A highly efficient absorptive and catalytic self-supporting Fe2O3/CC host for high performance Li-S batteries
-
摘要:
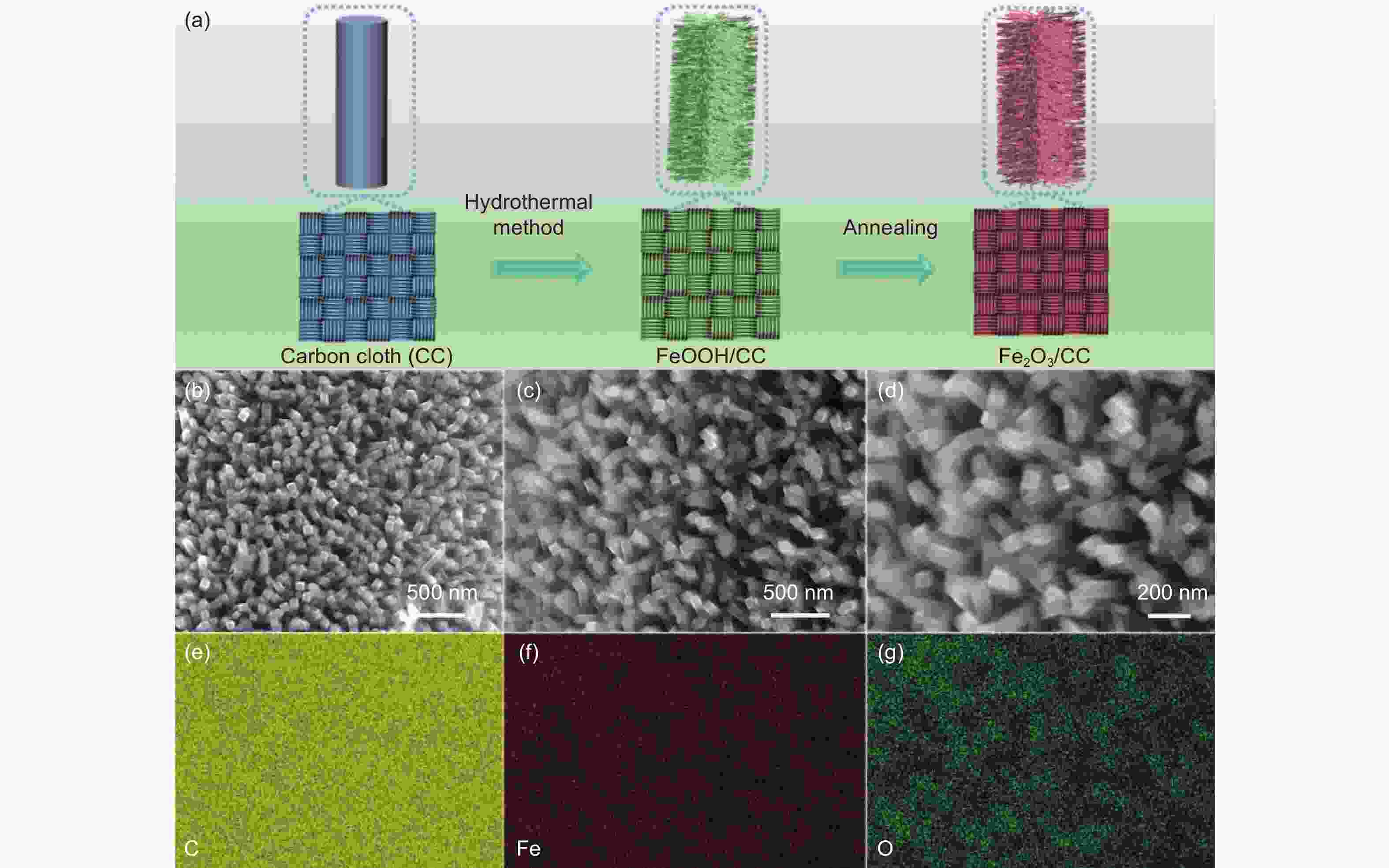
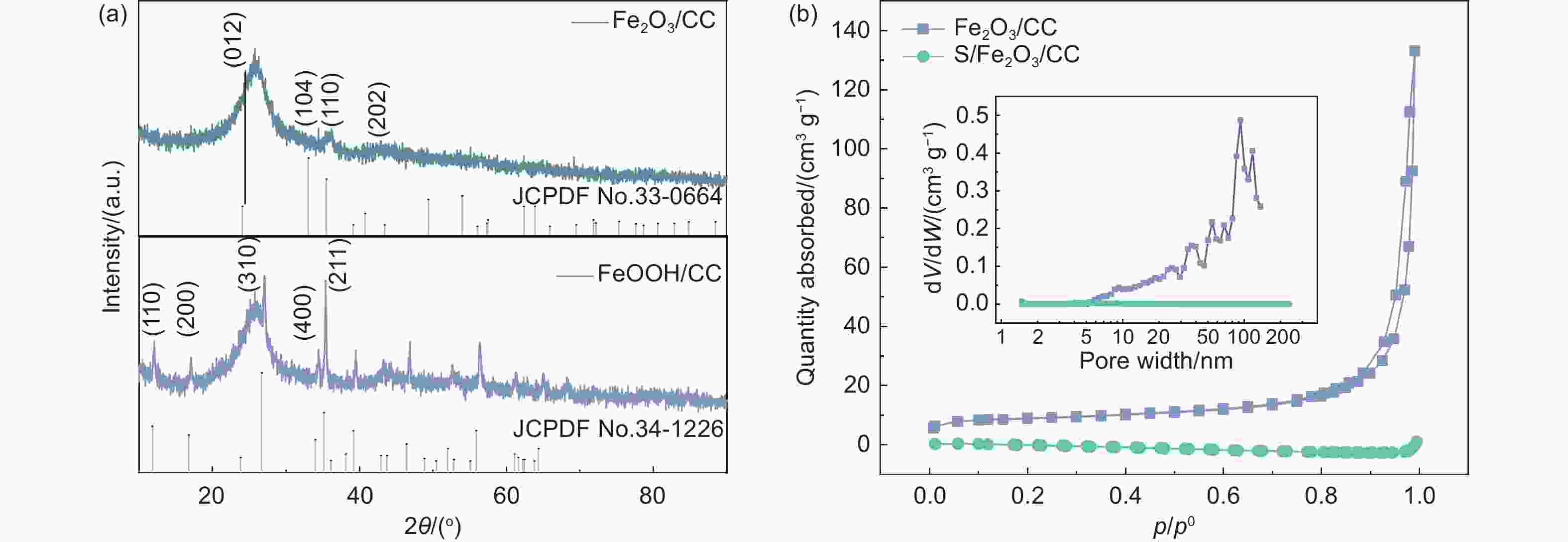
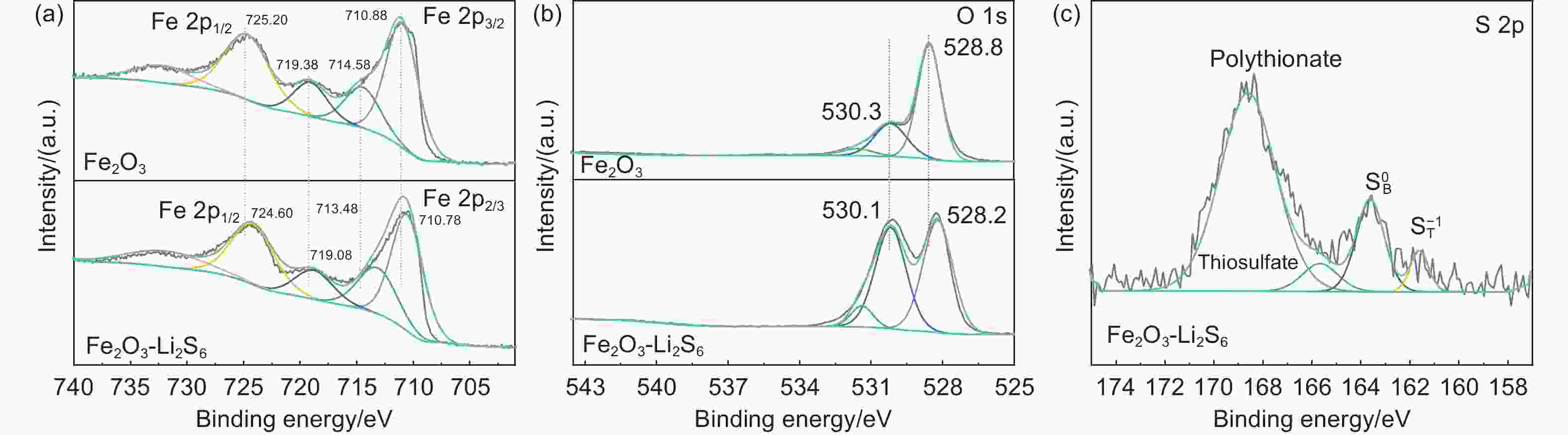
锂硫电池因其高能量密度和低成本而成为最有发展前景的电化学储能器件之一。然而,多硫化物的“穿梭效应”、硫导电率低是锂硫电池商业化面临的主要挑战。本工作中,以Fe(NO)3·9H2O为铁源,NH4F为表面活性剂,通过简单的水热及煅烧处理制备了Fe2O3纳米棒修饰炭布(CC)的柔性Fe2O3/CC复合材料。其中,Fe2O3中介孔的存在有利于电解质的渗透和充放电过程中锂离子的传输和扩散,同时其密集阵列暴露出的丰富活性位点可以实现多硫化物的高效吸附和快速转化,降低多硫化物的穿梭效应。电化学分析显示:Fe2O3/CC正极在0.1 C(1 C=1672 mA g−1)的电流密度下具有1250 mAh g−1的高放电比容量,经100圈循环后比容量保持在789 mAh g−1。在2 C的倍率下循环1000圈后仍能达到576 mAh g−1的放电比容量,容量保持率为70%,明显优于对比样品。因此,Fe2O3/CC能够很好地抑制多硫化物的穿梭,提高电池倍率性能和循环稳定性。
Abstract:The lithium−sulfur (Li-S) battery is a promising energy storage system because of its high energy density and low cost. However, the shuttling of lithium polysulfides (LiPSs) and low conductivity of the S cathode are barriers to its practical application. Fe2O3 nanorods were grown on a carbon cloth (Fe2O3/CC) by a solvothermal reaction and calcination to obtain a cathode for the battery. The mesoporous structure of the Fe2O3 and the CC conducting network facilitates lithium-ion and electron transport. Meanwhile, the nanorod arrangement results in the exposure of more Fe2O3 active sites, which improves the adsorption and rapid conversion of LiPSs. As a result, a Li–S cell using a Fe2O3/CC cathode has a high capacity of 1250 mAh g−1 at 0.1 C with an excellent life of over 100 cycles with a capacity retention of 67%. It also has a 70% capacity retention after 1000 cycles at 0.2 C. The excellent electrochemical performance of the Fe2O3/CC cathode indicates its potential applications in Li-S batteries.
-
Key words:
- Lithium-sulfur batteries /
- Fe2O3 /
- Cathode /
- Nanorod
-
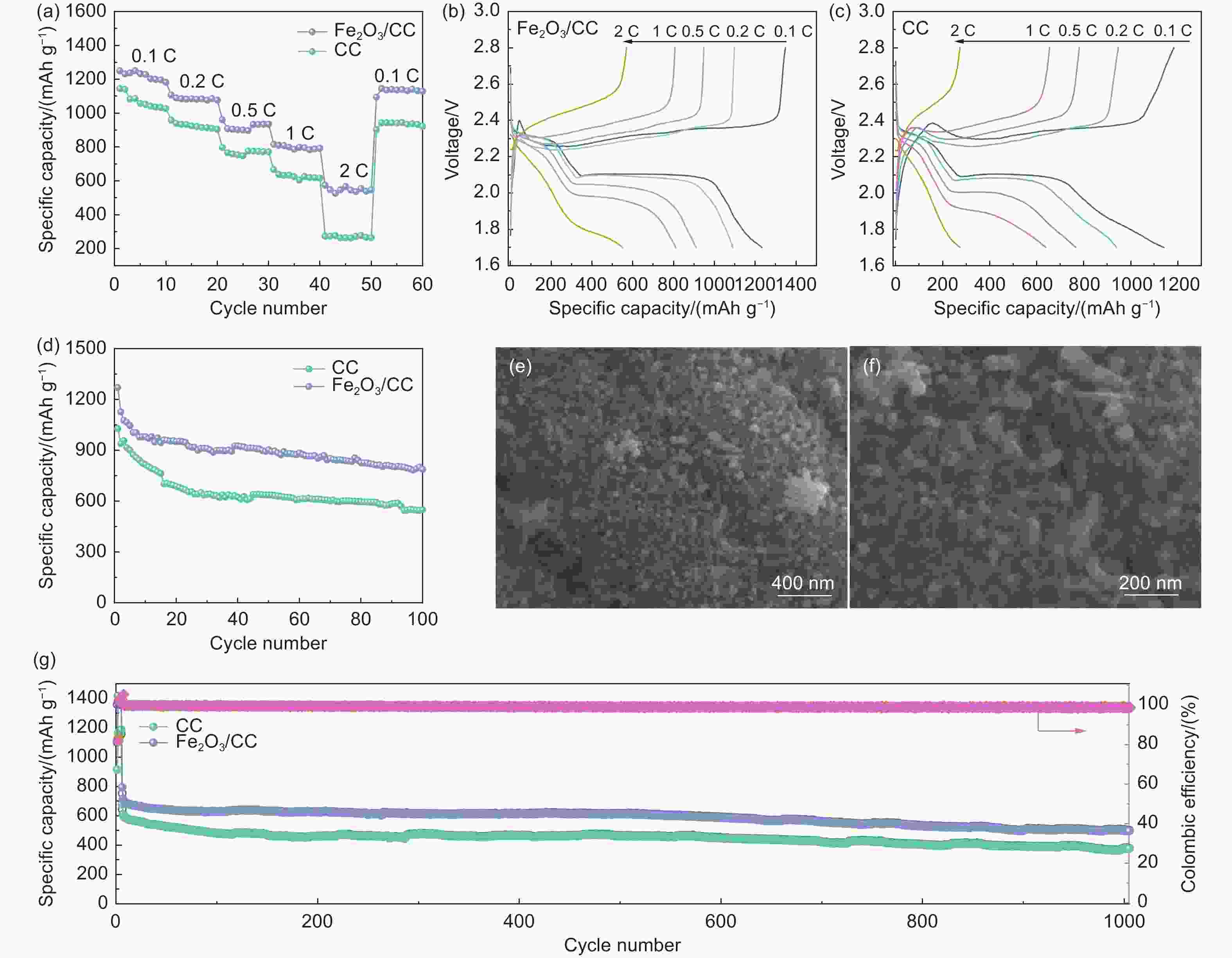
图 5 不同样品的电化学性能:(a) 倍率性能, (b) Fe2O3/CC和(c) CC在不同电流密度时的充放电曲线, (d) 0.1 C电流密度下Fe2O3/CC和CC的循环性能图, (e,f) 循环后Fe2O3/CC电极的SEM照片, (g) 1 C电流密度下Fe2O3/CC和CC的循环性能图
Figure 5. (a) Rate performance of the Li−S cell with different cathodes, discharge−charge profiles of the Li−S cell with Fe2O3/CC (b) and CC (c), (d) cycling performance of Fe2O3/CC and CC cathodes at 0.1 C for 100 cycles, (e,f) SEM images of Fe2O3/CC after cycling, (g) long-term cycle performance of Fe2O3/CC and CC cathodes at 1 C for 1000 cycles
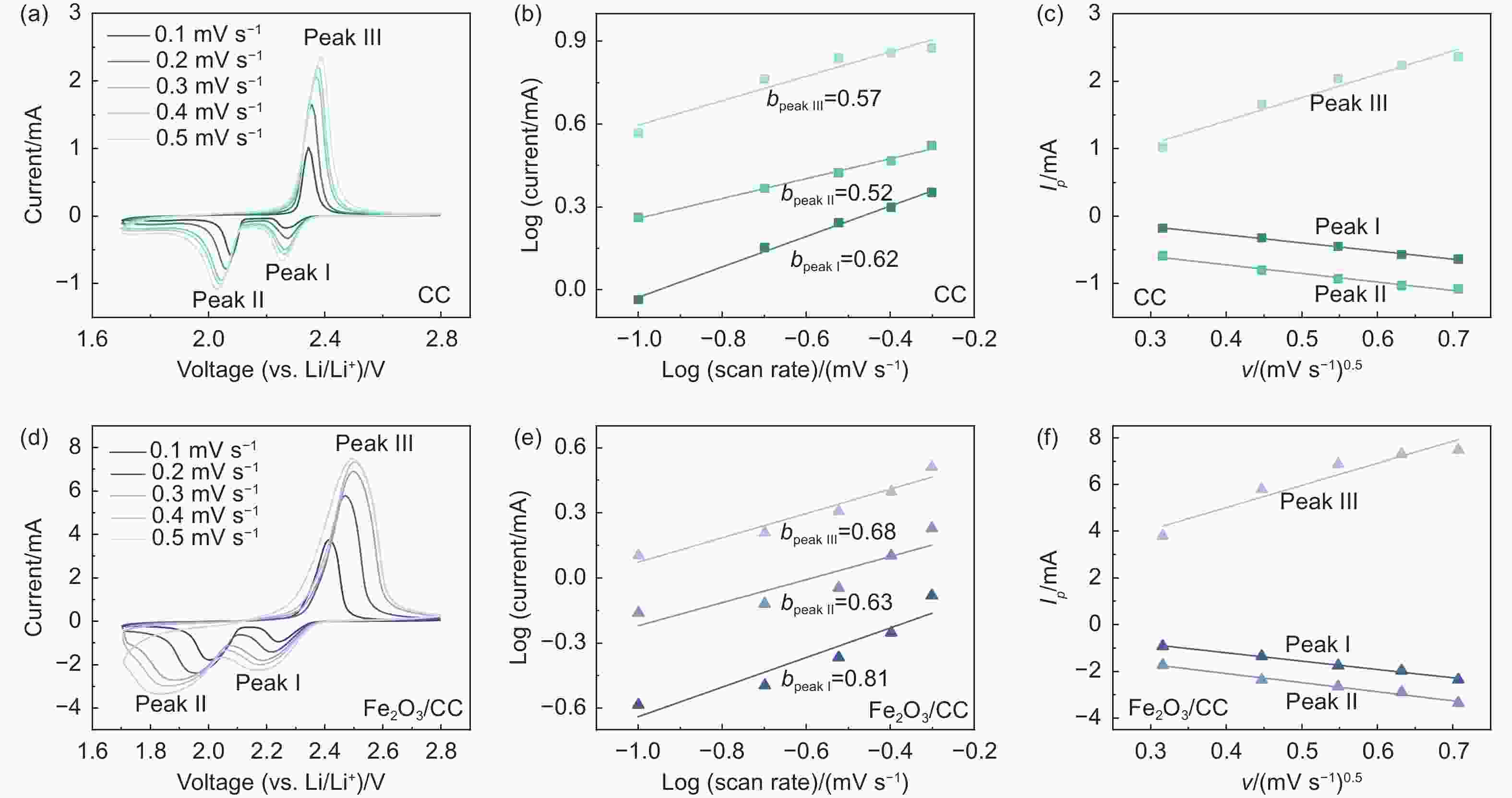
图 6 (a) CC电极在不同扫速下的CV曲线, (b) CC电极氧化还原峰对应的logi-logv曲线, (c) CC电极氧化还原峰对应的峰值电流与扫描速率的函数图, (d) Fe2O3/CC电极在不同扫速下的CV曲线, (e) Fe2O3/CC电极氧化还原峰对应的logi-logv曲线, (f) Fe2O3/CC电极氧化还原峰对应的峰值电流与扫描速率的函数图
Figure 6. (a) CV curves of CC at 0.1-0.5 mV s−1, (b) b values of CC calculated from the fittings of the relationship between peak currents and scan rates, (c) peak currents of CC at the positions of peak Ⅰ , peak Ⅱ and peak Ⅲ as a function of the scanning rate, (d) CV curves of Fe2O3/CC at 0.1-0.5 mV s−1, (e) b values of Fe2O3/CC calculated from the fittings of the relationship between peak currents and scan rates, (f) peak currents of Fe2O3/CC at the positions of peak Ⅰ , peak Ⅱ and peak Ⅲ as a function of the scanning rate
图 8 (a) 0.1 mV s−1下不同锂硫电池的CV曲线, (b) CC和Fe2O3/CC氧化峰Tafel拟合曲线, (c) CC和Fe2O3/CC还原峰Tafel拟合曲线, (d) 0.1 mV s−1下Li2S6对称电池的CV曲线, (e) 不同Li2S6对称电池的Nyquist图, (f) Fe2O3/CC和CC对多硫化物吸附实验的光学照片, (g) CC和 (h) Fe2O3/CC的多圈循环伏安曲线对比, (i)不同锂硫电池工作原理图
Figure 8. (a) CV curves of the first cycle of Fe2O3/CC and CC cathodes, (b) Tafel plots of anodic peak for CC and Fe2O3/CC, (c) Tafel plots of cathodic peak for CC and Fe2O3/CC, (d) CV curves of Fe2O3/CC and CC cathodes of the symmetric cell, (e) Nyquist plots of Fe2O3/CC and CC, (f) visualized adsorption of Li2S6 on Fe2O3/CC and CC after 12 h, the multi-CV curves of (g)CC and (h) Fe2O3/CC, (i) schematic diagram of different devices
表 1 Li+扩散系数
Table 1. Li+ diffusion coefficients of the CC and Fe2O3/CC
Sample DLi+ Peak Ⅰ
(cathodic peak)/(cm2 s−1)Peak Ⅱ
(cathodic peak)/(cm2 s−1)Peak Ⅲ
(anodic peak)/(cm2 s−1)CC 1.20×10−8 1.26×10−8 3.45×10−8 Fe2O3/CC 3.57×10−8 3.89×10−8 9.48×10−8 -
[1] Li Z N, Sami I, Yang J, et al. Lithiated metallic molybdenum disulfide nanosheets for high-performance lithium-sulfur batteries[J]. Nature Energy,2023,8:84-93. doi: 10.1038/s41560-022-01175-7 [2] Zhang P P, Zhao Y G, LI Y K, et al. Revealing the selective bifunctional electrocatalytic sites via in situ irradiated X-ray photoelectron spectroscopy for lithium–sulfur battery[J]. Advanced Science,2023,10(8):2206786. doi: 10.1002/advs.202206786 [3] Shi F Y, Onofrio N, Chen C H, et al. Stable liquid-sulfur generation on transition-metal dichalcogenides toward low-temperature lithium-sulfur batteries[J]. ACS Nano,2022,16(9):14412-14421. doi: 10.1021/acsnano.2c04769 [4] Zhao Z X, Yi Z L, Duan Y R, et al. Regulating the d-p band center of FeP/Fe2P heterostructure host with built-in electric field enabled efficient bidirectional electrocatalyst toward advanced lithium-sulfur batteries[J]. Chemical Engineering Journal,2023,463:142397. doi: 10.1016/j.cej.2023.142397 [5] Lee B J, Kang T H, Lee H Y, et al. Revisiting the role of conductivity and polarity of host materials for long-life lithium–sulfur battery[J]. Advanced Energy Materials,2020,10:1903934. doi: 10.1002/aenm.201903934 [6] Qin B, Wang Q, YAO W Q, et al. Heterostructured Mn3O4-MnS multi-shelled hollow spheres for enhanced polysulfide regulation in lithium-sulfur batteries [J]. Energy & Environmental Materials, 2023, 6, e12475. [7] Zhao Z X, Duan Y R, Chen F, et al. Multifunctional transitional metal-based phosphide nanoparticles towards improved polysulfide confinement and redox kinetics for highly stable lithium-sulfur batteries[J]. Chemical Engineering Journal,2022,450:138310. doi: 10.1016/j.cej.2022.138310 [8] Zhou C Y, Li X C, Jiang H L, et al. Pulverizing Fe2O3 nanoparticles for developing Fe3C/N-co doped carbon nanoboxes with multiple polysulfide anchoring and converting activity in Li-S batteries[J]. Advanced Functional Materials,2021,31(8):2011249. [9] Zheng C, Niu S Z, Lv W, et al. Propelling polysulfides transformation for high-rate and long-life lithium-sulfur batteries[J]. Nano Energy,2017,33:306-312. doi: 10.1016/j.nanoen.2017.01.040 [10] Zhang H, Gao Q M, Li Z Y, et al. A rGO-based Fe2O3 and Mn3O4 binary crystals nanocomposite additive for high performance Li–S battery[J]. Electrochimica Acta,2020,343:136079. doi: 10.1016/j.electacta.2020.136079 [11] Wang C, Sui G Z, Guo D X, et al. Oxygen vacancy-engineered Fe2O3 porous microspheres with large specific surface area for hydrogen evolution reaction and lithium-sulfur battery[J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects,2022,649:129476. doi: 10.1016/j.colsurfa.2022.129476 [12] Li J, Xu Y L, He C, et al. Three-dimensional embroidered ball-like α-Fe2O3 synthesized by a microwave hydrothermal method as a sulfur immobilizer for high-performance Li-S batteries[J]. Journal of Materials Chemistry C,2022,10:7066-7075. doi: 10.1039/D1TC05694H [13] Deng S G, Guo T Z, Heier J, et al. Unraveling polysulfide's adsorption and electrocatalytic conversion on metal oxides for Li-S batteries[J]. Advanced Science,2023,5:2204930. [14] Wang M, Emre A E, Kim J Y, et al. Multifactorial engineering of biomimetic membranes for batteries with multiple high-performance parameters[J]. Nature Communications,2022,13:278. doi: 10.1038/s41467-021-27861-w [15] Zhao Z X, Yi Z, Li H J, et al. Synergetic effect of spatially separated dual co-catalyst for accelerating multiple conversion reaction in advanced lithium sulfur batteries[J]. Nano Energy,2021,81:105621. doi: 10.1016/j.nanoen.2020.105621 [16] Niu S Z, Wu S D, Lv W, et al. A one-step hard-templating method for the preparation of a hierarchical microporous-mesoporous carbon for lithium-sulfur batteries[J]. New Carbon Materials,2017,32(4):289-296. doi: 10.1016/S1872-5805(17)60123-9 [17] Li F F, Lv W, Niu S Z, et al. Preparation and electrochemical performance of a graphene-wrapped carbon/sulphur composite cathode[J]. New Carbon Materials,2014,29(4):309-315. doi: 10.1016/S1872-5805(14)60140-2 [18] Zhao Z X, Yi Z, Li H J, et al. Understanding the modulation effect and surface chemistry in a heteroatom incorporated graphene-like matrix toward high-rate lithium-sulfur batteries[J]. Nanoscale,2021,13:14777-14784. doi: 10.1039/D1NR03390E [19] Li T T, Zhang Y, Chen J H, et al. Flexible binder for S@pPAN cathode of lithium sulfur battery[J]. Journal of Inorganic Materials,2022,37(2):182-188. doi: 10.15541/jim20210303 [20] Yao Y K, Zhao Z X, Ren R N, et al. Tailoring nickel-cobalt bimetallic alloy as highly effective catalyst in modified separators for high-performance lithium-sulfur batteries[J]. Journal of Alloys and Compounds,2023,945:169242. doi: 10.1016/j.jallcom.2023.169242 [21] Chen X R, Yu X F, He B, et al. Encapsulation of sulfur inside micro-nano carbon/molybdenum carbide by in-situ chemical transformation for high-performance Li-S batteries[J]. New Carbon Materials,2023,38(2):337-346. doi: 10.1016/S1872-5805(23)60713-9 [22] He J R, Bhargav A, Manthiram A. High-performance anode-free Li-S batteries with an integrated Li2S-electrocatalyst cathode[J]. ACS Energy Letters,2022,2(7):583-590. [23] Zhang C Y, Zhang C Q, Sun G W, et al. Spin effect to promote reaction kinetics and overall performance of lithium-sulfur batteries under external magnetic field[J]. Angewandte Chemie International Edition,2022,49(61):202211570. [24] Jiang B, Qiu Y, Tian D, et al. Crystal facet engineering induced active tin dioxide nanocatalysts for highly stable lithium–sulfur batteries[J]. Advanced Energy Materials,2021,11:2102995. doi: 10.1002/aenm.202102995 [25] Zhang K, Zhao Z X, Wang X M. Ni2P/rGO as a highly efficient sulfur host toward enhancing the polysulfides redox for lithium-sulfur batteries[J]. Journal of Alloys and Compounds,2022,906:164376. [26] Liu J Y, Ding Y Y, Shen Z H, et al. A lamellar yolk–shell lithium-sulfur battery cathode displaying ultralong cycling life, high-rate performance, and temperature tolerance[J]. Advanced Science,2022,9(3):2103517. doi: 10.1002/advs.202103517 [27] Yin F, Jin Q, Zhang X T, et al. Design of a 3D CNT/Ti3C2Tx aerogel-modified separator for Li–S batteries to eliminate both the shuttle effect and slow redox kinetics of polysulfides[J]. New Carbon Materials,2022,37(4):724-733. doi: 10.1016/S1872-5805(21)60085-9 -





 下载:
下载: