MOF-derived nanocarbon materials for electrochemical catalysis and their advanced characterization
-
摘要: 鉴于对清洁和可持续能源的需求,来自金属有机框架(MOFs)的纳米炭衍生物正崭露头角,成为电催化能量转化的独特催化剂。这些MOF衍生的纳米炭材料不仅保持了MOFs组成可定制和结构多样性等优势,而且在热解过程中可有效防止金属纳米颗粒和金属氧化物的聚集。因此,它们提高了电催化效率,改善了电导率,并在燃料电池和金属-空气电池等绿色能源技术中发挥了关键作用。该综述以MOF衍生碳基材料的炭化机制为起点,随后深入探讨了固有炭缺陷、金属和非金属原子掺杂,并研究了这些材料的合成策略。此外,全面介绍了先进的表征技术,包括原位映射和原位光谱学。最后,对MOF衍生碳基材料作为电催化剂的研究前景提供了见解。该综述的主要目标是为当前MOF衍生碳基电催化剂的状况提供更清晰的视角,鼓励更高效电催化材料的发展。Abstract: Because of the demand for clean and sustainable energy sources, nanocarbons, modified carbons and their composite materials derived from metal-organic frameworks (MOFs) are emerging as distinct catalysts for electrocatalytic energy conversion. These materials not only inherit the advantages of MOFs, like customizable dopants and structural diversity, but also effectively prevent the aggregation of nanoparticles of metals and metal oxides during pyrolysis. Consequently, they increase the electrocatalytic efficiency, improve electrical conductivity, and may play a pivotal role in green energy technologies such as fuel cells and metal-air batteries. This review first explores the carbonization mechanism of the MOF-derived carbon-based materials, and then considers 3 key aspects: intrinsic carbon defects, metal and non-metal atom doping, and the synthesis strategies for these materials. We also provide a comprehensive introduction to advanced characterization techniques to better understand the basic electrochemical catalysis processes, including mapping techniques for detecting localized active sites on electrocatalyst surfaces at the micro- to nano-scale and in-situ spectroscopy. Finally, we offer insights into future research concerning their use as electrocatalysts. Our primary objective is to provide a clearer perspective on the current status of MOF-derived carbon-based electrocatalysts and encourage the development of more efficient materials.
-
Key words:
- MOFs /
- Nanocarbon materials /
- Electrochemical catalysis /
- Advanced characterizations
-
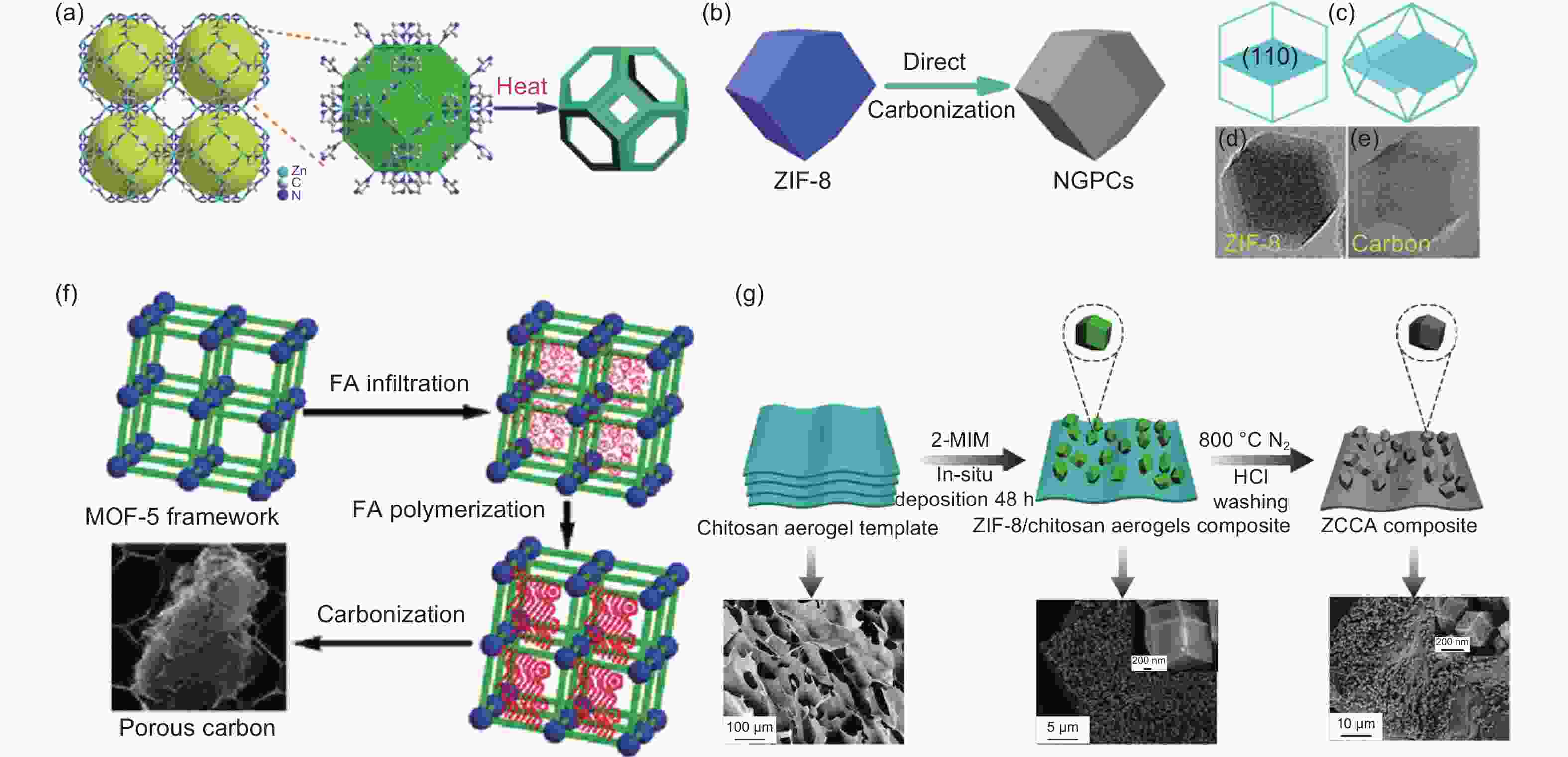
Figure 1. (a-b) Schematic illustration of the ZIF-8 derived highly graphitized NGPCs. (c) The corresponding rhombic dodecahedron-like structural models of ZIF-8 and NGPCs. (d-e) TEM images of typical ZIF-8 and NGPC polyhedron nanoparticle, respectively[14]. Reproduced with permission. Copyright @ 2014 Royal Society of Chemistry. (f) Schematic illustration of the FA/MOF-5 composites derived highly porous carbon and its corresponding SEM image[17]. Reproduced with permission Copyright © 2008 American Chemical Society. (g) Schematic of the self-assembly process of the ZIF-8 and chitosan aerogel for the formation of ZCCA composite(up) and their corresponding SEM images (bottom)[41]. Reproduced with permission. Copyright @ 2020 Elsevier Ltd.
Figure 2. (a) Synthetic process of the GO/ZIF-67 composites and GO/Co@CN nanosheets. (b) Randomly aligned GO/Co@CN nanosheets with electrode. (c) Horizontally aligned GO/Co@CN nanosheets with electrode. (d) The cross-sectional SEM image and (e) SAED pattern of the HAGO/Co@CN electrodes[19]. Reproduced with permission. Copyright@ 2022 The Royal Society of Chemistry. (f) Schematic illustration of the preparation of the Co@N–HCCs@NG derived from GO/ZIF-67 composites. (g-h) TEM images of Co@N–HCCs@NG. (i) HRTEM image of Co@N–HCCs@NG and (j) SAED pattern of the HAGO/Co@CN electrode[34]. Reproduced with permission. Copyright @ 2021 Elsevier Ltd.
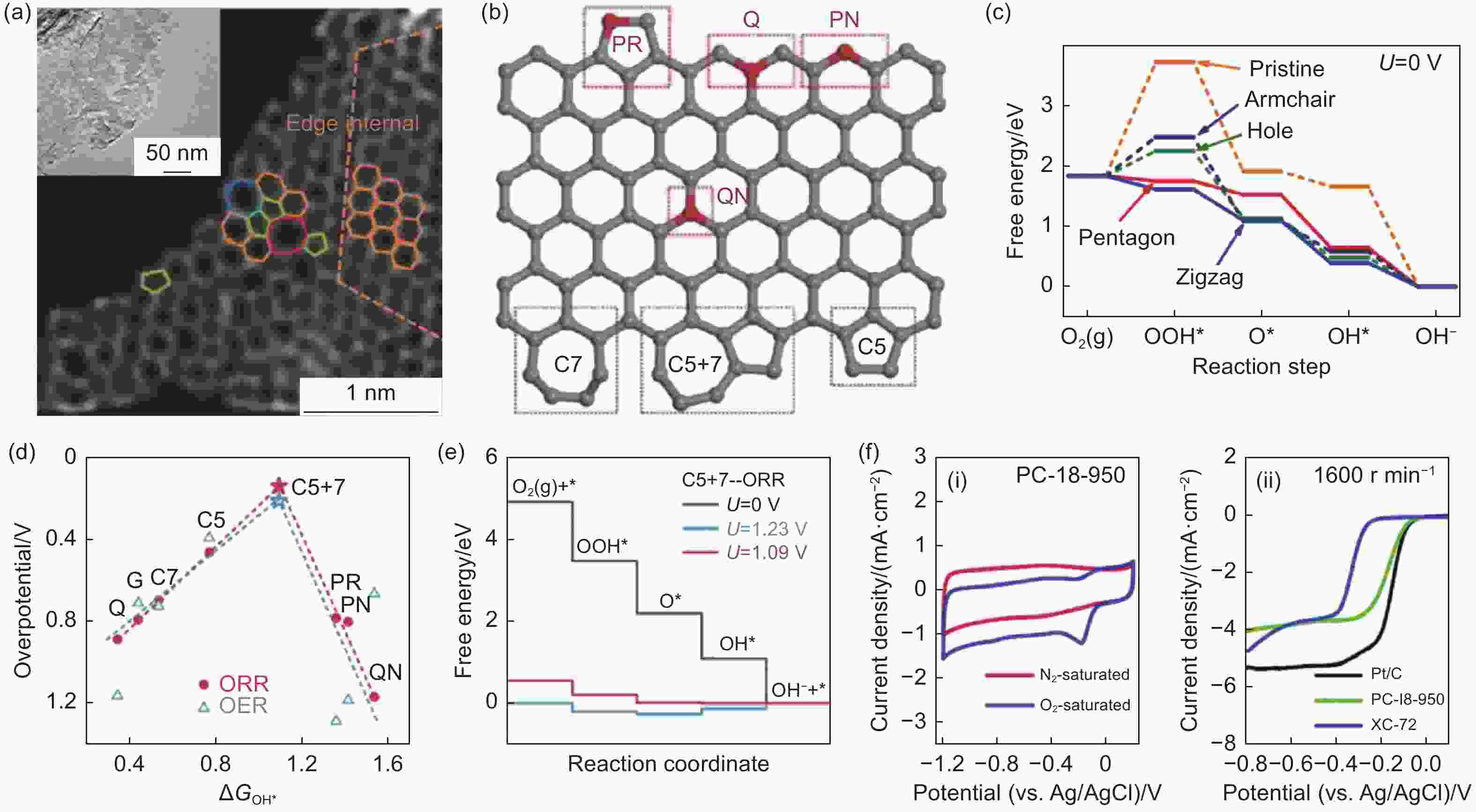
Figure 3. (a) High-angle annular dark-field imaging of various carbon structural defects[36]. Reproduced with permission. Copyright @ 2016 Wiley-VCH GmbH. (b) Scheme of the carbon structural defects[47]. Reproduced with permission. Copyright @ 2016 Wiley-VCH GmbH. (c) DFT calculation of free energy for ORR activities at different defects[48]. Reproduced with permission. Copyright © 2015 American Chemical Society. (d) Volcano plot of both ORR and OER for the adsorption energy of OH* at different carbon defects, (e) free energy diagrams of ORR substeps on C5+7 active site[47]. Reproduced with permission. Copyright @ 2016 Wiley-VCH GmbH. (f) Cyclic voltammetry and ORR performance of carbon structural defects [35]. Reproduced with permission. Copyright @ 2016 Royal Society of Chemistry
Figure 4. (a) N 1s XPS spectra of N-modified HOPG. (b) ORR results for corresponding N-modified HOPG in (a). (c) N 1s XPS of the N-modified HOPG sample catalyst before and after ORR. (d) Catalysis mechanism of the active site[53]. Reproduced with permission. Copyright @ 2016 American Association for the Advancement of Science. (e) Schematic of the formation of N-doped hollow carbon from ZIF-8. (f) Current density measured at 0.75 V (vs. RHE). (g) ORR curves and (h) discharge curve and corresponding power density of N-doped hollow carbon[21]. Reproduced with permission. Copyright @ 2020 Elsevier Ltd. (i) Schematic of the formation of N, P co-doped carbon from ZnO@ZIF-8. Linear sweep voltammetry curves of (j) ORR and (k) both ORR and OER at 1600 r min−1 in the whole in O2-saturated 0.1 mol L−1 KOH solution[59]. Reproduced with permission. Copyright @ 2018 Elsevier Ltd.
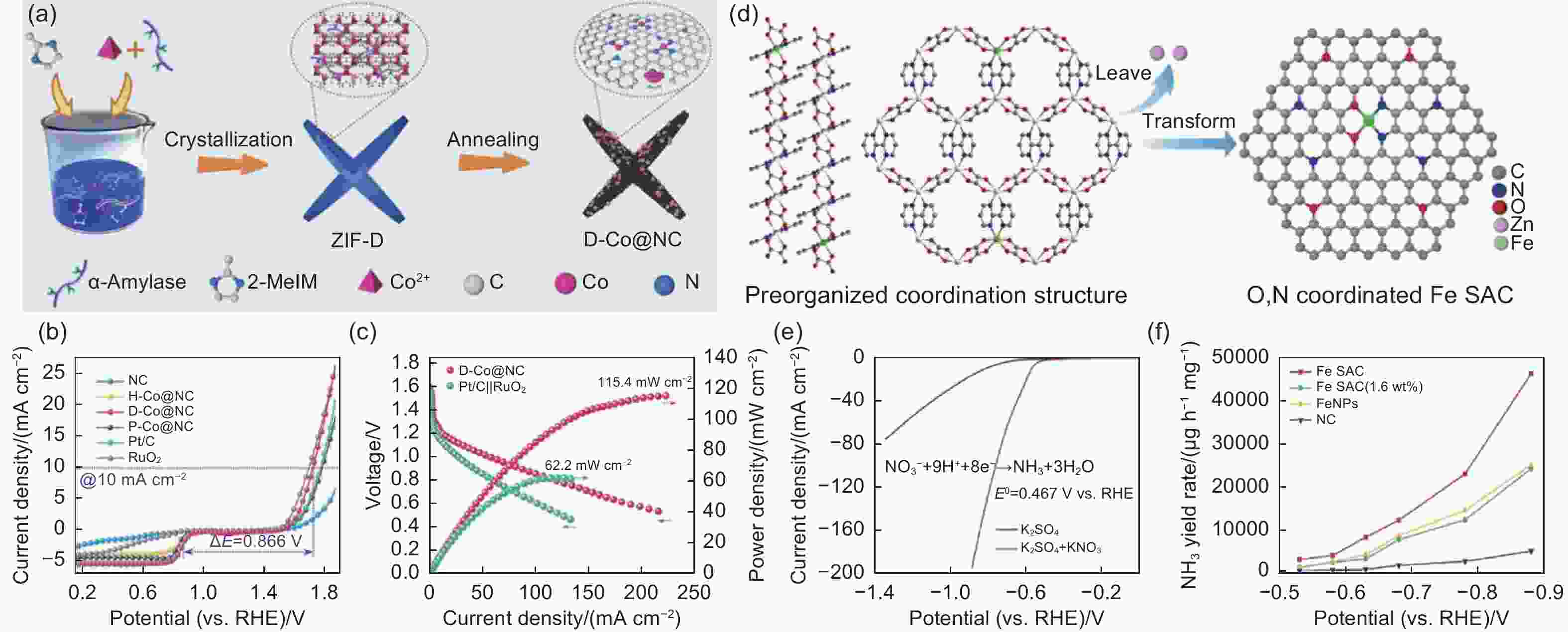
Figure 5. (a) Schematic of the formation of Co nanoparticle grafted on N-doped carbon. (b) The corresponding linear sweep voltammetry curves for both ORR and OER at 1600 r min−1. (c) Discharge curve and corresponding power density[63]. Reproduced with permission. Copyright @ 2022 Elsevier Ltd. (d) Schematic of the formation of Fe single-atom catalysts. (e) The corresponding linear sweep voltammetry and (f) NH3 yield[76]. Reproduced with permission. Copyright @ 2022 Elsevier Ltd.
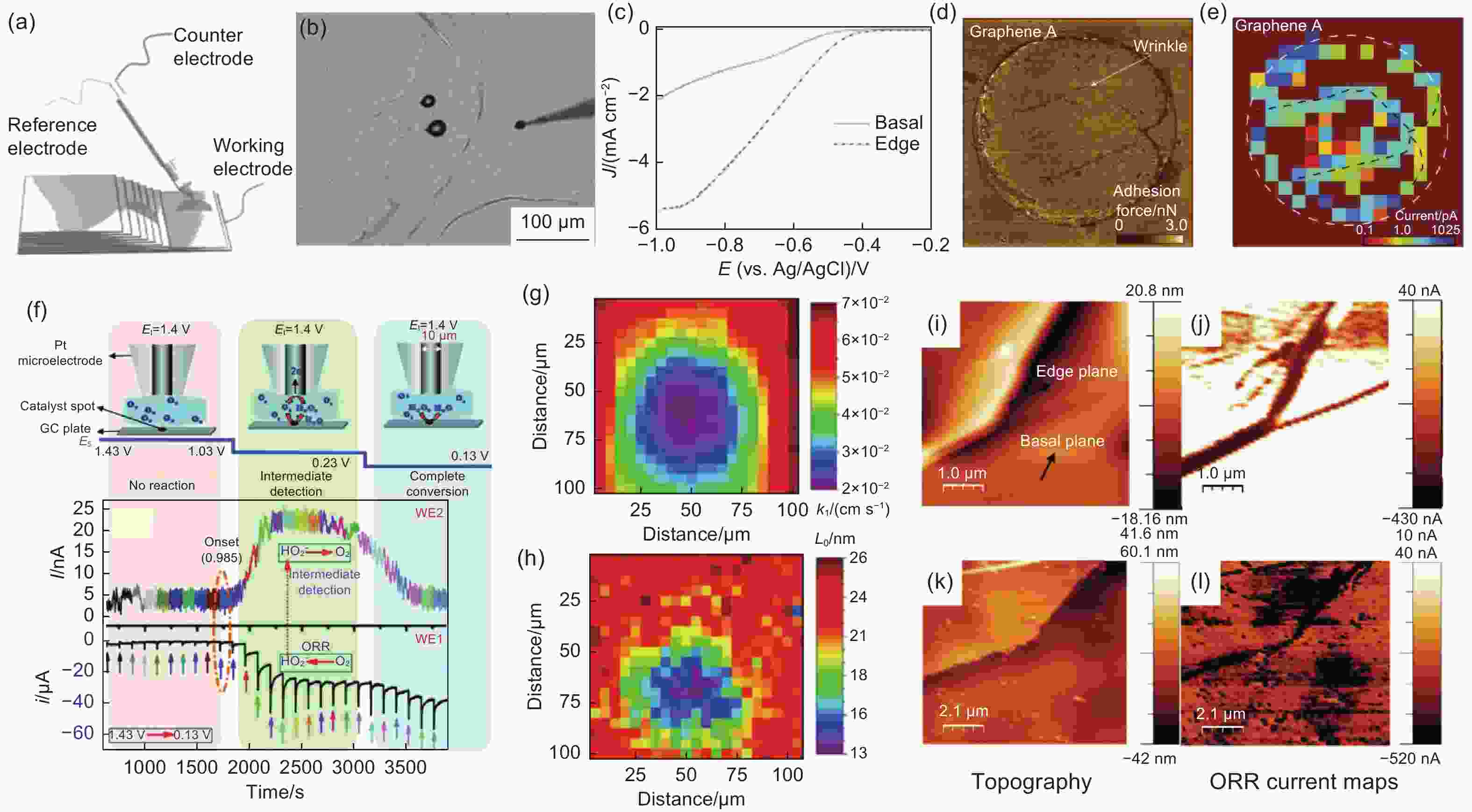
Figure 6. (a) Scheme and the (b) optical photograph of the SECCM measured on the HOPG, (c) ORR linear sweep voltammetry curves within a droplet on the edge and the basal region of the HOPG[15]. Reproduced with permission. Copyright @ 2014 Wiley-VCH GmbH. (d) Atomic Force Microscope (AFM) and (e) SECCM current density image of the graphene device of the proton transfer process[99]. Reproduced with permission. Copyright @ 2023 Macmillan Publisher Ltd.. (f) Schematic representation of the SECM measurement[104]. Reproduced with permission. Copyright @ 2017 Royal Society of Chemistry. (g) Current density from SECM and (h) G peak mapping from Raman of the graphene sample[107]. Reproduced with permission. Copyright @ 2018 American Chemical Society. (i) AMF image and (j) ORR current image measured from SECM of the HOPG sample, (k) AMF image and (l) ORR current image measured from SECM of Fe-N doped HOPG sample[108]. Reproduced with permission. Copyright @ 2018 Royal Society of Chemistry
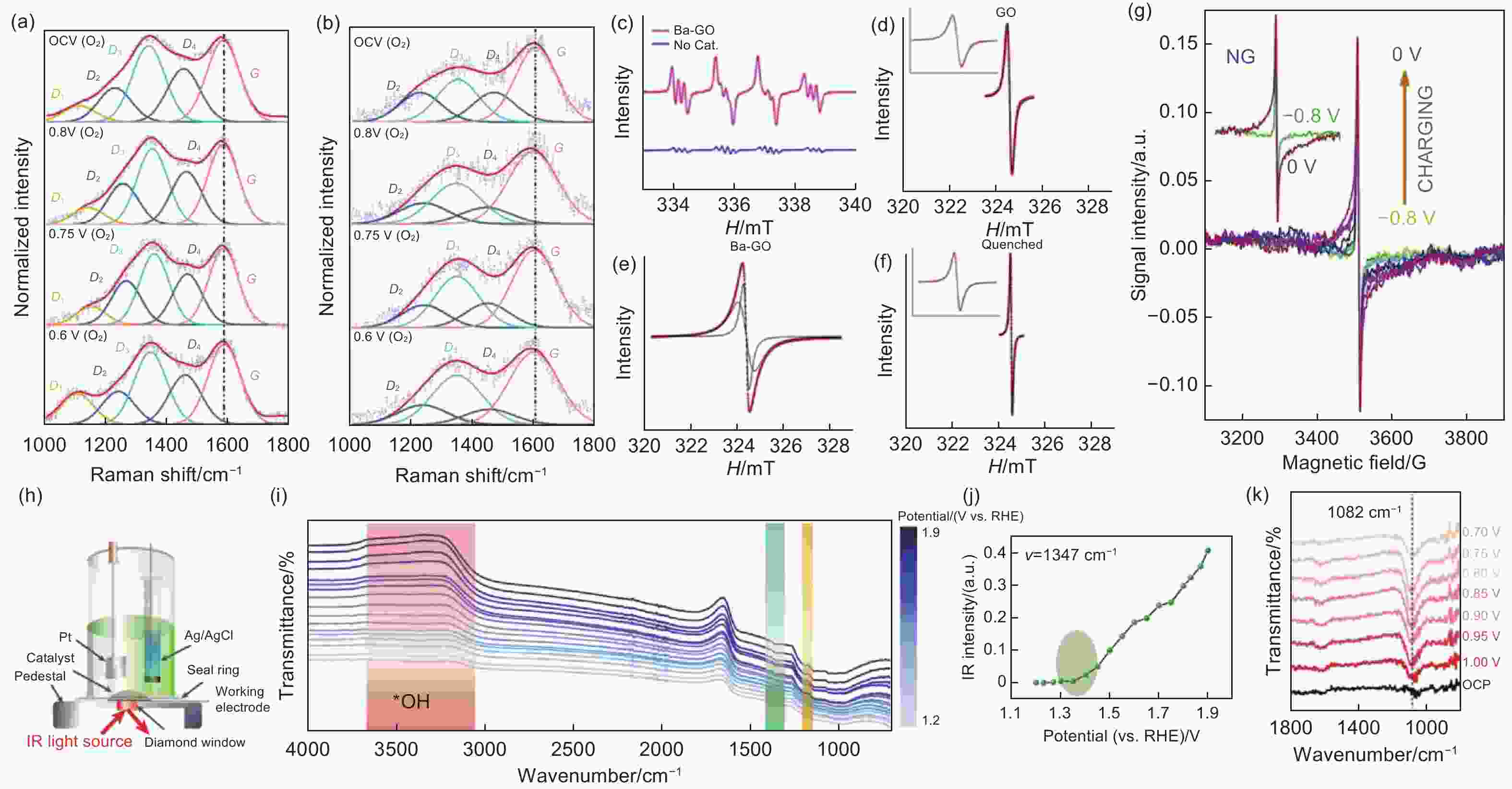
Figure 7. In-situ Raman spectra of (a) honeycomb carbon nanofibers with abundant oxygenated functional groups and (b) solid carbon nanofibers[113]. Reproduced with permission. Copyright @ 2021 Wiley-VCH GmbH. (c) Spin trapping results of the superoxide radicals using the DMPO, EPR spectra of (d) graphene oxide, (e) graphene oxide washed by base and acid solutions to remove Mn2+ impurities and oxidized debris and (f) quenched graphene oxide with phenyl carboxylic acid groups[117]. Reproduced with permission. Copyright @ 2012 Macmillan Publisher Ltd. (g) In situ EPR spectra of the N-doped graphene in KOH electrolyte[124]. Reproduced with permission. Copyright @ 2020 Elsevier Ltd. (h) Scheme of the in situ FTIR setup, (i) in situ FTIR spectra of high-entropy single-atom activated carbon catalysts, (j) summarised peak density at 1347 cm−1, (k) in situ FTIR spectra at 1082 cm−1[133]. Reproduced with permission. Copyright @ 2023 Macmillan Publisher Ltd.
Table 1. Summary of the recent MOF-derived carbon-based electrochemical catalysts for ORR
ORR Catalysts Electrolytes Onset potential
(Eonset (vs. RHE)/V)Half-wave potential
(E1/2 (vs. RHE)/V)Limiting current density
(JL/(mA cm−2)) at 1600 r min−1Durability Refs NHCP-1000 0.1 M KOH 0.98 0.86 ~5.7 36000 s (~96%) [21] Co@N–HCCs@NG 0.1 M KOH ~0.97 0.86 ~5.8 6 h (99%) [34] Carbon defects graphene 0.1 M KOH 0.91 0.76 ~4.6 5 h (90%) [36] Nitrogen-doped graphene mesh (NGM) 0.1 M KOH ~0.89 ~0.77 ~6.4 10 h (100%) [47] NPCTC-850 0.1 M KOH 0.92 0.83 ~5.4 20000 s (96%) [59] D-Co@NC 0.1 M KOH ~0.95 ~0.85 ~5.8 30000 s (98.4%) [63] ZnCo2@NCNTs-800 0.1 M KOH ~0.94 ~0.85 ~6.2 30000 s (97.5%) [77] Fe/Fe3C-N-CNTs 0.1 M KOH 1.02 0.88 ~5.7 20000 s (94%) [78] 0.05CoOx@PNC 0.1 M KOH 0.98 0.88 ~6.5 12 h (100%) [79] BTC-Co-O-Cu-BTA 0.1 M NaOH 1.06 0.95 ~6.0 20000 s (99%) [80] C-ZIF-CuPt 0.1 M KOH ~0.99 ~0.87 5.5 10000 cycles [81] CoP-NC@NFP 0.1 M KOH ~0.92 ~0.83 ~6.1 200 h (82%) [82] Fe3C-Co-NC 0.1 M KOH 1.20 0.89 ~4.5 20000 s (97%) [83] Note: M: mol L−1 Table 2. Summary of the recent MOF-derived carbon-based electrochemical catalysts for OER
Catalysts Electrolytes Tafel slopes/(mV dec−1) Over potential
(at 10 mA cm−2)Durability Refs Co@N–HCCs@NG 1 M KOH 114.4 1.6 V (onset potential) 21600 s (98%) [34] Carbon defects graphene 1 M KOH 97 1.57 V (onset potential) 35000 s (100%) [36] NPCTC-850 0.1 M KOH 115 210 mV [59] D-Co@NC 0.1 M KOH ~101 488 mV 10000 s (89%) [63] ZnCo2@NCNTs-800 0.1 M KOH ~76 1.58 V (onset potential) 30000 s (92.4%) [77] Fe/Fe3C-N-CNTs 0.1 M KOH ~78 1.57 (onset potential) 30000 s (95%) [78] CoNiP/CoNi 1 M KOH 72 300 mV 10 h (95%) [84] NiCoZnP/NC 1 M KOH 85 228 mV 45 h (91%) [85] CoP-NC@NFP 1 M KOH 84 320 mV 40 h (100%) [82] 2D Co@NC 1 M KOH 110 ~380 mV − [86] 2D Mo2C@NC 1 M KOH 100 ~370 mV − H-2D Co/Mo2C@NC 1 M KOH 48 ~256 mV 10 h (100%) CoNi/NC-YS 1 M KOH ~54 292 mV 24 h (100%) [87] Fe3C-Co-NC 1 M KOH 59 −338 mV 30000 s (100%) [83] Note: M: mol L−1 Table 3. Summary of the recent MOF-derived carbon-based electrochemical catalysts for HER
Catalysts Electrolytes Tafel slopes/(mV dec−1) Over potential/(mV at 10 mA cm−2) Durability Refs Carbon defects graphene 1 M KOH 118 320 35000 s [36] 0.5 M H2SO4 55 150 35000 s CoNiP/CoNi/N-RGO 1 M KOH 97 150 10 h [84] CoZnP/NC 1 M KOH ~121 308 at 100 mA cm−2 45 h [85] NiCoZnP/NC 1 M KOH ~48 133 at 100 mA cm−2 45 h Co3O4/C 0.1 M NaOH ~68 ~183 at 100 mA cm−2 12 h [88] 0.5 M H2SO4 ~72 ~195 12 h HNi/NiO/C 1 M KOH 91 87 at 100 mA cm−2 12 h [89] Ru–MoO2@PC/rGO 1 M KOH 43 126 8 h [90] C-ZIF-CuPt 0.1 M KOH 45 46 1000 cycles [81] PtCu-Mo2C@C (0.75:1) 0.5 M H2SO4 29 26 25 h [91] NiSe2/NiS2@NC 0.5 M H2SO4 46 188 2000 cycles [92] 1 M KOH ~93 211 2000 cycles CoP-NC@NFP 1 M KOH 108 200 50 h [82] Note: M: mol L−1 -
[1] Carpenter B P, Talosig A R, Rose B, et al. Understanding and controlling the nucleation and growth of metal-organic frameworks[J]. Chemical Society Reviews,2023,52(20):6918-6937. [2] Li H, Eddaoudi M, O'Keeffe M, et al. Design and synthesis of an exceptionally stable and highly porous metal-organic framework[J]. Nature,1999,402(6759):276-279. doi: 10.1038/46248 [3] Zhou H C J, Kitagawa S. Metal–Organic Frameworks (MOFs)[J]. Chemical Society Reviews,2014,43(16):5415-5418. doi: 10.1039/C4CS90059F [4] Ma Y, Lu W, Han X, et al. Direct observation of ammonia storage in UiO-66 incorporating Cu(II) binding sites[J]. Journal of the American Chemical Society,2022,144(19):8624-8632. doi: 10.1021/jacs.2c00952 [5] Luo T, Wang Z, Chen Y, et al. Photocatalytic dehalogenative deuteration of halides over a robust metal-organic framework[J]. Angewandte Chemie International Edition,2023,62 (48):e202306267. doi: 10.1002/anie.202306267 [6] Lin J, Nguyen T T, Vaidhyanathan R, et al. A scalable metal-organic framework as a durable physisorbent for carbon dioxide capture[J]. Science,2021,374(6574):1464-1469. doi: 10.1126/science.abi7281 [7] Lu X F, Xia B Y, Zang S Q, et al. Metal–organic frameworks based electrocatalysts for the oxygen reduction reaction[J]. Angewandte Chemie International Edition,2020,59(12):4634-4650. doi: 10.1002/anie.201910309 [8] Fu X, Ding B, D'Alessandro D. Fabrication strategies for metal-organic framework electrochemical biosensors and their applications[J]. Coordination Chemistry Reviews,2023,475:214814. doi: 10.1016/j.ccr.2022.214814 [9] Chen X, Sapchenko S, Lu W, et al. Impact of host–guest interactions on the dielectric properties of MFM-300 materials[J]. Inorganic Chemistry,2023,62(42):17157-17162. doi: 10.1021/acs.inorgchem.3c02110 [10] Zheng Z, Rong Z, Rampal N, et al. A GPT-4 reticular chemist for guiding MOF discovery[J]. Angewandte Chemie International Edition,2023,62 (46):e202311983. doi: 10.1002/anie.202311983 [11] Sun L, Campbell M G, Dincă M. Electrically conductive porous metal–organic frameworks[J]. Angewandte Chemie International Edition,2016,55(11):3566-3579. doi: 10.1002/anie.201506219 [12] Zaman N, Iqbal N, Noor T. Advances and challenges of MOF derived carbon-based electrocatalysts and photocatalyst for water splitting: A review[J]. Arabian Journal of Chemistry,2022,15(7):103906. doi: 10.1016/j.arabjc.2022.103906 [13] Tan X, Wu Y, Lin X, et al. Application of MOF-derived transition metal oxides and composites as anodes for lithium-ion batteries[J]. Inorganic Chemistry Frontiers,2020,7(24):4939-4955. doi: 10.1039/D0QI00929F [14] Zhang L, Su Z, Jiang F, et al. Highly graphitized nitrogen-doped porous carbon nanopolyhedra derived from ZIF-8 nanocrystals as efficient electrocatalysts for oxygen reduction reactions[J]. Nanoscale,2014,6(12):6590-6602. doi: 10.1039/C4NR00348A [15] Shen A, Zou Y, Wang Q, et al. Oxygen reduction reaction in a droplet on graphite: direct evidence that the edge is more active than the basal plane[J]. Angewandte Chemie International Edition,2014,53(40):10804-10808. doi: 10.1002/anie.201406695 [16] Yang C, Ma X, Zhou J, et al. Recent advances in metal-organic frameworks-derived carbon-based electrocatalysts for the oxygen reduction reaction[J]. International Journal of Hydrogen Energy,2022,47(51):21634-21661. doi: 10.1016/j.ijhydene.2022.05.025 [17] Liu B, Shioyama H, Akita T, et al. Metal-organic framework as a template for porous carbon synthesis[J]. Journal of the American Chemical Society,2008,130(16):5390-5391. doi: 10.1021/ja7106146 [18] Ren Q, Wang H, Lu X F, et al. Recent progress on MOF-derived heteroatom-doped carbon-based electrocatalysts for oxygen reduction reaction[J]. Advanced Science,2018,5(3):1700515. doi: 10.1002/advs.201700515 [19] Wang Y, Li J, Li X, et al. Metal-organic-framework derived Co@CN modified horizontally aligned graphene oxide array as free-standing anode for lithium-ion batteries[J]. Journal of Materials Chemistry A,2022,10(2):699-706. doi: 10.1039/D1TA07638H [20] Li L X, He S, Zeng S, et al. Equipping carbon dots in a defect-containing MOF via self-carbonization for explosive sensing[J]. Journal of Materials Chemistry C,2023,11(1):321-328. doi: 10.1039/D2TC04513C [21] Yan J, Zheng X, Wei C, et al. Nitrogen-doped hollow carbon polyhedron derived from salt-encapsulated ZIF-8 for efficient oxygen reduction reaction[J]. Carbon,2021,171:320-328. doi: 10.1016/j.carbon.2020.09.005 [22] Zheng S, Sun Y, Xue H, et al. Dual-ligand and hard-soft-acid-base strategies to optimize metal-organic framework nanocrystals for stable electrochemical cycling performance[J]. National Science Review,2021,9(7):197. [23] Yoo J M, Shin H, Chung D Y, et al. Carbon shell on active nanocatalyst for stable electrocatalysis[J]. Accounts of Chemical Research,2022,55(9):1278-1289. doi: 10.1021/acs.accounts.1c00727 [24] Gunaseelan H, Munde A V, Patel R, et al. Metal-organic framework derived carbon-based electrocatalysis for hydrogen evolution reactions: A review[J]. Materials Today Sustainability,2023,22:100371. doi: 10.1016/j.mtsust.2023.100371 [25] Jin H, Yu R, Hu C, et al. Size-controlled engineering of cobalt metal catalysts through a coordination effect for oxygen electrocatalysis[J]. Applied Catalysis B:Environmental,2022,317:121766. doi: 10.1016/j.apcatb.2022.121766 [26] Kim M, Xin R, Earnshaw J, et al. MOF-derived nanoporous carbons with diverse tunable nanoarchitectures[J]. Nature Protocols,2022,17(12):2990-3027. doi: 10.1038/s41596-022-00718-2 [27] Wang H F, Chen L, Pang H, et al. MOF-derived electrocatalysts for oxygen reduction, oxygen evolution and hydrogen evolution reactions[J]. Chemical Society Reviews,2020,49(5):1414-1448. doi: 10.1039/C9CS00906J [28] Liu B, Shioyama H, Jiang H, et al. Metal–organic framework (MOF) as a template for syntheses of nanoporous carbons as electrode materials for supercapacitor[J]. Carbon,2010,48(2):456-463. doi: 10.1016/j.carbon.2009.09.061 [29] Xu H, Zhou S, Xiao L, et al. Fabrication of a nitrogen-doped graphene quantum dot from MOF-derived porous carbon and its application for highly selective fluorescence detection of Fe3+[J]. Journal of Materials Chemistry C,2015,3(2):291-297. doi: 10.1039/C4TC01991A [30] Wang X, Li Y. Nanoporous carbons derived from MOFs as metal-free catalysts for selective aerobic oxidations[J]. Journal of Materials Chemistry A,2016,4(14):5247-5257. doi: 10.1039/C6TA00324A [31] Hu H, Han L, Yu M, et al. Metal–organic-framework-engaged formation of Co nanoparticle-embedded carbon@Co9S8 double-shelled nanocages for efficient oxygen reduction[J]. Energy & Environmental Science,2016,9(1):107-111. [32] Lu X F, Chen Y, Wang S, et al. Interfacing manganese oxide and cobalt in porous graphitic carbon polyhedrons boosts oxygen electrocatalysis for Zn-Air batteries[J]. Advanced Materials,2019,31(39):1902339. doi: 10.1002/adma.201902339 [33] Ding B, Fan Z, Lin Q, et al. Confined pyrolysis of ZIF-8 polyhedrons wrapped with graphene oxide nanosheets to prepare 3d porous carbon heterostructures[J]. Small Methods,2019,3(11):1900277. doi: 10.1002/smtd.201900277 [34] Zhang Y, Wang P, Yang J, et al. Decorating ZIF-67-derived cobalt–nitrogen doped carbon nanocapsules on 3D carbon frameworks for efficient oxygen reduction and oxygen evolution[J]. Carbon,2021,177:344-356. doi: 10.1016/j.carbon.2021.02.052 [35] Zhao X, Zou X, Yan X, et al. Defect-driven oxygen reduction reaction (ORR) of carbon without any element doping[J]. Inorganic Chemistry Frontiers,2016,3(3):417-421. doi: 10.1039/C5QI00236B [36] Jia Y, Zhang L, Du A, et al. Defect graphene as a trifunctional catalyst for electrochemical reactions[J]. Advanced Materials,2016,28(43):9532-9538. doi: 10.1002/adma.201602912 [37] Jiao Y, Qu C, Zhao B, et al. High-performance electrodes for a hybrid supercapacitor derived from a metal-organic framework/graphene composite[J]. ACS Applied Energy Materials,2019,2(7):5029-5038. doi: 10.1021/acsaem.9b00700 [38] Wang R, Chen Z, Sun Y, et al. Three-dimensional graphene network-supported Co, N-codoped porous carbon nanocages as free-standing polysulfides mediator for lithium-sulfur batteries[J]. Chemical Engineering Journal,2020,399:125686. doi: 10.1016/j.cej.2020.125686 [39] Wang Y Z, Tang Z H, Shen S L, et al. The influence of heteroatom doping on the performance of carbon-based electrocatalysts for oxygen evolution reactions[J]. New Carbon Materials,2022,37(2):321-336. doi: 10.1016/S1872-5805(22)60591-2 [40] Zhong H W, Wang J, Zhang Y W, et al. ZIF-8 derived graphene-based nitrogen-doped porous carbon sheets as highly efficient and durable oxygen reduction electrocatalysts[J]. Angewandte Chemie International Edition,2014,53(51):14235-14239. doi: 10.1002/anie.201408990 [41] Wang M, Zhang J, Yi X, et al. Nitrogen-doped hierarchical porous carbon derived from ZIF-8 supported on carbon aerogels with advanced performance for supercapacitor[J]. Applied Surface Science,2020,507:145166. doi: 10.1016/j.apsusc.2019.145166 [42] Ma R, Lin G, Zhou Y, et al. A review of oxygen reduction mechanisms for metal-free carbon-based electrocatalysts[J]. npj Computational Materials,2019,5(1):78. doi: 10.1038/s41524-019-0210-3 [43] Lu Z, Chen G, Siahrostami S, et al. High-efficiency oxygen reduction to hydrogen peroxide catalysed by oxidized carbon materials[J]. Nature Catalysis,2018,1(2):156-162. doi: 10.1038/s41929-017-0017-x [44] San Roman D, Krishnamurthy D, Garg R, et al. Engineering three-dimensional (3D) out-of-plane graphene edge sites for highly selective two-electron oxygen reduction electrocatalysis[J]. ACS Catalysis,2020,10(3):1993-2008. doi: 10.1021/acscatal.9b03919 [45] Deng D, Yu L, Pan X, et al. Size effect of graphene on electrocatalytic activation of oxygen[J]. Chemical Communications,2011,47(36):10016-10018. doi: 10.1039/c1cc13033a [46] Jeon I Y, Choi H J, Jung S M, et al. Large-scale production of edge-selectively functionalized graphene nanoplatelets via ball milling and their use as metal-free electrocatalysts for oxygen reduction reaction[J]. Journal of the American Chemical Society,2013,135(4):1386-1393. doi: 10.1021/ja3091643 [47] Tang C, Wang H F, Chen X, et al. Topological defects in metal-free nanocarbon for oxygen electrocatalysis[J]. Advanced Materials,2016,28(32):6845-6851. doi: 10.1002/adma.201601406 [48] Jiang Y, Yang L, Sun T, et al. Significant contribution of intrinsic carbon defects to oxygen reduction activity[J]. ACS Catalysis,2015,5(11):6707-6712. doi: 10.1021/acscatal.5b01835 [49] Parvez K, Yang S, Hernandez Y, et al. Nitrogen-doped graphene and its iron-based composite as efficient electrocatalysts for oxygen reduction reaction[J]. ACS Nano,2012,6(11):9541-9550. doi: 10.1021/nn302674k [50] Borghei M, Kanninen P, Lundahl M, et al. High oxygen reduction activity of few-walled carbon nanotubes with low nitrogen content[J]. Applied Catalysis B:Environmental,2014,158:233-241. [51] Vikkisk M, Kruusenberg I, Ratso S, et al. Enhanced electrocatalytic activity of nitrogen-doped multi-walled carbon nanotubes towards the oxygen reduction reaction in alkaline media[J]. RSC Advances,2015,5(73):59495-59505. doi: 10.1039/C5RA08818F [52] Zhu Y, Zhang Z, Li W, et al. Highly exposed active sites of defect-enriched derived MOFs for enhanced oxygen reduction reaction[J]. ACS Sustainable Chemistry & Engineering,2019,7(21):17855-17862. [53] Guo D, Shibuya R, Akiba C, et al. Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts[J]. Science,2016,351(6271):361-365. doi: 10.1126/science.aad0832 [54] Xuan C, Hou B, Xia W, et al. From a ZIF-8 polyhedron to three-dimensional nitrogen doped hierarchical porous carbon: an efficient electrocatalyst for the oxygen reduction reaction[J]. Journal of Materials Chemistry A,2018,6(23):10731-10739. doi: 10.1039/C8TA02385A [55] Yang M, Hu X, Fang Z, et al. Bifunctional MOF-derived carbon photonic crystal architectures for advanced Zn-Air and Li-S batteries: Highly Exposed Graphitic Nitrogen Matters[J]. Advanced Functional Materials,2017,27(36):1701971. doi: 10.1002/adfm.201701971 [56] Wang N, Wang B, Wang W, et al. Structural design of electrospun nanofibers for electrochemical energy storage and conversion[J]. Journal of Alloys and Compounds,2023,935:167920. doi: 10.1016/j.jallcom.2022.167920 [57] Song Z, Liu W, Cheng N, et al. Origin of the high oxygen reduction reaction of nitrogen and sulfur Co-doped MOF-derived nanocarbon electrocatalysts[J]. Materials Horizons,2017,4(5):900-907. doi: 10.1039/C7MH00244K [58] Najam T, Shah S S A, Ding W, et al. An efficient anti-poisoning catalyst against SOx, NOx and POx: P, N-doped carbon for oxygen reduction in acidic media[J]. Angewandte Chemie International Edition,2018,57(46):15101-15106. doi: 10.1002/anie.201808383 [59] Li Y, Yan Z, Wang Q, et al. Ultrathin, highly branched carbon nanotube cluster with outstanding oxygen electrocatalytic performance[J]. Electrochimica Acta,2018,282:224-232. doi: 10.1016/j.electacta.2018.06.058 [60] Gupta S, Tryk D, Bae I, et al. Heat-treated polyacrylonitrile-based catalysts for oxygen electroreduction[J]. Journal of Applied Electrochemistry,1989,19(1):19-27. doi: 10.1007/BF01039385 [61] Kuk Y, Ahmed S, Sun H J, et al. Synthesis of porous carbon-coated cobalt catalyst through pyrolyzing metal–organic framework and their bifunctional OER/ORR catalytic activity for Zn-Air rechargeable batteries[J]. Bulletin of the Korean Chemical Society,2020,41(3):310-316. doi: 10.1002/bkcs.11973 [62] Gao B, Tan M, Xi W, et al. Co-embedded carbon nanotubes modified N-doped carbon derived from poly (Schiff base) and zeolitic imidazole frameworks as efficient oxygen electrocatalyst towards rechargeable Zn-air battery[J]. Journal of Power Sources,2022,527:231205. doi: 10.1016/j.jpowsour.2022.231205 [63] Zhang F, Chen L, Yang H, et al. Ultrafine Co nanoislands grafted on tailored interpenetrating N-doped carbon nanoleaves: An efficient bifunctional electrocatalyst for rechargeable Zn-air batteries[J]. Chemical Engineering Journal,2022,431:133734. doi: 10.1016/j.cej.2021.133734 [64] Chang H, Shi L N, Chen Y H, et al. Advanced MOF-derived carbon-based non-noble metal oxygen electrocatalyst for next-generation rechargeable Zn-air batteries[J]. Coordination Chemistry Reviews,2022,473:214839. doi: 10.1016/j.ccr.2022.214839 [65] Li L, He J, Wang Y, et al. Metal-organic frameworks: A promising platform for constructing non-noble electrocatalysts for the oxygen-reduction reaction[J]. Journal of Materials Chemistry A,2019,7(5):1964-1988. doi: 10.1039/C8TA11704G [66] Cao X, Tan C, Sindoro M, et al. Hybrid micro-/nano-structures derived from metal–organic frameworks: preparation and applications in energy storage and conversion[J]. Chemical Society Reviews,2017,46(10):2660-2677. doi: 10.1039/C6CS00426A [67] Ni B, Ouyang C, Xu X, et al. Modifying commercial carbon with trace amounts of ZIF to prepare derivatives with superior ORR activities[J]. Advanced Materials,2017,29(27):1701354. doi: 10.1002/adma.201701354 [68] Liu J, Zhu D, Guo C, et al. Design strategies toward advanced mof-derived electrocatalysts for energy-conversion reactions[J]. Advanced Energy Materials,2017,7(23):1700518. doi: 10.1002/aenm.201700518 [69] Ren S, Duan X, Lei M, et al. Energetic MOF-derived cobalt/iron nitrides embedded into N, S-codoped carbon nanotubes as superior bifunctional oxygen catalysts for Zn-air batteries[J]. Applied Surface Science,2021,569:151030. doi: 10.1016/j.apsusc.2021.151030 [70] Zhao T, Wang D, Cheng C, et al. Preparation of a dual-MOF heterostructure (ZIF@MIL) for enhanced oxygen evolution reaction activity[J]. Chemistry-An Asian Journal,2021,16(1):64-71. doi: 10.1002/asia.202001235 [71] Lei Z, Tan Y, Zhang Z, et al. Defects enriched hollow porous Co-N-doped carbons embedded with ultrafine CoFe/Co nanoparticles as bifunctional oxygen electrocatalyst for rechargeable flexible solid zinc-air batteries[J]. Nano Research,2021,14(3):868-878. doi: 10.1007/s12274-020-3127-8 [72] Gong H, Zheng X, Zeng K, et al. Ni3Fe nanoalloys embedded in N-doped carbon derived from dual-metal ZIF: Efficient bifunctional electrocatalyst for Zn-air battery[J]. Carbon,2021,174:475-483. doi: 10.1016/j.carbon.2020.12.053 [73] Xia P, Wang C, He Q, et al. MOF-derived single-atom catalysts: The next frontier in advanced oxidation for water treatment[J]. Chemical Engineering Journal,2023,452:139446. doi: 10.1016/j.cej.2022.139446 [74] Ye J, Yan J, Peng Y, et al. Metal-organic framework-based single-atom catalysts for efficient electrocatalytic CO2 reduction reactions[J]. Catalysis Today,2023,410:68-84. doi: 10.1016/j.cattod.2022.09.005 [75] Song Z, Zhang L, Doyle-Davis K, et al. Recent advances in MOF-derived single atom catalysts for electrochemical applications[J]. Advanced Energy Materials,2020,10(38):2001561. doi: 10.1002/aenm.202001561 [76] Zhang W D, Dong H, Zhou L, et al. Fe single-atom catalysts with pre-organized coordination structure for efficient electrochemical nitrate reduction to ammonia[J]. Applied Catalysis B: Environmental,2022,317:121750. doi: 10.1016/j.apcatb.2022.121750 [77] Qin T, Li F, Liu X, et al. Template-assisted synthesis of high-efficiency bifunctional catalysts with roller-comb-like nanostructure for rechargeable zinc-air batteries[J]. Chemical Engineering Journal,2022,429:132199. doi: 10.1016/j.cej.2021.132199 [78] Zong L, Chen X, Dou S, et al. Stable confinement of Fe/Fe3C in Fe, N-codoped carbon nanotube towards robust zinc-air batteries[J]. Chinese Chemical Letters,2021,32(3):1121-1126. doi: 10.1016/j.cclet.2020.08.029 [79] Tan Y, Zhu W, Zhang Z, et al. Electronic tuning of confined sub-nanometer cobalt oxide clusters boosting oxygen catalysis and rechargeable Zn–air batteries[J]. Nano Energy,2021,83:105813. doi: 10.1016/j.nanoen.2021.105813 [80] Sanad M F, Puente Santiago A R, Tolba S A, et al. Co–Cu bimetallic metal organic framework catalyst outperforms the Pt/C benchmark for oxygen reduction[J]. Journal of the American Chemical Society,2021,143(10):4064-4073. doi: 10.1021/jacs.1c01096 [81] Wang C, Kuai L, Cao W, et al. Highly dispersed Cu atoms in MOF-derived N-doped porous carbon inducing Pt loads for superior oxygen reduction and hydrogen evolution[J]. Chemical Engineering Journal,2021,426:130749. doi: 10.1016/j.cej.2021.130749 [82] Vijayakumar E, Ramakrishnan S, Sathiskumar C, et al. MOF-derived CoP-nitrogen-doped carbon@NiFeP nanoflakes as an efficient and durable electrocatalyst with multiple catalytically active sites for OER, HER, ORR and rechargeable zinc-air batteries[J]. Chemical Engineering Journal,2022,428:131115. doi: 10.1016/j.cej.2021.131115 [83] Wang H, Sun C, Zhu E, et al. Core-shell MOF-derived Fe3C-Co-NC as high-performance ORR/OER bifunctional catalyst[J]. Journal of Alloys and Compounds,2023,948:169728. doi: 10.1016/j.jallcom.2023.169728 [84] Arunkumar P, Gayathri S, Han J H. A complementary Co−Ni phosphide/bimetallic alloy-interspersed N-doped graphene electrocatalyst for overall alkaline water splitting[J]. ChemSusChem,2021,14(8):1921-1935. doi: 10.1002/cssc.202100116 [85] Chen B, Kim D, Zhang Z, et al. MOF-derived NiCoZnP nanoclusters anchored on hierarchical N-doped carbon nanosheets array as bifunctional electrocatalysts for overall water splitting[J]. Chemical Engineering Journal,2021,422:130533. doi: 10.1016/j.cej.2021.130533 [86] Hyoun Ahn C, Seok Yang W, Jae Kim J, et al. Design of hydrangea-type Co/Mo bimetal MOFs and MOF-derived Co/Mo2C embedded carbon composites for highly efficient oxygen evolution reaction[J]. Chemical Engineering Journal,2022,435:134815. doi: 10.1016/j.cej.2022.134815 [87] Hou G, Jia X, Kang H, et al. CoNi nano-alloys modified yolk-shell structure carbon cage via Saccharomycetes as carbon template for efficient oxygen evolution reaction[J]. Applied Catalysis B:Environmental,2022,315:121551. doi: 10.1016/j.apcatb.2022.121551 [88] Gothandapani K, Grace A N, Venugopal V. Mesoporous carbon-supported CO3O4 derived from Zif-67 metal organic framework (MOF) for hydrogen evolution reaction in acidic and alkaline medium[J]. International Journal of Energy Research,2022,46(3):3384-3395. doi: 10.1002/er.7388 [89] Do H H, Tekalgne M A, Le Q V, et al. Hollow Ni/NiO/C composite derived from metal-organic frameworks as a high-efficiency electrocatalyst for the hydrogen evolution reaction[J]. Nano Convergence,2023,10(1):6. doi: 10.1186/s40580-023-00354-w [90] Han J Y, Cai S H, Zhu J Y, et al. MOF-derived ruthenium-doped amorphous molybdenum dioxide hybrid for highly efficient hydrogen evolution reaction in alkaline media[J]. Chemical Communications,2022,58(1):100-103. doi: 10.1039/D1CC05683B [91] Zhang C, Liu Q, Wang P, et al. Molybdenum Carbide-PtCu Nanoalloy Heterostructures on MOF-Derived Carbon toward Efficient Hydrogen Evolution[J]. Small,2021,17(51):2104241. doi: 10.1002/smll.202104241 [92] Lu K, Sun J, Xu H, et al. Electronic structure regulation of an ultra-thin MOF-derived NiSe2/NiS2@NC heterojunction for promoting the hydrogen evolution reaction[J]. Materials Advances,2022,3(4):2139-2145. doi: 10.1039/D1MA01168E [93] Da Y, Li X, Zhong C, et al. Advanced characterization techniques for identifying the key active sites of gas-involved electrocatalysts[J]. Advanced Functional Materials,2020,30(35):2001704. doi: 10.1002/adfm.202001704 [94] Cao X, Tan D, Wulan B, et al. In situ characterization for boosting electrocatalytic carbon dioxide reduction[J]. Small Methods,2021,5(10):2100700. doi: 10.1002/smtd.202100700 [95] Limani N, Batsa Tetteh E, Kim M, et al. Scrutinizing Intrinsic Oxygen Reduction Reaction Activity of a Fe−N−C Catalyst via Scanning Electrochemical Cell Microscopy[J]. ChemElectroChem,2023,10(6):e202201095. doi: 10.1002/celc.202201095 [96] Yule L C, Bentley C L, West G, et al. Scanning electrochemical cell microscopy: A versatile method for highly localised corrosion related measurements on metal surfaces[J]. Electrochimica Acta,2019,298:80-88. doi: 10.1016/j.electacta.2018.12.054 [97] Wahab O J, Kang M, Unwin P R. Scanning electrochemical cell microscopy: A natural technique for single entity electrochemistry[J]. Current Opinion in Electrochemistry,2020,22:120-128. doi: 10.1016/j.coelec.2020.04.018 [98] Tetteh E B, Banko L, Krysiak O A, et al. Zooming-in-Visualization of active site heterogeneity in high entropy alloy electrocatalysts using scanning electrochemical cell microscopy[J]. Electrochemical Science Advances,2022,2(3):e2100105. doi: 10.1002/elsa.202100105 [99] Wahab O J, Daviddi E, Xin B, et al. Proton transport through nanoscale corrugations in two-dimensional crystals[J]. Nature,2023,620(7975):782-786. doi: 10.1038/s41586-023-06247-6 [100] Valavanis D, Ciocci P, Meloni Gabriel N, et al. Hybrid scanning electrochemical cell microscopy-interference reflection microscopy (SECCM-IRM): tracking phase formation on surfaces in small volumes[J]. Faraday Discussions,2022,233(0):122-148. [101] Preet A, Lin T E. A Review: Scanning electrochemical microscopy (SECM) for visualizing the real-time local catalytic activity[J]. Catalysts,2021,11(5):594. doi: 10.3390/catal11050594 [102] Gupta D, Chakraborty S, Amorim R G, et al. Local electrocatalytic activity of PtRu supported on nitrogen-doped carbon nanotubes towards methanol oxidation by scanning electrochemical microscopy[J]. Journal of Materials Chemistry A,2021,9(37):21291-21301. doi: 10.1039/D1TA04962C [103] Zhang X, Han C, Xu W. Imaging analysis of scanning electrochemical microscopy in energy catalysis[J]. Chemical & biomedical imaging,2023,1(3):205-219. [104] Tiwari A, Singh V, Mandal D, et al. Nitrogen containing carbon spheres as an efficient electrocatalyst for oxygen reduction: Microelectrochemical investigation and visualization[J]. Journal of Materials Chemistry A,2017,5(37):20014-20023. doi: 10.1039/C7TA05503J [105] Hatfield K O, Gole M T, Schorr N B, et al. Surface-enhanced raman spectroscopy-scanning electrochemical microscopy: Observation of real-time surface pH perturbations[J]. Analytical Chemistry,2021,93(22):7792-7796. doi: 10.1021/acs.analchem.1c00888 [106] Mayer F D, Hosseini-Benhangi P, Sánchez-Sánchez C M, et al. Scanning electrochemical microscopy screening of CO2 electroreduction activities and product selectivities of catalyst arrays[J]. Communications Chemistry,2020,3(1):155. doi: 10.1038/s42004-020-00399-6 [107] Schorr N B, Jiang A G, Rodríguez-López J. Probing graphene interfacial reactivity via simultaneous and colocalized Raman-scanning electrochemical microscopy imaging and interrogation[J]. Analytical Chemistry,2018,90(13):7848-7854. doi: 10.1021/acs.analchem.8b00730 [108] Kolagatla S, Subramanian P, Schechter A. Nanoscale mapping of catalytic hotspots on Fe, N-modified HOPG by scanning electrochemical microscopy-atomic force microscopy[J]. Nanoscale,2018,10(15):6962-6970. doi: 10.1039/C8NR00849C [109] Kolagatla S, Subramanian P, Schechter A. Catalytic current mapping of oxygen reduction on isolated Pt particles by atomic force microscopy-scanning electrochemical microscopy[J]. Applied Catalysis B:Environmental,2019,256:117843. doi: 10.1016/j.apcatb.2019.117843 [110] Chen M, Liu D, Qiao L, et al. In-situ/operando Raman techniques for in-depth understanding on electrocatalysis[J]. Chemical Engineering Journal,2023,461:141939. doi: 10.1016/j.cej.2023.141939 [111] Li H, Wei P, Gao D, et al. In situ Raman spectroscopy studies for electrochemical CO2 reduction over Cu catalysts[J]. Current Opinion in Green and Sustainable Chemistry,2022,34:100589. doi: 10.1016/j.cogsc.2022.100589 [112] Dix S T, Linic S. In-operando surface-sensitive probing of electrochemical reactions on nanoparticle electrocatalysts: Spectroscopic characterization of reaction intermediates and elementary steps of oxygen reduction reaction on Pt[J]. Journal of Catalysis,2021,396:32-39. doi: 10.1016/j.jcat.2021.02.009 [113] Dong K, Liang J, Wang Y, et al. Honeycomb carbon nanofibers: a superhydrophilic O2-entrapping electrocatalyst enables ultrahigh mass activity for the two-electron oxygen reduction reaction[J]. Angewandte Chemie International Edition,2021,60(19):10583-10587. doi: 10.1002/anie.202101880 [114] Chen S, Chen Z, Siahrostami S, et al. Defective carbon-based materials for the electrochemical synthesis of hydrogen peroxide[J]. ACS Sustainable Chemistry & Engineering,2018,6(1):311-317. [115] Wang B, Wang W, Sun K, et al. Developing in situ electron paramagnetic resonance characterization for understanding electron transfer of rechargeable batteries[J]. Nano Research, 2023, 16: 11992–12012 DOI: 10.1007/s12274-023-5855-z [116] Kang X, Wang B, Hu K, et al. Quantitative electro-reduction of CO2 to liquid fuel over electro-synthesized metal-organic frameworks[J]. Journal of the American Chemical Society,2020,142(41):17384-17392. doi: 10.1021/jacs.0c05913 [117] Su C, Acik M, Takai K, et al. Probing the catalytic activity of porous graphene oxide and the origin of this behaviour[J]. Nature Communications,2012,3(1):1298. doi: 10.1038/ncomms2315 [118] Barbon A. EPR spectroscopy in the study of 2D graphene-based nanomaterials and nanographites. Electron Paramagnetic Resonance: Volume 26[M]. The Royal Society of Chemistry. 2019: 38-65. [119] Wang B, Fielding A J, Dryfe R A W. Electron Paramagnetic resonance investigation of the structure of graphene oxide: pH-dependence of the spectroscopic response[J]. ACS Applied Nano Materials,2019,2(1):19-27. doi: 10.1021/acsanm.8b01329 [120] Risse T, Hollmann D, Brückner A. Chapter 1 In situ electron paramagnetic resonance (EPR) – a unique tool for analysing structure and reaction behaviour of paramagnetic sites in model and real catalysts[J]. Catalysis: Volume 27[M]. The Royal Society of Chemistry. 2015: 1-32. [121] Jones A E, Ejigu A, Wang B, et al. Quinone voltammetry for redox-flow battery applications[J]. Journal of Electroanalytical Chemistry,2022,920:116572. doi: 10.1016/j.jelechem.2022.116572 [122] Wang B, Fielding A J, Dryfe R A W. Electron paramagnetic resonance as a structural tool to study graphene oxide: potential dependence of the EPR response[J]. The Journal of Physical Chemistry C,2019,123(36):22556-22563. doi: 10.1021/acs.jpcc.9b04292 [123] Wang B, Fielding A J, Dryfe R A W. In situ electrochemical electron paramagnetic resonance spectroscopy as a tool to probe electrical double layer capacitance[J]. Chemical Communications,2018,54(31):3827-3830. doi: 10.1039/C8CC00450A [124] Wang B, Likodimos V, Fielding A J, et al. In situ Electron paramagnetic resonance spectroelectrochemical study of graphene-based supercapacitors: Comparison between chemically reduced graphene oxide and nitrogen-doped reduced graphene oxide[J]. Carbon,2020,160:236-246. doi: 10.1016/j.carbon.2019.12.045 [125] Rotonnelli B, Fernandes M S D, Bournel F, et al. In situ/operando X-ray absorption and photoelectron spectroscopies applied to water-splitting electrocatalysis[J]. Current Opinion in Electrochemistry,2023,40:101314. doi: 10.1016/j.coelec.2023.101314 [126] Varsha M V, Nageswaran G. Operando X-Ray spectroscopic techniques: a focus on hydrogen and oxygen evolution reactions[J]. Frontiers in Chemistry, 2020, 8: 497887. [127] Liu C, Dong Q, Han Y, et al. Understanding fundamentals of electrochemical reactions with tender X-rays: A new lab-based operando X-ray photoelectron spectroscopy method for probing liquid/solid and gas/solid interfaces across a variety of electrochemical systems[J]. Chinese Journal of Catalysis,2022,43(11):2858-2870. doi: 10.1016/S1872-2067(22)64092-0 [128] Streibel V, Hävecker M, Yi Y, et al. In situ electrochemical cells to study the oxygen evolution reaction by near ambient pressure X-ray photoelectron spectroscopy[J]. Topics in Catalysis,2018,61(20):2064-2084. doi: 10.1007/s11244-018-1061-8 [129] Genovese C, Schuster M E, Gibson E K, et al. Operando spectroscopy study of the carbon dioxide electro-reduction by iron species on nitrogen-doped carbon[J]. Nature Communications,2018,9(1):935. doi: 10.1038/s41467-018-03138-7 [130] Zhang Y, Katayama Y, Tatara R, et al. Revealing electrolyte oxidation via carbonate dehydrogenation on Ni-based oxides in Li-ion batteries by in situ fourier transform infrared spectroscopy[J]. Energy & Environmental Science,2020,13(1):183-199. [131] Tremolet de Villers B J, Bak S M, Yang J, et al. In situ ATR-FTIR study of the cathode-electrolyte interphase: Electrolyte solution structure, transition metal redox, and surface layer evolution[J]. Batteries & Supercaps,2021,4(5):778-784. [132] Nesselberger M, Arenz M. In situ FTIR spectroscopy: Probing the electrochemical interface during the oxygen reduction reaction on a commercial platinum high-surface-area catalyst[J]. ChemCatChem,2016,8(6):1125-1131. doi: 10.1002/cctc.201501193 [133] Lei X, Tang Q, Zheng Y, et al. High-entropy single-atom activated carbon catalysts for sustainable oxygen electrocatalysis[J]. Nature Sustainability,2023,6(7):816-826. doi: 10.1038/s41893-023-01101-z -






 下载:
下载: