-
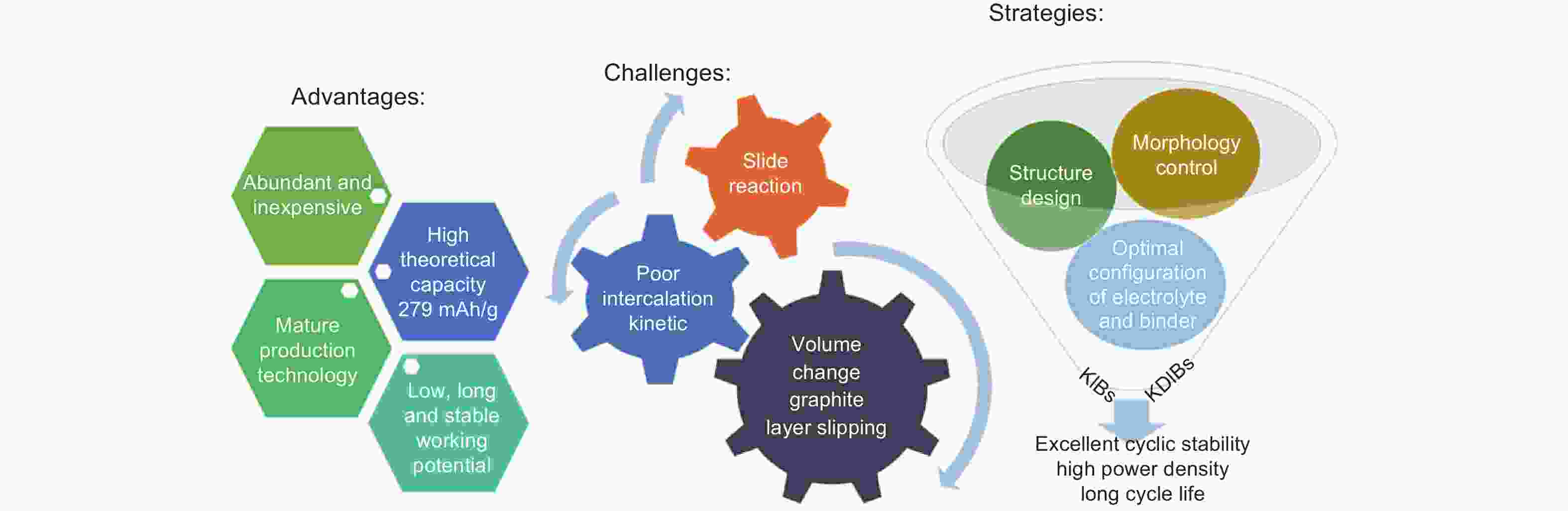
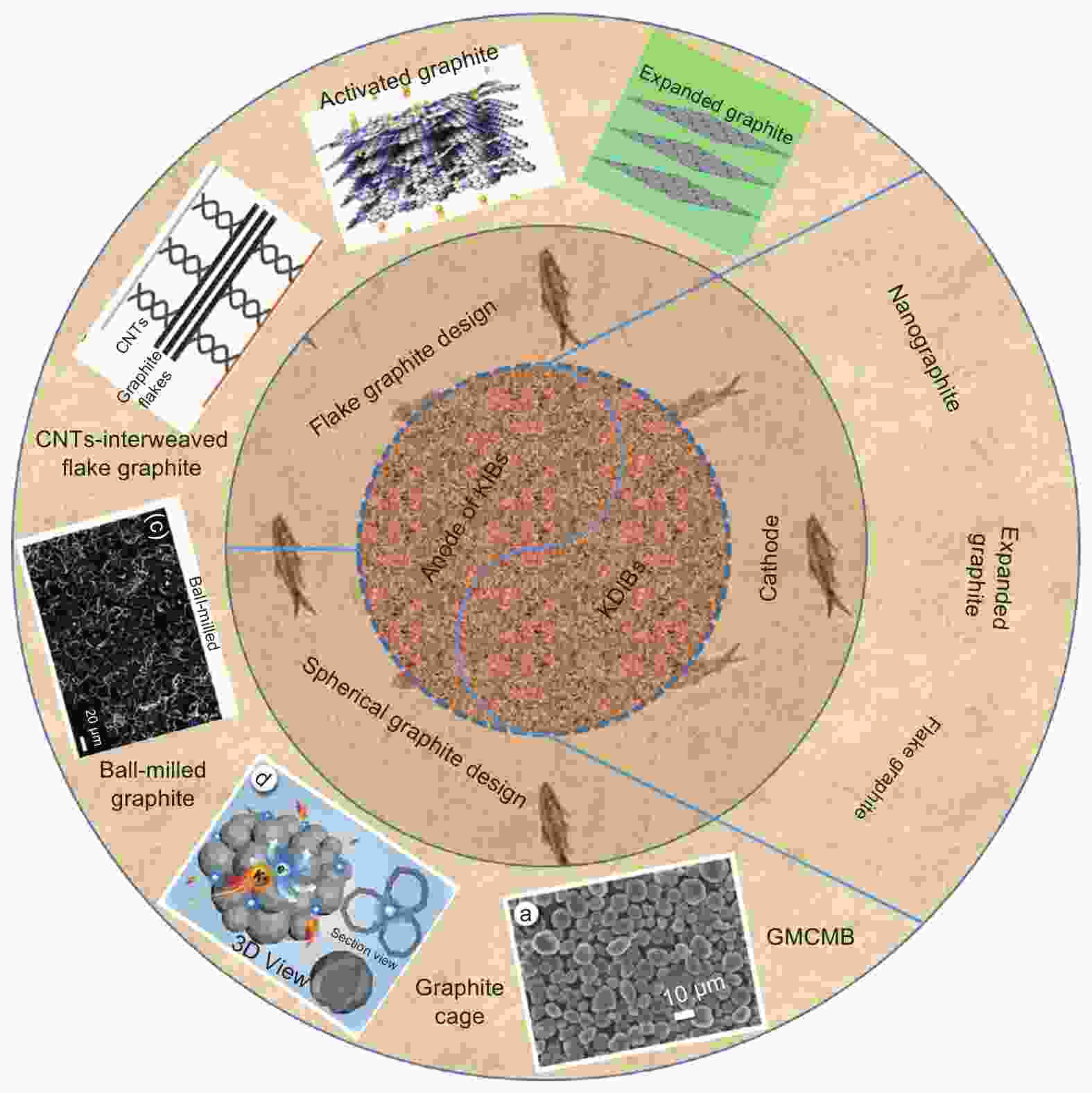
摘要: 由于钾离子电池成本低和其电化学性能与锂离子电池相当,钾离子电池和钾基双离子电池成为非常有潜力的新兴储能器件。另外,石墨作为已成功商业化应用的锂离子电池负极材料,也可容纳半径较大的钾离子和一些阴离子的插层并表现出较高的理论容量。但是,石墨材料在钾基能源存储器件中的应用依然面临一些挑战,如半径较大的离子插层会造成较大的体积膨胀(K+>61%;阴离子>130%),导致在循环过程中石墨层间滑移,电池容量衰减;同时由于石墨材料有限的层间距,半径较大的离子会表现出缓慢的插层反应动力学而导致较差的倍率性能。因此,针对存在的问题,本文总结了近年来石墨材料应用于钾基能源存储的研究进展,分析了插层机理并揭示了电化学性能与石墨结构,电解液和黏结剂之间的关系。最后,总结并展望了石墨材料在钾基能源存储中应用的发展方向。Abstract: Potassium ion batteries (KIBs) and potassium-based dual ion batteries (KDIBs) are newly-emerging energy storage devices that have attracted considerable attention owing to the low-cost of potassium resources and their comparable performance to lithium-ion batteries (LIBs). Graphite materials, as the successful commercialized anode materials of LIBs, can also be used as anodic and cathodic host materials for the intercalation of the large potassium cations and other anions, respectively. However, there are still some challenges hindering the practical application of graphite materials in the anode for KIBs and the cathode for KDIBs. The huge volume changes after intercalation (61% for K and 130% for anions) result in graphite interlayer slipping and structural collapse, causing capacity fade and a short cycle life. Moreover, the intercalation of large K+ and anions have poor kinetics due to the small graphite interlayer spacing, restricting the rate capability. To solve these issues of the use of graphite materials, this review attempts to provide a better understanding of the intercalation mechanisms for K+ and anions, and to correlate the electrochemical performance of KIBs and KDIBs to the microstructure of graphite, and the physicochemical properties of electrolytes and binders. Finally, research prospects are provided to guide the future development of graphite materials for potassium-based energy storage.
-
Key words:
- Graphite /
- Potassium ion battery /
- Potassium-based dual ion battery
-
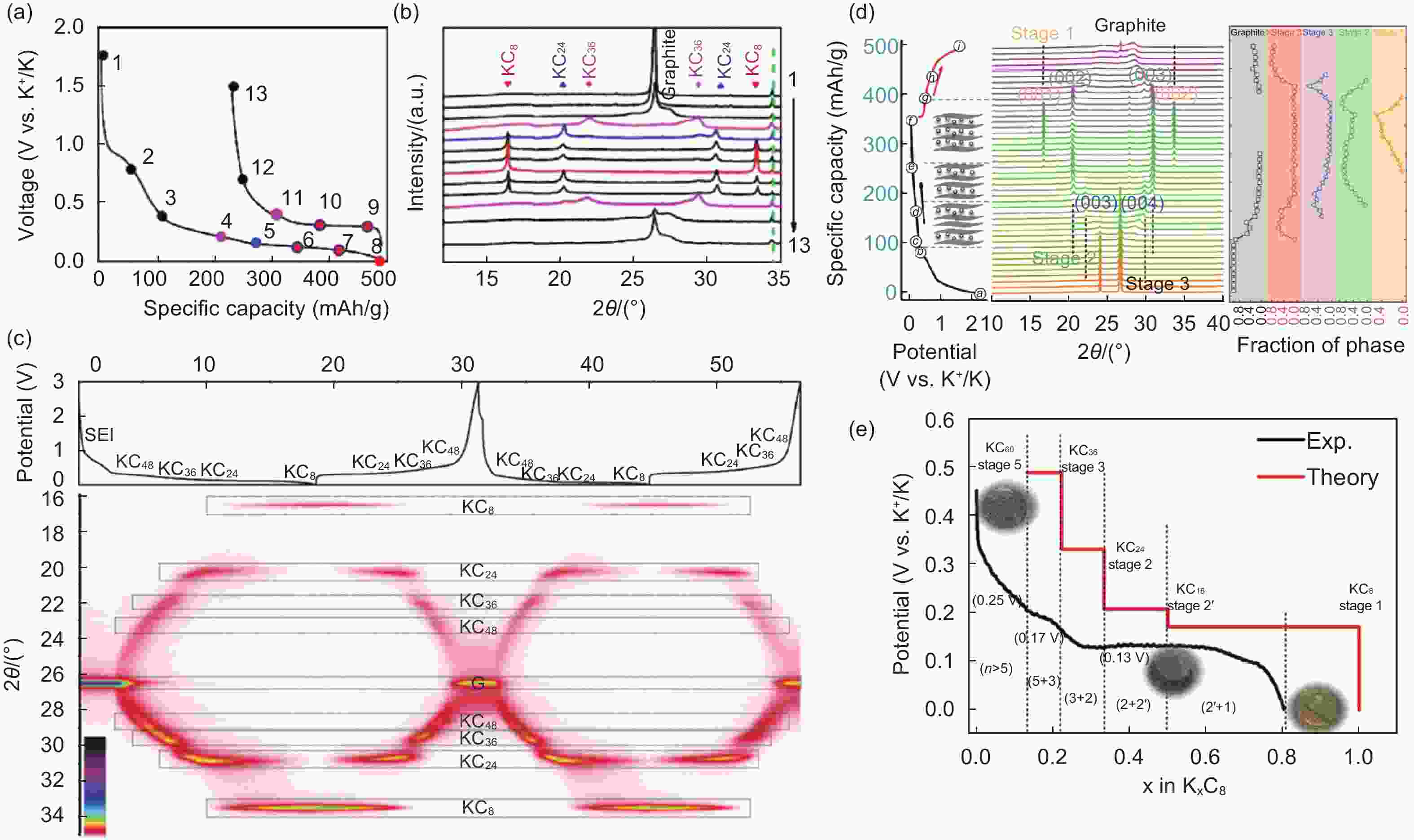
Figure 2. Charge/discharge potential profiles and corresponding XRD patterns (ex or in situ) with different graphite electrodes. (a-b) Commercial graphite at C/10 (1 C=279 mAh g−1) (Copyright 2015, American Chemical Society[36]), (c) graphite at C/14 (1st and 2nd cycle) ( Reproduced with permission, Copyright 2019, Willey[37), (d) graphite foam at C/20 (1st cycle) and (e) the discharging curve and corresponding intercalation compounds at each stage (Reproduced with permission, Copyright 2019, Willey[38]).
Figure 3. (a) The ex-situ XRD patterns of an expanded graphite cathode with the different intercalation/de intercalation stages (Reproduced with permission, Copyright 2017, Willey[42]), (b) the in-plane structure of C24PF6 (Reproduced with permission, Copyright 2014, Willey[43]) and (c-d) schematics of the staging mechanism of intercalation of FSI- anion into the graphite and the in-situ XRD of the graphite electrode during the first two cycles[44].
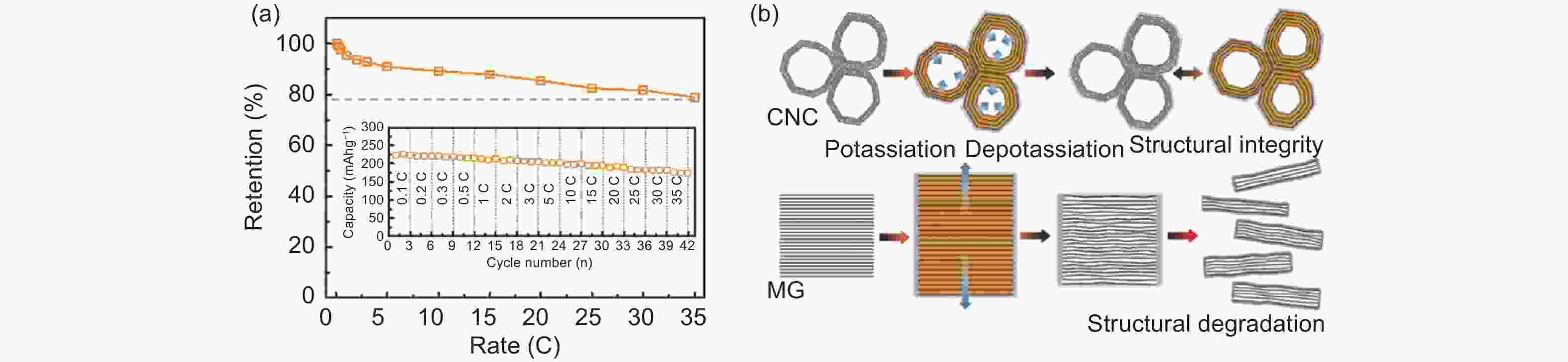
Figure 5. (a) The rate retentions and capacities of the CNC anode at different current densities, (b) schematic illustration of structural variations of CNC and MG electrodes during potassium storage (Reproduced with permission, Copyright 2019, Willey[48]).
Table 1. The overview of three ions (cation and anion) for potassium-based energy storage.
Cation/
anionIon radius/
dimensions
(Å)GIC
stoichiometryVolume
changeSpecific
capacity/
mAh g−1K+ 1.38 KC8 61% 279 PF6- 3.5×3.5 C24PF6 136% 93 FSI- 5.4×2.6 C6[FSI]0.5 134% 186 Note: Å- 0.1 nm Table 2. Electrochemical performance of different graphite anodes in KIBs (1 C=279 mAh g−1).
Materials Electrolyte Binder Initial coulombic efficiency Initial charge capacity (mAh g−1) Cyclability Rate performance (mAh g−1) Refs. Graphite 0.5 M KPF6 in EC/DEC=1∶1 PVDF 74.3% 208 at 5 mA g−1 141 at 200 mA g−1 [23] Natural graphite powder (particle size 3 μm) 1 M KFSI in EC:DEC = 1∶1 PANa 79% ~240 at C/10 ~250 at C/10
after 50 cycles[31] CMCNa 89% ~230 at C/10 ~240 at C/10
after 8 cyclesPVDF 59% ~225 at C/10 ~230 at C/10
after 20 cyclesCommercially available synthetic graphite 0.8 M KPF6 in EC:DEC = 1∶1 PVDF 57.4% 273 at C/40 100 at C/2
after 50 cycles80 at 1 C [36] KS4 graphite 1 M KPF6 in EC:PC = 1∶1 Na-alginate 66.5% 246 at 20 mA g−1 220 at 20 mA g−1
after 200 cycles~0 at 500 mA g−1 [45] PVDF 44.5% ~240 at 20 mA g−1 200 at 20 mA g−1
after 15 cycles1 M KPF6 in EC:DEC = 1∶1 Na-alginate 47.0% ~240 at 20 mA g−1 200 at 20 mA g−1
after 100 cycles1 M KPF6 in EC:DMC = 1∶1 42.7% ~240 at 20 mA g−1 ~0 at 20 mA g−1
after 140 cyclesActivated carbon from the graphite 0.8 M KPF6 in EC:DEC = 1∶1 PVDF 260 at 50 mA g−1 100.3 at 200 mA g−1
after 100 cycles30 at 1000 mA g−1 [46] Commercial graphite 1 M KFSI in EC:DEC = 1∶1 CMC 80.83% 163 at 50 mA g−1 61 at 50 mA g−1
after 200 cycles44 at 200 mA g−1 [47] Commercial expanded graphite 81.56% 218 at 50 mA g−1 228 at 50 mA g−1
after 100 cycles175 at 200 mA g−1 Mesophase graphite 1 M KFSI in
EC:PC = 1∶13 wt.% CMC and 6 wt.% PAA 248 at 0.2 C 154 at 0.2 C
after 50 cycles30 at 1 C [48] Graphitic Carbon Nanocage 40% 212 at 0.2 C 195 at 0.2 C after
100 cycles99 at 1 C
56 at 3 CBall-milled graphite 0.8 M KPF6 in EC:DEC = 1∶1 PVDF 61% 211 at 25 mA g−1 100 at 25 mA g−1
after 200 cycles[49] Graphite 0.8 M KPF6 in EC:EMC, 1∶1 CMC 20 at C/3 after
300 cycles[37] KFSI:EMC, 1:2.5, molar ratio ~170 at C/3 255 at C/3 after
2000 cyclesCarbon nanotubes-interweaved layer on graphite flakes 0.8 M KPF6 in EC:PC, 1∶1 CMC-Na 40.7% 293.8 at 200 mA g−1 245 at 200 mA g−1 after 600 cycles 222.3 at
4000 mA g−1[50] Graphite 1 M KPF6 in DME CMC 87.4% ~95 at 1 C 80 at 1 C after
700 cycles90 at 0.5 C
82 at 10 C[51] 1 M KPF6 in EC/DMC = 1∶1 69.6% ~95 at 1 C 17 at 1 C after
700 cycles220 at 0.5 C
8 at 10 CPolynanocrystalline Graphite 0.8 M KPF6 in EC:DEC = 1∶1 CMC 54.1% 224 at 20 mA g−1 ~70 at 100 mA
after 300 cycles99 at 200 mA g−1
13.6 at 1000 mA g−1[52] Graphitic Nanocarbon 0.8 M KPF6 in EC:DEC = 1∶1 CMC-Na 369 at 50 mA g−1 189 at 200 mA g−1 after 200 cycles 152 at 1000 mA g−1 [53] Note: M- mol L−1 Table 3. Electrochemical performance of KDIBs.
Cathode Anode Electrolyte Voltage window
(V)Energy density (Wh kg−1) Cyclability
(mAh g−1)Rate performance
(mAh g−1)Refs. Available synthetic
flake-type graphiteAvailable synthetic
flake-type graphiteionic liquid (Pyr14TFSI +
0.3 M KTFSI +
2 wt% ES)3.4–5.0 47 at 50 mA g−1 after
100 cycles42 at 250 mA g−1 [32] Nanographite Nanographite 0.8 M KPF6 in
EC:DMC =1∶13.0–5.0 62 at 100 mA g−1 after
60 cycles45 at 250 mA g−1 [64] Expanded graphite Metal foil 1 M KPF6 in EC+DMC+
EMC = 4∶3∶23.0–5.0 116 66 at 50 mA g−1 after
300 cycles52 at 300 mA g−1g [65] Expanded graphite Mesocarbon microbead 1 M KPF6 in EC+DMC+
EMC = 4∶3∶23.0–5.2 61 at 100 mA g−1 after
100 cycles52 at 300 mA g−1 [42] Expanded graphite Porous carbon 1 M KPF6 in EC+DMC+
EMC = 4∶3∶21.0–3.8 117 63 at 1000 mA g−1 after
2000 cycles82 at 3000 mA g−1 [69] KS6 graphite Natural graphite 0.8 M KPF6 in
EC:DEC =1∶12.4–5.4 158.3 50.5 at 100 mA g−1 after
400 cycles16.3 at 300 mA g−1 [66] Note: M- mol L−1 -
[1] Chu S, Cui Y, Liu N. The path towards sustainable energy[J]. Nature Materials,2016,16(1):16-22. [2] Feng X, Ouyanga M, Liu X, et al. Thermal runaway mechanism of lithium ion battery for electric vehicles: A review[J]. Energy Storage Materials,2018,10:246-267. doi: 10.1016/j.ensm.2017.05.013 [3] Deng J, Bae C, Denlinger A, et al. Electric vehicles batteries: requirements and challenges[J]. Joule,2020,4(3):511-515. doi: 10.1016/j.joule.2020.01.013 [4] Pomerantseva E, Bonaccorso F, Feng X, et al. Energy storage: The future enabled by nanomaterials[J]. Science,2019,366(6468):eaan8285. doi: 10.1126/science.aan8285 [5] Lu L, Han X, Li J, et al. A review on the key issues for lithium-ion battery management in electric vehicles[J]. Journal of Power Sources,2013,226:272-288. doi: 10.1016/j.jpowsour.2012.10.060 [6] Fan E, Li L, Wang Z, et al. Sustainable recycling technology for Li-ion batteries and beyond: challenges and future prospects[J]. Chemical Reviews,2020,120(14):7020-7063. doi: 10.1021/acs.chemrev.9b00535 [7] Eshraghi N, Berardo L, Schrijnemakers A, et al. recovery of nano-structured silicon from end-of-life photovoltaic wafers with value-added applications in lithium-ion battery[J]. ACS Sustainable Chemistry & Engineering,2020,8:5868−5879. [8] Eftekhari A, Jian Z, Ji X. Potassium secondary batteries[J]. ACS Applied Materials & Interfaces,2017,9(5):4404-4419. [9] Liu S, Zhou J, Song H. 2D Zn-hexamine coordination frameworks and their derived N-rich porous carbon nanosheets for ultrafast sodium storage[J]. Advanced Energy Materials,2018,8(22):1800569. doi: 10.1002/aenm.201800569 [10] You C, Wu X, Yuan X, et al. Advances in rechargeable Mg batteries[J]. Journal of Materials Chemistry A,2020,8(48):25601-25625. doi: 10.1039/D0TA09330K [11] An Y, Liu Y, Tian Y, et al. Recent development and prospect of potassium-ion batteries with high energy and high safety for post-lithium batteries[J]. Functional Materials Letters,2019,12(04):1930002. doi: 10.1142/S1793604719300020 [12] Liu F, Wang T, Liu X, et al. Challenges and recent progress on key materials for rechargeable magnesium batteries[J]. Advanced Energy Materials,2020,11(2):2000787. [13] Loaiza LC, Monconduit L, Seznec V. Si and Ge-based anode materials for Li-, Na-, and K-ion batteries: A perspective from structure to electrochemical mechanism[J]. Small,2020,16(5):1905260. doi: 10.1002/smll.201905260 [14] Wang H, Wu X, Qi X, et al. Sb nanoparticles encapsulated in 3D porous carbon as anode material for lithium-ion and potassium-ion batteries[J]. Materials Research Bulletin,2018,103:32-37. doi: 10.1016/j.materresbull.2018.03.018 [15] Ying H, Han WQ. Metallic Sn-based anode materials: application in high-performance lithium-ion and sodium-ion batteries[J]. Advanced Science (Weinh),2017,4(11):1700298. doi: 10.1002/advs.201700298 [16] Abel PR, Fields MG, Heller A, et al. Tin-germanium alloys as anode materials for sodium-ion batteries[J]. ACS Applied Materials & Interfaces,2014,6(18):15860-15867. [17] Niu X, Zhang Y, Tan L, et al. Amorphous FeVO4 as a promising anode material for potassium-ion batteries[J]. Energy Storage Materials,2019,22:160-167. doi: 10.1016/j.ensm.2019.01.011 [18] Lei K, Li F, Mu C, et al. High K-storage performance based on the synergy of dipotassium terephthalate and ether-based electrolytes[J]. Energy & Environmental Science,2017,10(2):552-557. [19] Wei Z, Wang D, Li M, et al. Fabrication of hierarchical potassium titanium phosphate spheroids: a host material for sodium-ion and potassium-ion storage[J]. Advanced Energy Materials,2018,8(27):1801102. doi: 10.1002/aenm.201801102 [20] Du J, Gao S, Shi P, et al. Three-dimensional carbonaceous for potassium ion batteries anode to boost rate and cycle life performance[J]. Journal of Power Sources,2020,451:227727. doi: 10.1016/j.jpowsour.2020.227727 [21] Zhang Z, Jia B, Liu L, et al. Hollow multihole carbon bowls: a stress-release structure design for high-stability and high-volumetric-capacity potassium-ion batteries[J]. ACS Nano,2019,13(10):11363-11371. doi: 10.1021/acsnano.9b04728 [22] Wu X, Chen Y, Xing Z, et al. Advanced carbon‐based anodes for potassium‐ion batteries[J]. Advanced Energy Materials,2019,9(21):1900343. doi: 10.1002/aenm.201900343 [23] Luo W, Wan J, Ozdemir B, et al. Potassium ion batteries with graphitic materials[J]. Nano Letters,2015,15(11):7671-7677. doi: 10.1021/acs.nanolett.5b03667 [24] Li J, Qin W, Xie J, et al. Sulphur-doped reduced graphene oxide sponges as high-performance free-standing anodes for K-ion storage[J]. Nano Energy,2018,53:415-424. doi: 10.1016/j.nanoen.2018.08.075 [25] Ju Z, Li P, Ma G, et al. Few layer nitrogen-doped graphene with highly reversible potassium storage[J]. Energy Storage Materials,2018,11:38-46. doi: 10.1016/j.ensm.2017.09.009 [26] Jian Z, Hwang S, Li Z, et al. Hard-soft composite carbon as a long-cycling and high-rate anode for potassium-ion batteries[J]. Advanced Functional Materials,2017,27(26):1700324. doi: 10.1002/adfm.201700324 [27] Chen J, Feng J, Dong L, et al. Nanoporous coal via Ni-catalytic graphitization as anode materials for potassium ion battery[J]. Journal of Electroanalytical Chemistry,2020,862:113902. doi: 10.1016/j.jelechem.2020.113902 [28] Naylor A J, Carboni M, Valvo M, et al. Interfacial reaction mechanisms on graphite anodes for K-ion batteries[J]. ACS Applied Materials & Interfaces,2019,11(49):45636-45645. [29] Zhang Y, Yang L, Tian Y, et al. Honeycomb hard carbon derived from carbon quantum dots as anode material for K-ion batteries[J]. Materials Chemistry and Physics,2019,229:303-309. doi: 10.1016/j.matchemphys.2019.03.021 [30] Sun Y, Xiao H, Li H, et al. Nitrogen/oxygen co-doped hierarchically porous carbon for high-performance potassium atorage[J]. Chemistry,2019,25(30):7359-7365. doi: 10.1002/chem.201900448 [31] Komaba S, Hasegawa T, Dahbi M, et al. Potassium intercalation into graphite to realize high-voltage/high-power potassium-ion batteries and potassium-ion capacitors[J]. Electrochemistry Communications,2015,60:172-175. doi: 10.1016/j.elecom.2015.09.002 [32] Beltrop K, Beuker S, Heckmann A, et al. Alternative electrochemical energy storage: Potassium-based dual-graphite batteries[J]. Energy & Environmental Science,2017,10(10):2090-2094. [33] Wang M, Tang Y. A review on the features and progress of dual-ion batteries[J]. Advanced Energy Materials,2018,8(19):1703320. doi: 10.1002/aenm.201703320 [34] Heidrich B, Heckmann A, Beltrop K, et al. Unravelling charge/discharge and capacity fading mechanisms in dual-graphite battery cells using an electron inventory model[J]. Energy Storage Materials,2019,21:414-426. doi: 10.1016/j.ensm.2019.05.031 [35] Wu X, Xing Z, Hu Y, et al. Effects of functional binders on electrochemical performance of graphite anode in potassium-ion batteries[J]. Ionics,2018,25(6):2563-2574. [36] Jian Z, Luo W, Ji X. Carbon electrodes for K-ion batteries[J]. Journal American Chemical Society,2015,137(36):11566-11569. doi: 10.1021/jacs.5b06809 [37] Fan L, Ma R, Zhang Q, et al. Graphite anode for potassium ion battery with unprecedented performance[J]. Angewandte Chemie Int Ed Engl,2019,58(31):10500-10505. doi: 10.1002/anie.201904258 [38] Liu J, Yin T, Tian B, et al. Unraveling the potassium storage mechanism in graphite foam[J]. Advanced Energy Materials,2019,22(9):1900579. [39] Yu D, Cheng L, Chen M, et al. High-performance phosphorus-graphite dual-ion battery[J]. ACS Applied Materials & Interfaces,2019,11(49):45755-45762. [40] Jiang C, Xiang L, Miao S, et al. Flexible interface design for stress regulation of a silicon anode toward highly stable dual-ion batteries[J]. Advanced Materials,2020,32(17):1908470. doi: 10.1002/adma.201908470 [41] Zhang M, Song X, Ou X, et al. Rechargeable batteries based on anion intercalation graphite cathodes[J]. Energy Storage Materials,2019,16:65-84. doi: 10.1016/j.ensm.2018.04.023 [42] Ji B, Zhang F, Wu N, et al. A dual-carbon battery based on potassium-ion electrolyte[J]. Advanced Energy Materials,2017,7(20):1700920. doi: 10.1002/aenm.201700920 [43] Placke T, Schmuelling G, Kloepsch R, et al. In situ X-ray diffraction studies of cation and anion intercalation into graphitic carbons for electrochemical energy storage applications[J]. Zeitschrift Für Anorganische und Allgemeine Chemie,2014,640(10):1996-2006. [44] Kravchyk KV, Bhauriyal P, Piveteau L, et al. High-energy-density dual-ion battery for stationary storage of electricity using concentrated potassium fluorosulfonylimide[J]. Nature Communications,2018,9(1):4469. doi: 10.1038/s41467-018-06923-6 [45] Zhao J, Zou X, Zhu Y, et al. Electrochemical intercalation of potassium into graphite[J]. Advanced Functional Materials,2016,26(44):8103-8110. doi: 10.1002/adfm.201602248 [46] Tai Z, Zhang Q, Liu Y, et al. Activated carbon from the graphite with increased rate capability for the potassium ion battery[J]. Carbon,2017,123:54-61. doi: 10.1016/j.carbon.2017.07.041 [47] An Y, Fei H, Zeng G, et al. Commercial expanded graphite as a low-cost, long-cycling life anode for potassium-ion batteries with conventional carbonate electrolyte[J]. Journal of Power Sources,2018,378:66-72. doi: 10.1016/j.jpowsour.2017.12.033 [48] Cao B, Zhang Q, Liu H, et al. Graphitic carbon nanocage as a stable and high power anode for potassium-ion batteries[J]. Advanced Energy Materials,2018,25(8):1801149. [49] Carboni M, Naylor AJ, Valvo M, et al. Unlocking high capacities of graphite anodes for potassium-ion batteries[J]. RSC Advances,2019,9(36):21070-21074. doi: 10.1039/C9RA01931F [50] Jiang S, Li Y, Qian Y, et al. Constructing a buffering and conducting carbon nanotubes-interweaved layer on graphite flakes for high-rate and long-term K-storage properties[J]. Journal of Power Sources,2019,436:226847. doi: 10.1016/j.jpowsour.2019.226847 [51] Wang L, Yang J, Li J, et al. Graphite as a potassium ion battery anode in carbonate-based electrolyte and ether-based electrolyte[J]. Journal of Power Sources,2019,409:24-30. doi: 10.1016/j.jpowsour.2018.10.092 [52] Xing Z, Qi Y, Jian Z, et al. Polynanocrystalline graphite: a new carbon anode with superior cycling performance for K-ion batteries[J]. ACS Applied Materials & Interfaces,2017,9(5):4343-4351. [53] Zhang W, Ming J, Zhao W, et al. Graphitic nanocarbon with engineered defects for high-performance potassium-ion battery anodes[J]. Advanced Functional Materials,2019,29(35):1903641. doi: 10.1002/adfm.201903641 [54] Rahman M M, Hou C, Mateti S, et al. Documenting capacity and cyclic stability enhancements in synthetic graphite potassium-ion battery anode material modified by low-energy liquid phase ball milling[J]. Journal of Power Sources,2020,476:228733. doi: 10.1016/j.jpowsour.2020.228733 [55] Zhang W, Liu Y, Guo Z. Approaching high-performance potassium-ion batteries via advanced design strategies and engineering[J]. Science Advances,2019,5(5):7412. doi: 10.1126/sciadv.aav7412 [56] Zhang W, Wu Z, Zhang J, et al. Unraveling the effect of salt chemistry on long-durability high-phosphorus-concentration anode for potassium ion batteries[J]. Nano Energy,2018,53:967-974. doi: 10.1016/j.nanoen.2018.09.058 [57] Wang W, Yang S. Enhanced overall electrochemical performance of silicon/carbon anode for lithium-ion batteries using fluoroethylene carbonate as an electrolyte additive[J]. Journal of Alloys and Compounds,2017,695:3249-3255. doi: 10.1016/j.jallcom.2016.11.248 [58] Etacheri V, Haik O, Goffer Y, et al. Effect of fluoroethylene carbonate (FEC) on the performance and surface chemistry of Si-nanowire Li-ion battery anodes[J]. Langmuir: the ACS Journal of Surfaces and Colloids,2012,28(1):965-976. doi: 10.1021/la203712s [59] Yoon G, Kim H, Park I, et al. Conditions for reversible Na intercalation in graphite: Theoretical studies on the interplay among guest ions, solvent, and graphite host[J]. Advanced Energy Materials,2017,7(2):1601519. doi: 10.1002/aenm.201601519 [60] Zhang Q, Mao J, Pang W K, et al. Boosting the potassium storage performance of alloy-based anode materials via electrolyte salt chemistry[J]. Advanced Energy Materials,2018,8(15):1703288. doi: 10.1002/aenm.201703288 [61] Niu X, Li L, Qiu J, et al. Salt-concentrated electrolytes for graphite anode in potassium ion battery[J]. Solid State Ionics,2019,341:115050. doi: 10.1016/j.ssi.2019.115050 [62] Qin L, Xiao N, Zheng J, et al. Localized high‐concentration electrolytes boost potassium storage in high‐loading graphite[J]. Advanced Energy Materials,2019,9(44):1902618. doi: 10.1002/aenm.201902618 [63] Kravchyk K V, Kovalenko M V. Rechargeable dual‐ion batteries with graphite as a cathode: key challenges and opportunities[J]. Advanced Energy Materials,2019,9(35):1901749. doi: 10.1002/aenm.201901749 [64] Fan L, Liu Q, Chen S, et al. Potassium-based dual ion battery with dual-graphite electrode[J]. Small,2017,13(30):1701011. doi: 10.1002/smll.201701011 [65] Ji B, Zhang F, Song X, et al. A novel potassium-ion-based dual-ion battery[J]. Advanced Materials,2017,29(19):1700519. doi: 10.1002/adma.201700519 [66] Zhu J, Li Y, Yang B, et al. A dual carbon-based potassium dual ion battery with robust comprehensive performance[J]. Small,2018,14(31):1801836. doi: 10.1002/smll.201801836 [67] Münster P, Heckmann A, Nölle R, et al. Enabling high performance potassium‐based dual‐graphite battery cells by highly concentrated electrolytes[J]. Batteries & Supercaps,2019,2(12):992-1006. [68] Meister P, Siozios V, Reiter J, et al. Dual-ion cells based on the electrochemical intercalation of asymmetric fluorosulfonyl-(trifluoromethanesulfonyl) imide anions into graphite[J]. Electrochimica Acta,2014,130:625-633. doi: 10.1016/j.electacta.2014.03.070 [69] Ding X, Zhang F, Ji B, et al. Potassium dual-ion hybrid batteries with ultrahigh rate performance and excellent cycling stability[J]. ACS Applied Materials & Interfaces,2018,10(49):42294-42300. -






 下载:
下载: