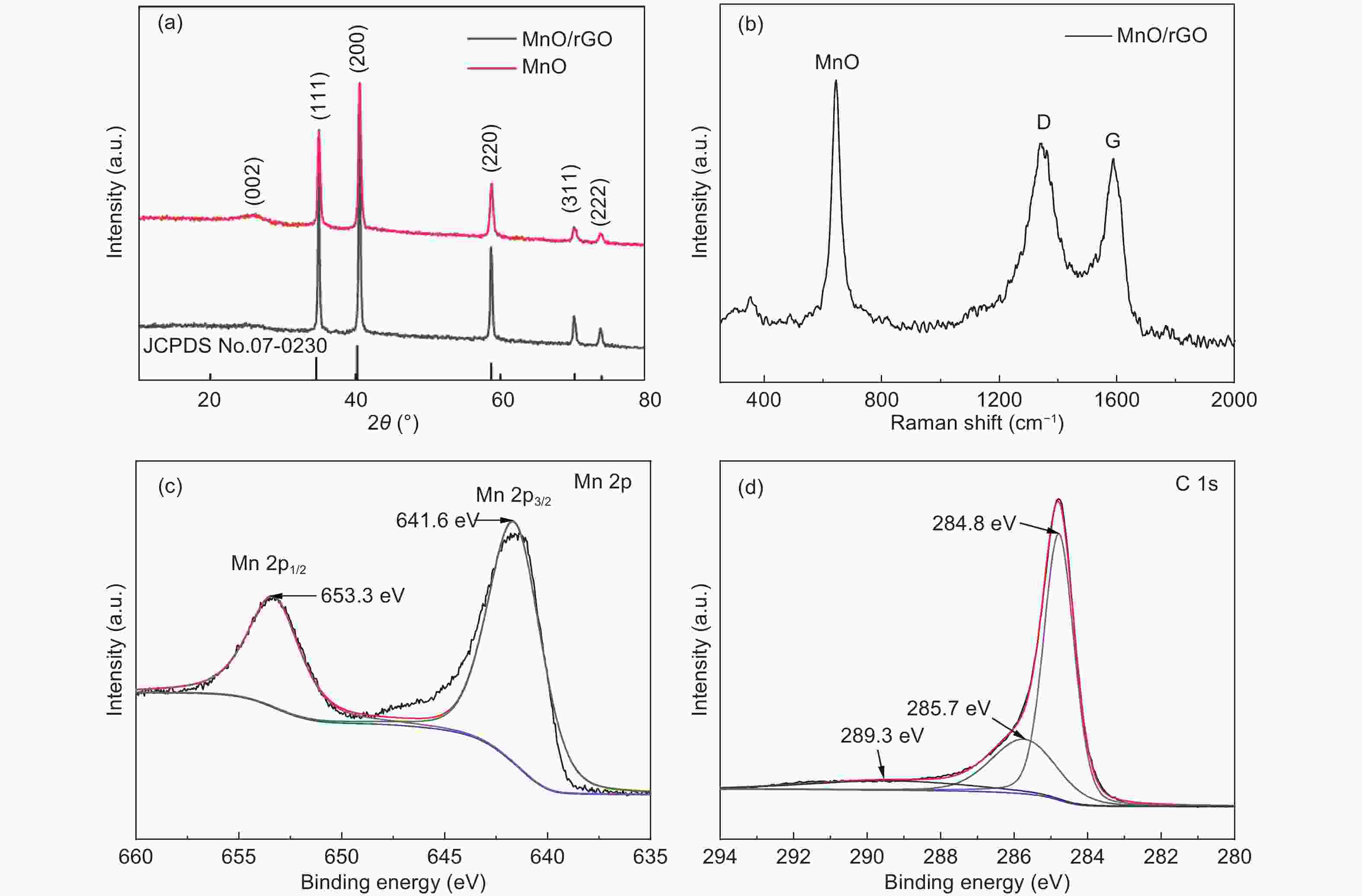
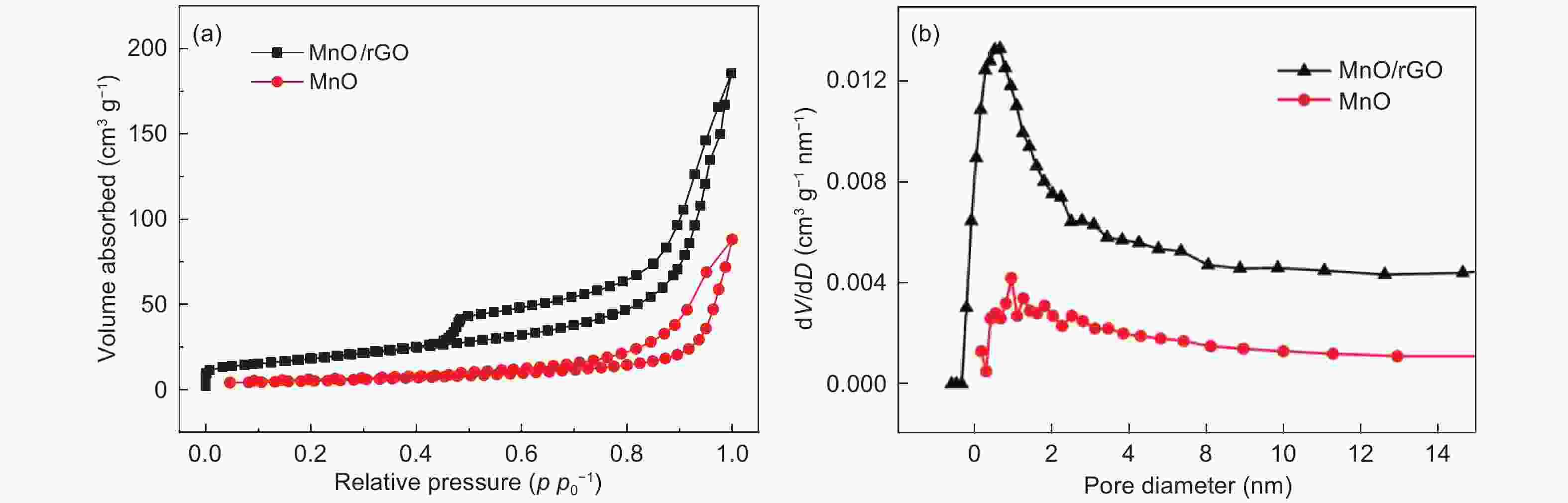
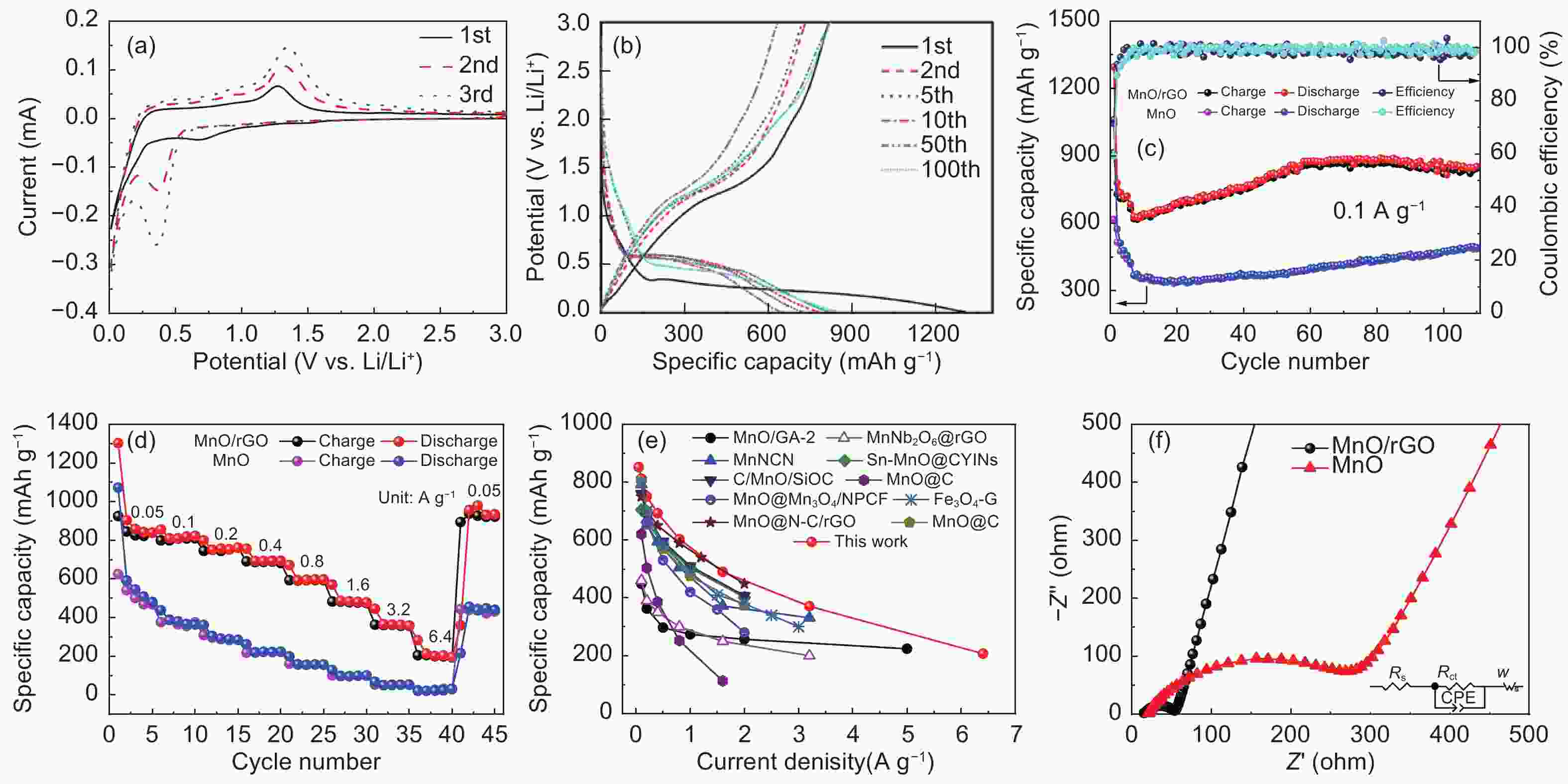
摘要: 发展一种具有优异脱/嵌锂能力且存在稳定放电平台的负极材料是解决锂离子电容器(LICs)负极动力学性能较差以及提升循环稳定性的关键。本文通过溶剂热和热处理制备了一种还原氧化石墨烯(rGO)包覆MnO微球(~2 μm)的复合材料(MnO/rGO)。电化学测试表明,MnO/rGO材料表现出较好的循环稳定性(在0.1 A g−1的电流密度下循环110圈后比容量为846 mAh g−1)和良好的倍率性能(在6.2 A g−1时比容量为207 mAh g−1)。通过对锂离子存储的动力学行为进行分析,表明赝电容性贡献对容量存储起主要作用。以MnO/rGO为阳极,活性炭(AC)为阴极组装的MnO/rGO//AC LICs,在10350 W kg−1的功率密度下,具有98 Wh kg−1的高能量密度,并且在1.6 A g−1的电流密度下循环5 000圈后容量保持率为71%。Abstract: Developing an anode material with high-rate Li+ intercalation and stable charge/discharge platform is important for achieving high performance lithium ion capacitors (LICs). Reduced graphene oxide (rGO)-encapsulated MnO microspheres (~2 μm) are obtained by a simple process including solvothermal and calcination techniques. The material contains a large number of mesopores (~2.8 nm diameter). The MnO/rGO has a favorable cycling stability (846 mAh g−1 at 0.1 A g−1 after 110 cycles) and an outstanding rate performance (207 mAh g−1 at 6.4 A g−1). Kinetic analysis reveals that a pseudocapacitive contribution plays a dominant role for the energy storage. The improvement in the pseudocapacitive behavior is ascribed to the fact that the uniform rGO coating on the MnO provides continuous pathways for electron transport, and the mesoporous structure provides numerous migration paths for Li-ions. Furthermore, MnO/rGO//activated carbon (AC) LICs have a high energy density of 98 Wh kg−1 at a relatively high power density of 10350 W kg−1, and have a capacity retention of 71% after 5 000 cycles at 1.6 A g−1. These outstanding results indicate that the enhanced Li+ intercalation of the anode offsets the kinetic imbalance between the two electrodes.
-
Key words:
- MnO microspheres /
- Reduced graphene oxide /
- Rate capability /
- Lithium ion capacitors
-
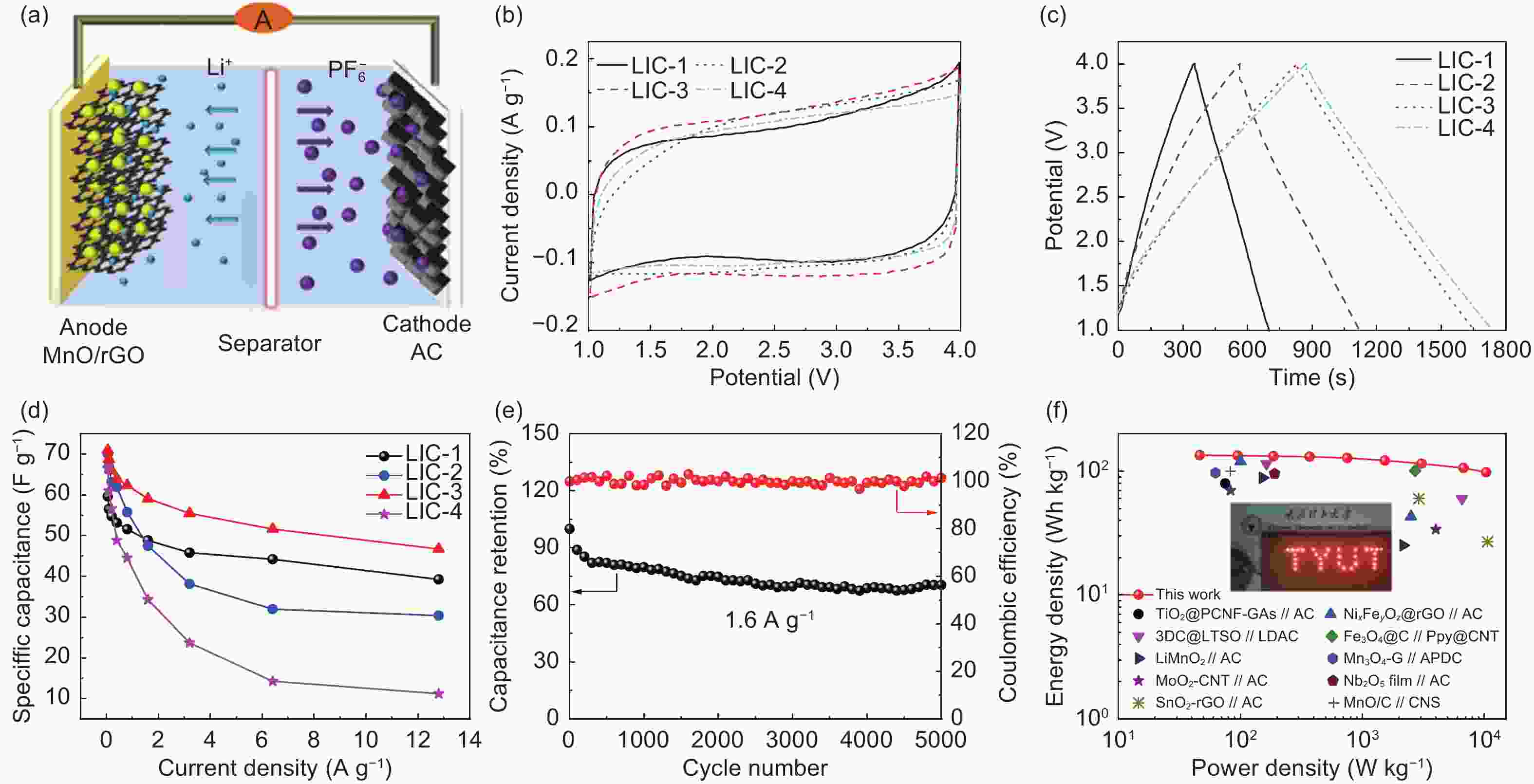
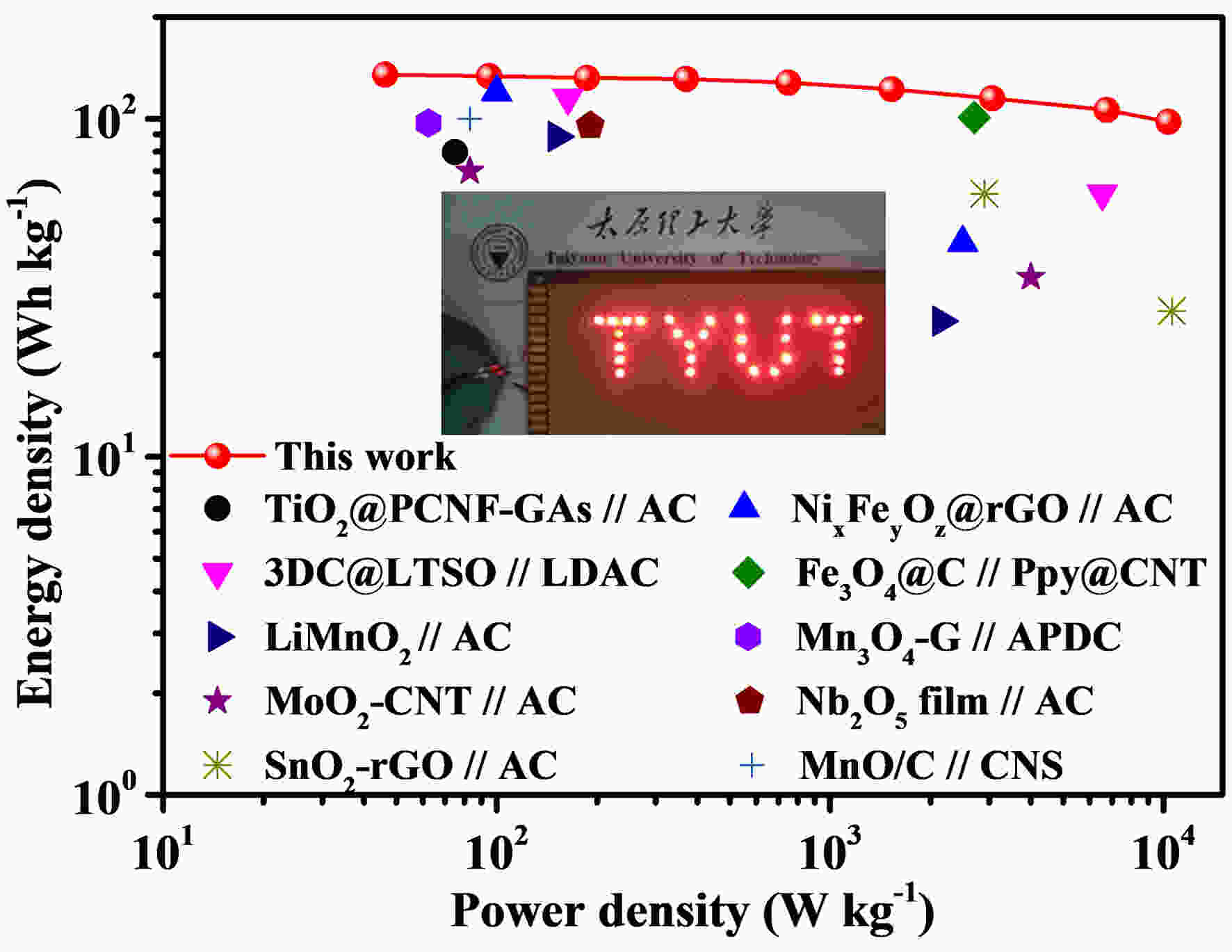
Figure 7. Electrochemical performance of MnO/rGO//AC LICs: (a) Schematic illustration of working principle; (b) CV curves at 2 mV s−1; (c) GCD curves at 0.8 A g−1 and (d) Rate capability of LICs; (e) Cyclic performance of LIC-3 at 1.6 A g−1; (f) Ragone plots of LIC-3 and similar LICs reported in literature, inset (f) is the digital photo of the LED lighting application.
-
[1] Ding J, Hu W, Paek E, et al. Review of hybrid ion capacitors: From aqueous to lithium to sodium[J]. Chemical Reviews,2018,118(14):6457-6498. doi: 10.1021/acs.chemrev.8b00116 [2] Li B, Tian Z, Li H, et al. Self-supporting graphene aerogel electrode intensified by NiCo2S4 nanoparticles for asymmetric supercapacitor[J]. Electrochimica Acta,2019,314:32-39. doi: 10.1016/j.electacta.2019.05.040 [3] Shen C W, Ko T H, Chiu K F, et al. Recycled silicon powder coated on carbon paper used as the anode of lithium ion batteries[J]. New Carbon Materials,2019,34(2):140-145. doi: 10.1016/S1872-5805(19)60007-7 [4] Wang S, Zhao X, Yan X, et al. Regulating fast anionic redox for high-voltage aqueous hydrogen-ion-based energy storage[J]. Angewandte Chemie International Edition,2019,58(1):205-210. doi: 10.1002/anie.201811220 [5] Zhang W, Wang J, Ruan D, et al. Water-based synthesis of spiro-(1,1′)-bipyrrolidinium bis(fluorosulfonyl)imide electrolyte for high-voltage and low-temperature supercapacitor[J]. Chemical Engineering Journal,2019,373:1012-1019. doi: 10.1016/j.cej.2019.05.039 [6] Zhang J, Liu X, Wang J, et al. Different types of pre-lithiated hard carbon as negative electrode material for lithium-ion capacitors[J]. Electrochimica Acta,2016,187:134-142. doi: 10.1016/j.electacta.2015.11.055 [7] Auxilia F M, Jang J, Jang K, et al. Au@TiO2/reduced graphene oxide nanocomposites for lithium-ion capacitors[J]. Chemical Engineering Journal,2019,362:136-143. doi: 10.1016/j.cej.2019.01.008 [8] Yang Z, Guo H, Li X, et al. Graphitic carbon balanced between high plateau capacity and high rate capability for lithium ion capacitors[J]. Journal of Materials Chemistry A,2017,5(29):15302-15309. doi: 10.1039/C7TA03862C [9] Wang H, Zhu C, Chao D, et al. Nonaqueous hybrid lithium-ion and sodium-ion capacitors[J]. Advanced Materials,2017,29(46):1702093-1702115. doi: 10.1002/adma.201702093 [10] Zhang J, Zhang H, Zhang Y, et al. Unveiling of the energy storage mechanisms of multi -modified (Nb2O5@C)/rGO nanoarrays as anode for high voltage supercapacitors with formulated ionic liquid electrolytes[J]. Electrochimica Acta,2019,313:532-543. doi: 10.1016/j.electacta.2019.04.160 [11] Chen L, Chen L, Zhai W, et al. Tunable synthesis of LixMnO2 nanowires for aqueous Li-ion hybrid supercapacitor with high rate capability and ultra-long cycle life[J]. Journal of Power Sources,2019,413:302-309. doi: 10.1016/j.jpowsour.2018.12.026 [12] Jin L, Gong R, Zhang W, et al. Toward high energy-density and long cycling-lifespan lithium ion capacitors: A 3D carbon modified low-potential Li2TiSiO5 anode coupled with a lignin-derived activated carbon cathode[J]. Journal of Materials Chemistry A,2019,7(14):8234-8244. doi: 10.1039/C8TA12000E [13] Li C, Zhang X, Wang K, et al. A 29.3 Wh kg−1 and 6 kW kg−1 pouch-type lithium-ion capacitor based on SiOx/graphite composite anode[J]. Journal of Power Sources,2019,414:293-301. doi: 10.1016/j.jpowsour.2018.12.090 [14] Li G, Yin Z, Guo H, et al. Metalorganic quantum dots and their graphene-like derivative porous graphitic carbon for advanced lithium-ion hybrid supercapacitor[J]. Advanced Energy Materials,2019,9(2):1802878-1802886. doi: 10.1002/aenm.201802878 [15] Qian Y, Jiang S, Li Y, et al. In situ revealing the electroactivity of P-O and P-C bonds in hard carbon for high‐capacity and long‐life Li/K‐ion batteries[J]. Advanced Energy Materials,2019,9(34):1901676-1901686. doi: 10.1002/aenm.201901676 [16] Han C, Shi R, Zhou D, et al. High-energy and high-power nonaqueous lithium-ion capacitors based on polypyrrole/carbon nanotube composites as pseudocapacitive cathodes[J]. ACS Applied Materials Interfaces,2019,11(17):15646-15655. doi: 10.1021/acsami.9b02781 [17] Yang C, Lan J L, Ding C, et al. Three-dimensional hierarchical ternary aerogels of ultrafine TiO2 nanoparticles@porous carbon nanofibers-reduced graphene oxide for high-performance lithium-ion capacitors[J]. Electrochimica Acta,2019,296:790-798. doi: 10.1016/j.electacta.2018.10.037 [18] Deng B, Lei T, Zhu W, et al. In- plane assembled orthorhombic Nb2O5 oanorod films with high-rate Li+ intercalation for high-performance flexible Li-ion capacitors[J]. Advanced Functional Materials,2018,28(1):1704330-1704341. doi: 10.1002/adfm.201704330 [19] Zhao Y, Cui Y, Shi J, et al. Two-dimensional biomass-derived carbon nanosheets and MnO/carbon electrodes for high-performance Li-ion capacitors[J]. Journal of Materials Chemistry A,2017,5(29):15243-15252. doi: 10.1039/C7TA04154C [20] Zheng F, Yin Z, Xia H, et al. Porous MnO@C nanocomposite derived from metal-organic frameworks as anode materials for long-life lithium-ion batteries[J]. Chemical Engineering Journal,2017,327:474-480. doi: 10.1016/j.cej.2017.06.097 [21] Liu C, Fu H, Pei Y, et al. Understanding the electrochemical potential and diffusivity of MnO/C nanocomposites at various charge/discharge states[J]. Journal of Materials Chemistry A,2019,7(13):7831-7842. doi: 10.1039/C9TA00056A [22] Guo J, Liang J, Cui C, et al. Oleic acid-treated synthesis of MnO@C with superior electrochemical properties[J]. Journal of Energy Chemistry,2017,26(3):340-345. doi: 10.1016/j.jechem.2017.03.003 [23] Yang Y, Wang H, Liu W, et al. Polymer salt-derived carbon-based nanomaterials for high-performance hybrid Li-ion capacitors[J]. Journal of Materials Science,2019,54(10):7811-7822. doi: 10.1007/s10853-019-03423-w [24] Yang H, Zhang C, Meng Q, et al. Pre-lithiated manganous oxide/graphene aerogel composites as anode materials for high energy density lithium ion capacitors[J]. Journal of Power Sources,2019,431:114-124. doi: 10.1016/j.jpowsour.2019.05.060 [25] Sheng L, Jiang H, Liu S, et al. Nitrogen-doped carbon-coated MnO nanoparticles anchored on interconnected graphene ribbons for high-performance lithium-ion batteries[J]. Journal of Power Sources,2018,397:325-333. doi: 10.1016/j.jpowsour.2018.07.021 [26] Wang F, Li C, Zhong J, Yang Z. A flexible core-shell carbon layer MnO nanofiber thin film via host-guest interaction: construction, characterization, and electrochemical performances[J]. Carbon,2018,128:277-286. doi: 10.1016/j.carbon.2017.11.075 [27] Leng J, Wang Z, Wang J, et al. Advances in nanostructures fabricated via spray pyrolysis and their applications in energy storage and conversion[J]. Chemical Society Reviews,2019,48(11):3015-3072. doi: 10.1039/C8CS00904J [28] Xiao Z, Ning G, Yu Z, et al. MnO@graphene nanopeapods derived via a one-pot hydrothermal process for a high performance anode in Li-ion batteries[J]. Nanoscale,2019,11(17):8270-8280. doi: 10.1039/C8NR10294E [29] Wang Y, Wu H, Liu Z, et al. Tailoring sandwich-like CNT@MnO@N-doped carbon hetero-nanotubes as advanced anodes for boosting lithium storage[J]. Electrochimica Acta,2019,304:158-167. doi: 10.1016/j.electacta.2019.03.002 [30] Pei X, Mo D, Lyu S, et al. Facile preparation of N-doped MnO/rGO composite as an anode material for high-performance lithium-ion batteries[J]. Applied Surface Science,2019,465:470-477. doi: 10.1016/j.apsusc.2018.09.151 [31] Zhang F, Wang Y, Guo W, et al. Synthesis of Sn-MnO@nitrogen-doped carbon yolk-shelled three-dimensional interconnected networks as a high-performance anode material for lithium-ion batteries[J]. Chemical Engineering Journal,2019,360:1509-1516. doi: 10.1016/j.cej.2018.11.004 [32] Li D, Muller M B, Gilje S, et al. Processable aqueous dispersions of graphene nanosheets[J]. Nature Nanotechnology,2008,3(2):101-105. doi: 10.1038/nnano.2007.451 [33] Wei Y, Zi Z, Chen B, et al. Facile synthesis of hollow MnO microcubes as superior anode materials for lithium-ion batteries[J]. Journal of Alloys and Compounds,2018,756:93-102. doi: 10.1016/j.jallcom.2018.04.331 [34] Zhao J, Tang M, Cao J, et al. Structurally tunable reduced graphene oxide substrate maintains mouse embryonic stem cell pluripotency[J]. Advanced Science,2019,6(12):1802136-1802150. doi: 10.1002/advs.201802136 [35] Xu Q, Xue H, Guo S. FeS2 walnut-like microspheres wrapped with rGO as anode material for high-capacity and long-cycle lithium-ion batteries[J]. Electrochimica Acta,2018,292:1-9. doi: 10.1016/j.electacta.2018.09.135 [36] Zhu G, Wang L, Lin H, et al. Walnut-like multicore-shell MnO encapsulated nitrogen-rich carbon nanocapsules as anode material for long-cycling and soft-packed lithium-ion batteries[J]. Advanced Functional Materials,2018,28(18):1800003-1800010. doi: 10.1002/adfm.201800003 [37] Zhang Y, Chen P, Gao X, et al. Nitrogen- doped graphene ribbon assembled core-sheath MnO@graphene scrolls as hierarchically ordered 3D porous electrodes for fast and durable lithium storage[J]. Advanced Functional Materials,2016,26(43):7754-7765. doi: 10.1002/adfm.201603716 [38] Liu D, Liu D, Hou B, et al. 1D porous MnO@N-doped carbon nanotubes with improved Li-storage properties as advanced anode material for lithium-ion batteries[J]. Electrochimica Acta,2018,264:292-300. doi: 10.1016/j.electacta.2018.01.129 [39] Hou C, Tai Z, Zhao L, et al. High performance MnO@C microcages with a hierarchical structure and tunable carbon shell for efficient and durable lithium storage[J]. Journal of Materials Chemistry A,2018,6(20):9723-9736. doi: 10.1039/C8TA02863J [40] Liu R, Chen X, Zhou C, et al. Controlled synthesis of porous 3D interconnected MnO/C composite aerogel and their excellent lithium-storage properties[J]. Electrochimica Acta,2019,306:143-150. doi: 10.1016/j.electacta.2019.03.129 [41] Zhang X, Zhang J, Kong S, et al. A novel calendula-like MnNb2O6 anchored on graphene sheet as high-performance intercalation pseudocapacitive anode for lithium-ion capacitors[J]. Journal of Materials Chemistry A,2019,7(6):2855-2863. doi: 10.1039/C8TA10233C [42] Liu C, Zhang C, Fu H, et al. Exploiting high-performance anode through tuning the character of chemical bonds for Li-ion batteries and capacitors[J]. Advanced Energy Materials,2017,7(1):1601127-1601136. doi: 10.1002/aenm.201601127 [43] Huang H, Shi C, Fang R, et al. Bio-templated fabrication of MnO nanoparticles in SiOC matrix with lithium storage properties[J]. Chemical Engineering Journal,2019,359:584-593. doi: 10.1016/j.cej.2018.11.166 [44] Chu Y, Guo L, Xi B, et al. Embedding MnO@Mn3O4 nanoparticles in an N-doped-carbon framework derived from Mn-organic clusters for efficient lithium storage[J]. Advanced Materials,2018,30(6):1704244-1704256. doi: 10.1002/adma.201704244 [45] Zhang S, Li C, Zhang X, et al. High Performance performance lithium-ion hybrid capacitors employing Fe3O4-graphene composite anode and activated carbon cathode[J]. ACS Applied Materials Interfaces,2017,9(20):17136-17144. doi: 10.1021/acsami.7b03452 [46] Zhang W, Li J, Zhang J, et al. Top- down strategy to synthesize mesoporous dual carbon armored MnO nanoparticles for lithium-ion battery anodes[J]. ACS Applied Materials Interfaces,2017,9(14):12680-12686. doi: 10.1021/acsami.6b16576 [47] Wang J, Liu H, Liu H, et al. Facile synthesis of microsized MnO/C composites with high tap density as high performance anodes for Li-ion batteries[J]. Chemical Engineering Journal,2017,328:591-598. doi: 10.1016/j.cej.2017.07.039 [48] Tian Z, Wang X, Li B, et al. High rate capability electrode constructed by anchoring CuCo2S4 on graphene aerogel skeleton toward quasi-solid-state supercapacitor[J]. Electrochimica Acta,2019,298:321-329. doi: 10.1016/j.electacta.2018.12.103 [49] Zhang J, Lv W, Zheng D, et al. The interplay of oxygen functional groups and folded texture in densified graphene electrodes for compact sodium-ion capacitors[J]. Advanced Energy Materials,2018,8(11):1702395-1702402. doi: 10.1002/aenm.201702395 [50] Li H, Hao S, Tian Z, et al. Flexible self-supporting Ni2P@N-doped carbon anode for superior rate and durable sodium-ion storage[J]. Electrochimica Acta,2019,321:134624-134635. doi: 10.1016/j.electacta.2019.134624 [51] Hu Z, Sayed S, Jiang T, et al. Self- assembled binary organic granules with multiple lithium uptake mechanisms toward high-energy flexible lithium-lon hybrid supercapacitors[J]. Advanced Energy Materials,2018,8(30):1802273-1802285. doi: 10.1002/aenm.201802273 [52] Huang H, Wang X, Tervoort E, et al. Nano- sized structurally disordered metal oxide composite aerogels as high-power anodes in hybrid supercapacitors[J]. ACS Nano,2018,12(3):2753-2763. doi: 10.1021/acsnano.7b09062 [53] Liu C, Ren Q, Zhang S, et al. High energy and power lithium-ion capacitors based on Mn3O4/3D-graphene as anode and activated polyaniline-derived carbon nanorods as cathode[J]. Chemical Engineering Journal,2019,370:1485-1492. doi: 10.1016/j.cej.2019.04.044 [54] Fleischmann S, Zeiger M, Quade A, et al. Atomic layer-deposited molybdenum oxide/carbon nanotube hybrid electrodes: the influence of crystal ctructure on lithium-ion capacitor performance[J]. ACS Applied Materials Interfaces,2018,10(22):18675-18684. doi: 10.1021/acsami.8b03233 [55] Arnaiz M, Botas C, Carriazo D, et al. Reduced graphene oxide decorated with SnO2 nanoparticles as negative electrode for lithium ion capacitors[J]. Electrochimica Acta,2018,284:542-550. doi: 10.1016/j.electacta.2018.07.189 -
 20200035-SI.pdf
20200035-SI.pdf

-





 下载:
下载: