A review of graphdiyne: A new material for synthesizing effective adsorbents for aqueous contaminants
-
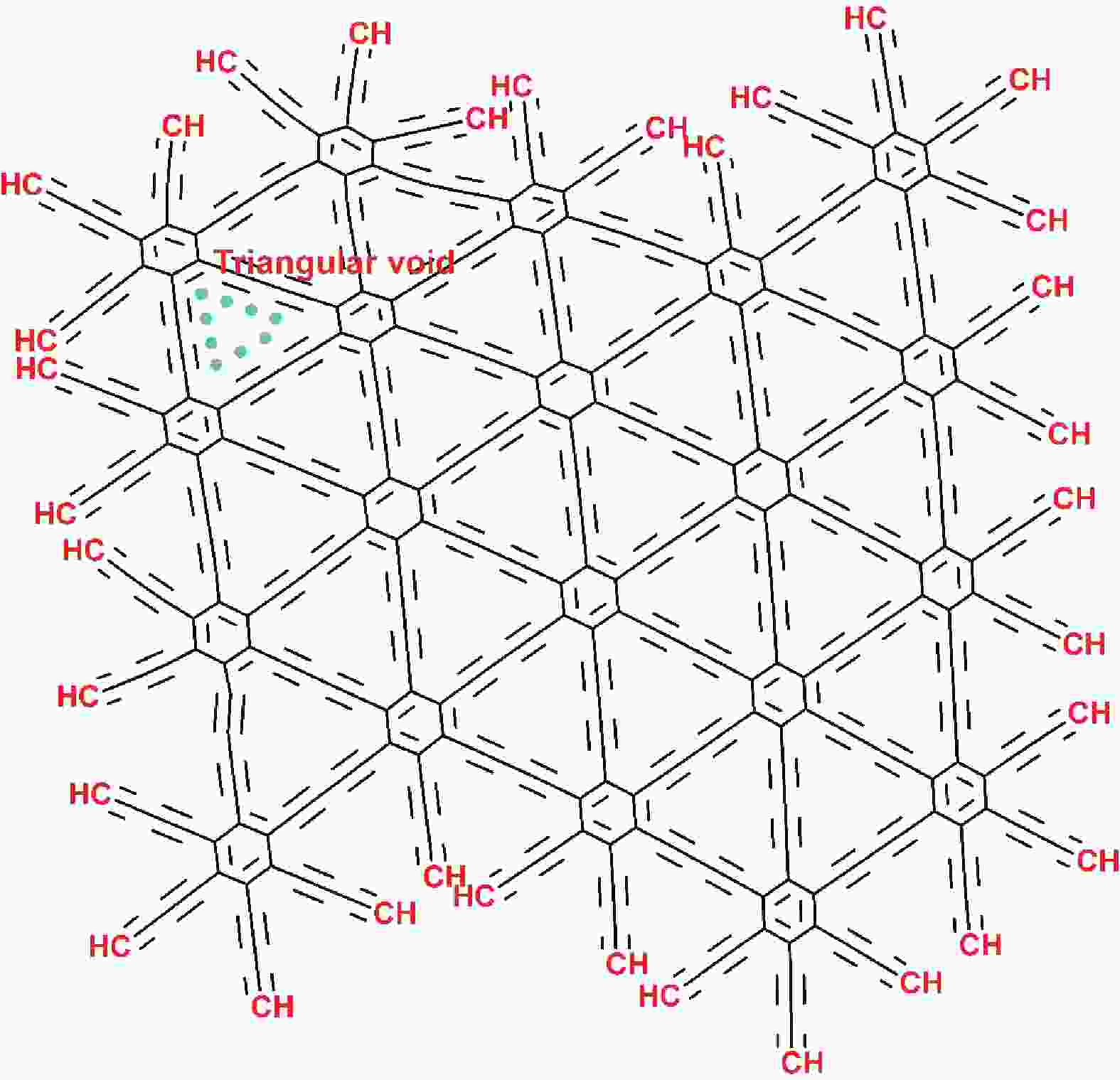
摘要: 石墨炔(GDY)是一种新生的二维材料,其在去除水溶液中污染物的研究方面引起了广泛关注。GDY是sp和sp2杂化碳原子的框架,其在二维对称网络中存在苯环和二乙基键,因此具有优异的共轭性、独特的可调谐电子性能、以及优异的化学和热稳定性。GDY的分子中有C≡C键,且具有均匀分布的三角形孔,可提供更多的反应位点和多种反应路径。因而,GDY具有吸附性,其作为吸附剂时在去除污染水中的油、有机污染物、染料和金属方面表现优异。在已发表的文献中,GDY被用作吸附剂的报道十分有限。本文综述了GDY的合成方法、GDY作为吸附剂的应用以及GDY基吸附剂的表征,并展望了GDY在污染物修复中的应用前景。Abstract: Graphdiyne (GDY), a new two-dimensional (2D) carbon molecule, is expected to have applications in the removal of contaminants from aqueous media. It has superior conjugation, unusual and varied electronic properties, and exceptional chemical and thermal stability because of its framework of sp and sp2 hybridized carbon bonds that are combined to produce benzene rings and diacetylenic bonds in a two-dimensional symmetrical network. Its molecular chemistry is the result of it having carbon-carbon triple bonds, with a regular distribution of triangular pores in its structure, which provide reaction sites and various reaction pathways. GDY is an adsorbent with an excellent efficiency for the removal of oil, organic pollutants, dyes, and metals from contaminated water, but there is limited evidence of it being used as an adsorbent in the literature. This review discusses its synthesis and its use as an adsorbent together with its prospects for pollutant removal.
-
Key words:
- Graphdiyne /
- Synthesis /
- Adsorption /
- Contaminants /
- Plausible mechanism
-
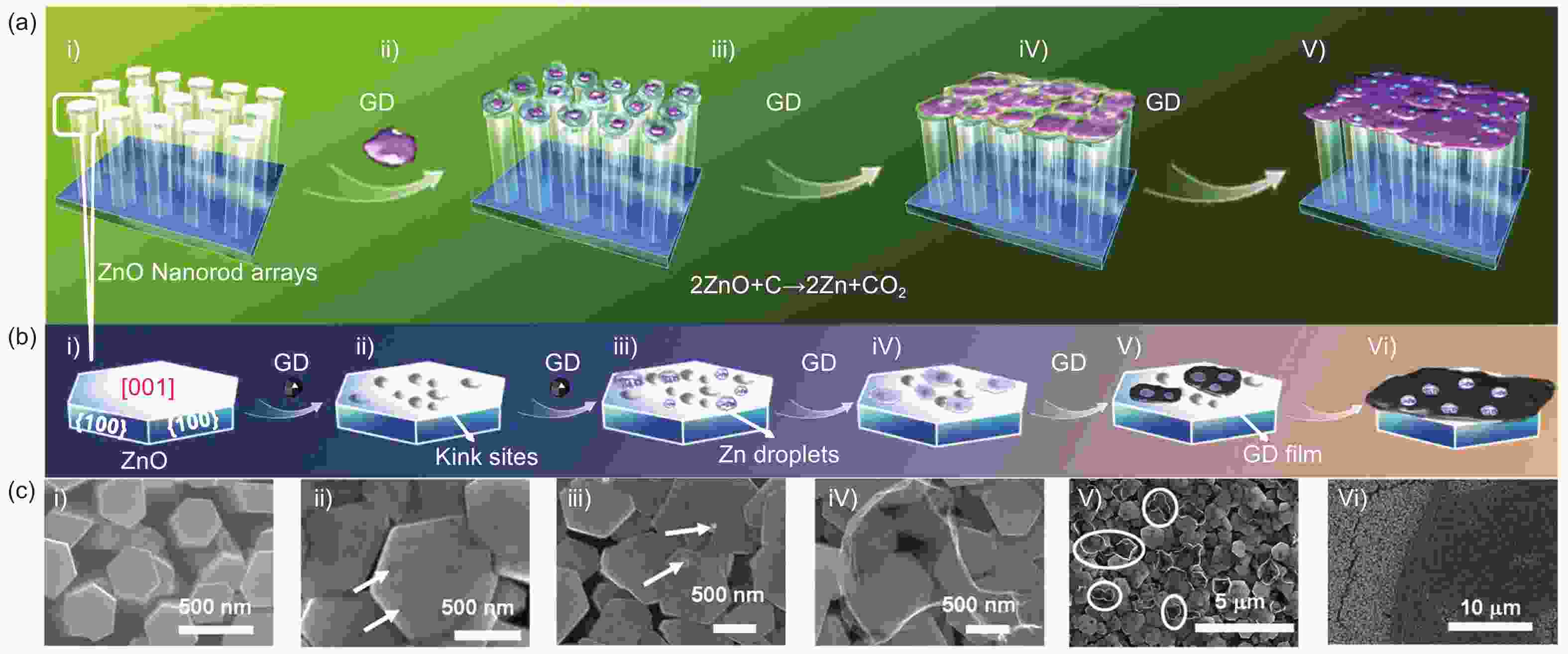
Figure 4. (a,b) Schematic representation of the self-catalyzed vapor-liquid-solid (VLS) growth technique to fabricate GDY thin films on ZnO nano-rod arrays. (c) SEM images. Reprinted with permission from[47]
Figure 5. (a) Lead ion adsorption in GDY. (b,c) SEM images of GDY. (d) Spectra of an aqueous solution with lead ions and xylenol orange added prior to and thereafter adsorption. Reprinted with permission from[77]
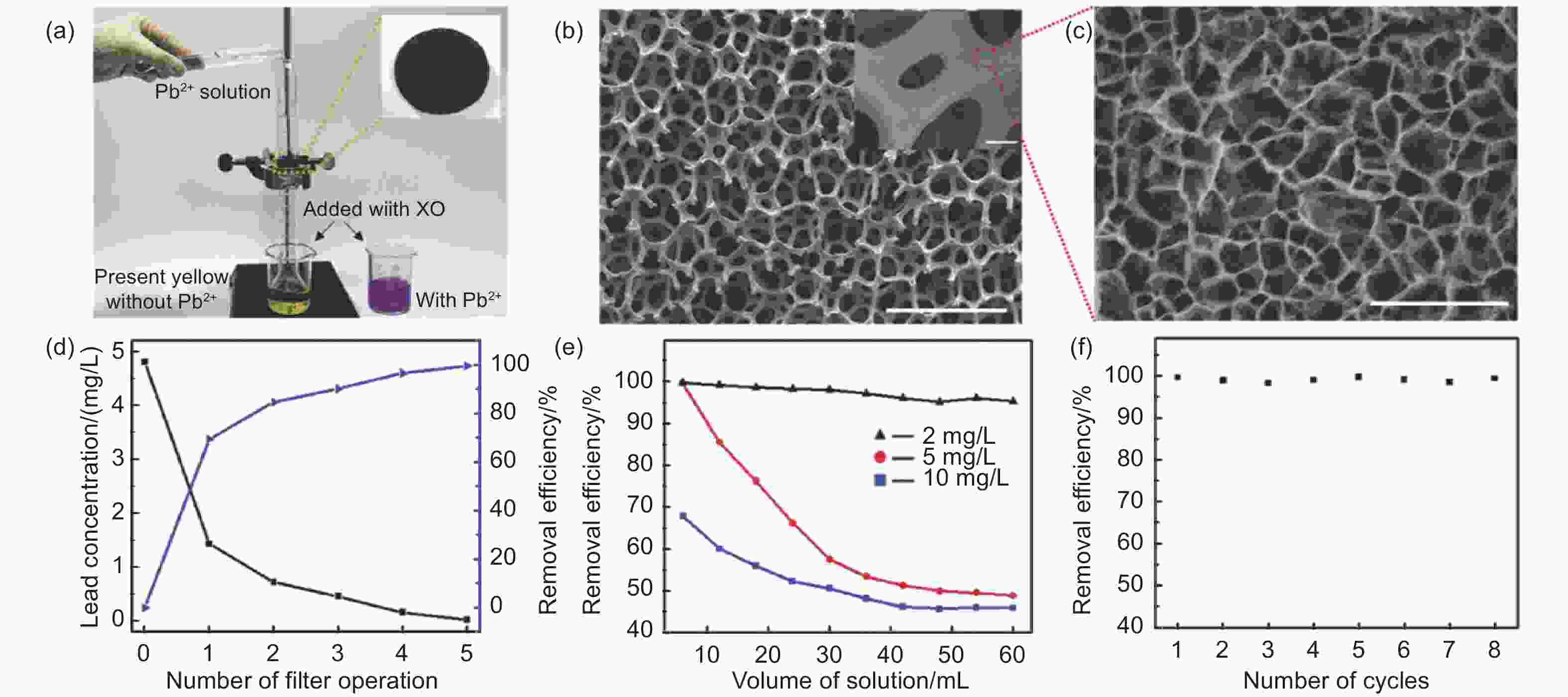
Figure 6. (a) Lead ion filtration experimental setup. (b,c) SEM images of GDY filter. (d) Concentration of Pb2+ and effectiveness of removal by GDY after varied numbers of filter operations. (e) Lead ion percentage removal as a function of the volume of aqueous medium with varying lead ion concentrations. (f) Recyclability. Reprinted with permission from[77]
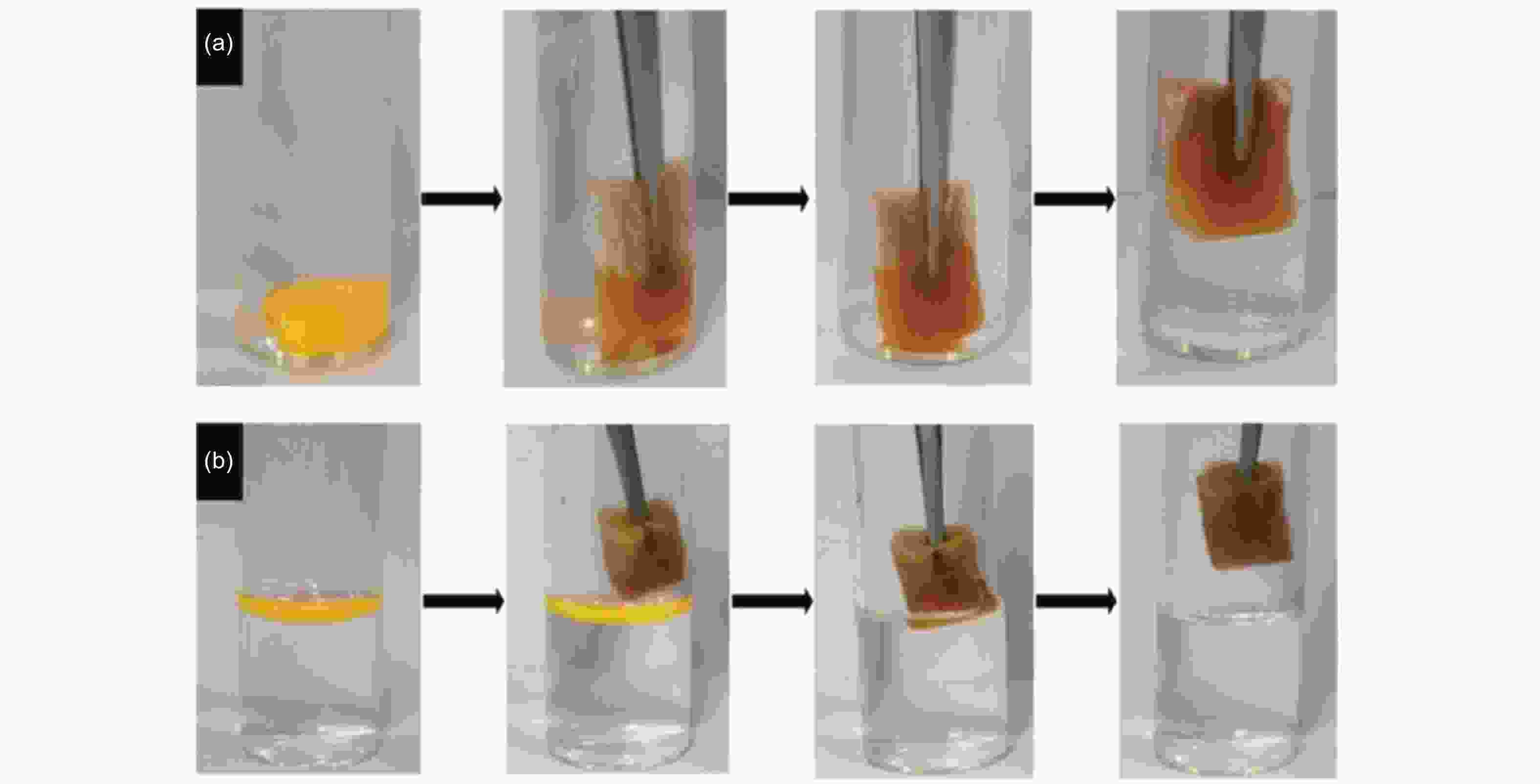
Figure 7. Graphdiyne melamine sponge adsorbing oil: (a) chloroform (organic solvent) and (b) gasoline (oil). Reprinted with permission from[80]. Copyright © 2019, American Chemical Society
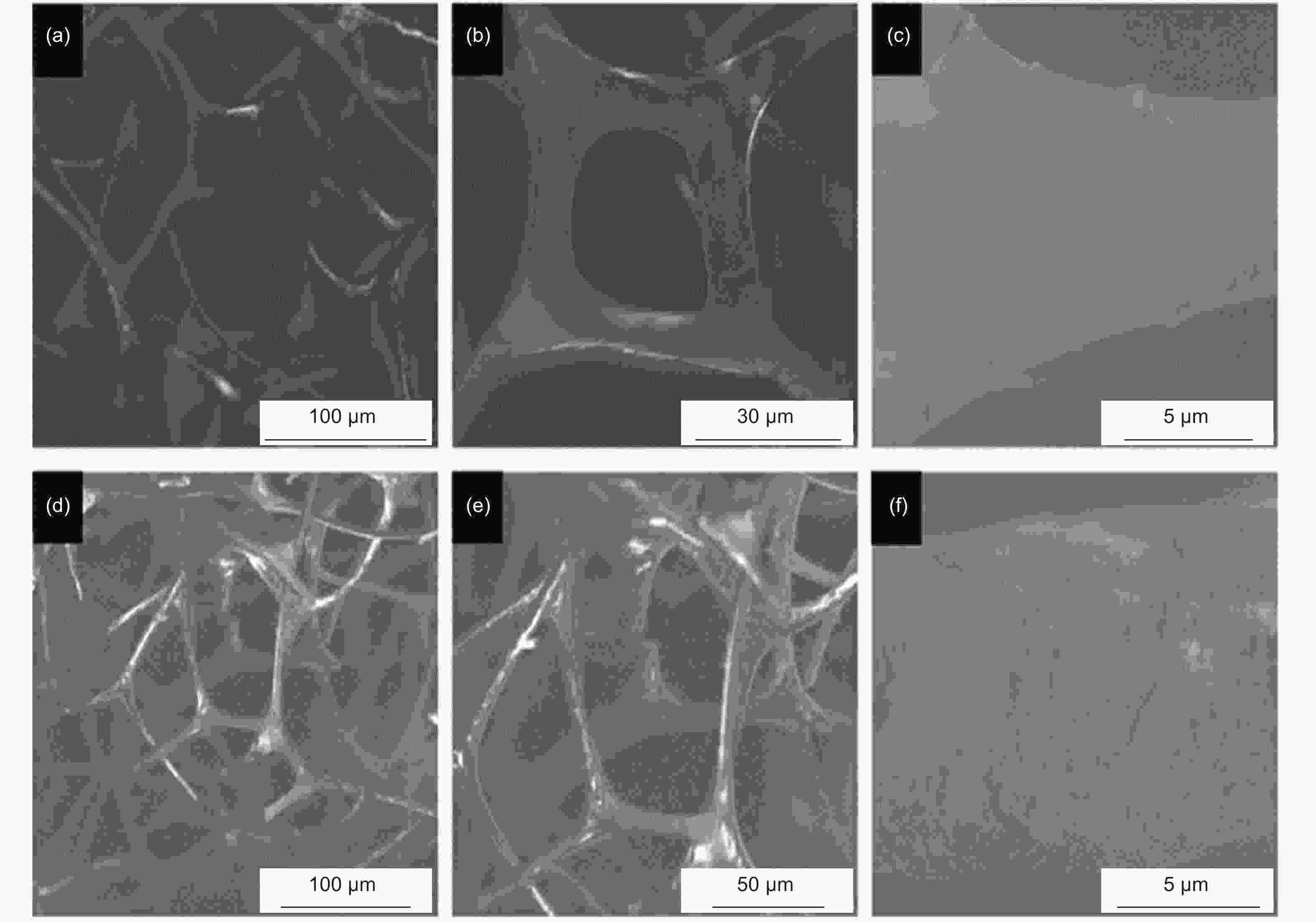
Figure 8. SEM images of melamine sponge (a-c) and graphdiyne coated on melamine sponge (d-f).Reprinted with permission from [80]. Copyright © 2019, American Chemical Society
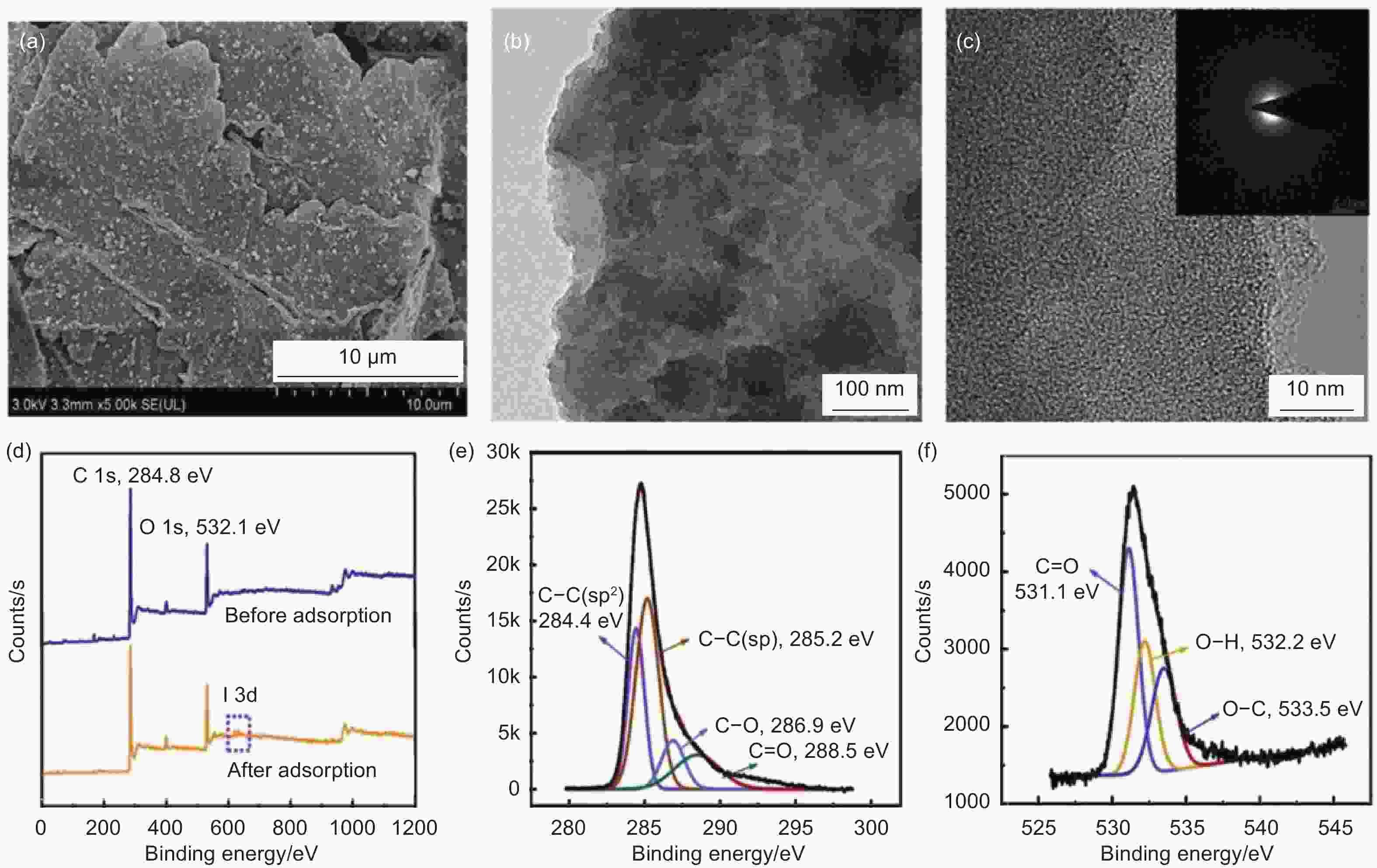
Figure 9. Images of (a) SEM and (b-c) TEM. XPS spectra of (c) orange line (iodosulfuron-methyl sodium), blue line (GDY), (e) C 1s and (f) O 1s. Reprinted with permission from[81]
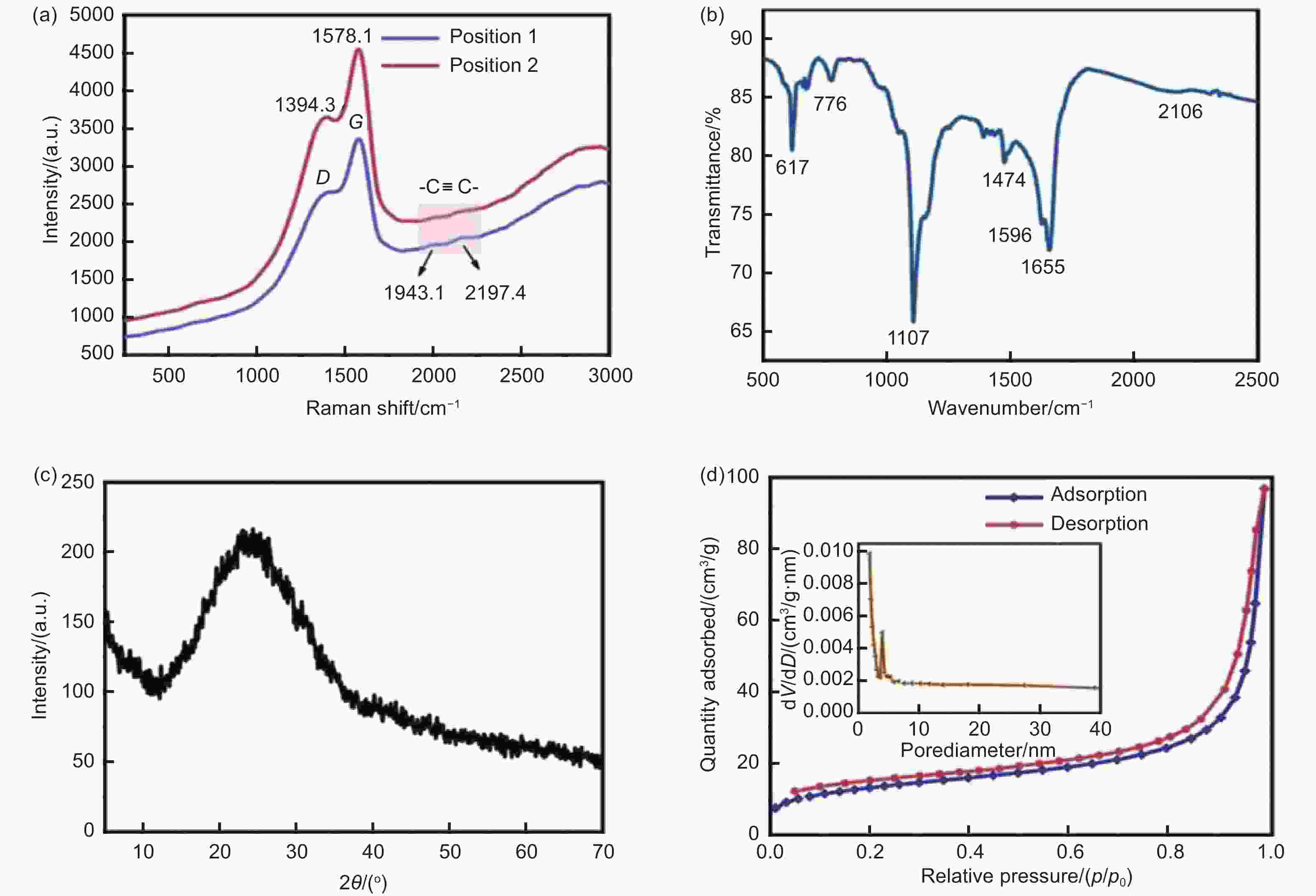
Figure 10. Oxygen-defective GDY (a) Raman spectra, (b) FTIR spectrum, (c) XRD spectra and (d) N2: adsorption-desorption isotherm. Reprinted with the permission from[81]
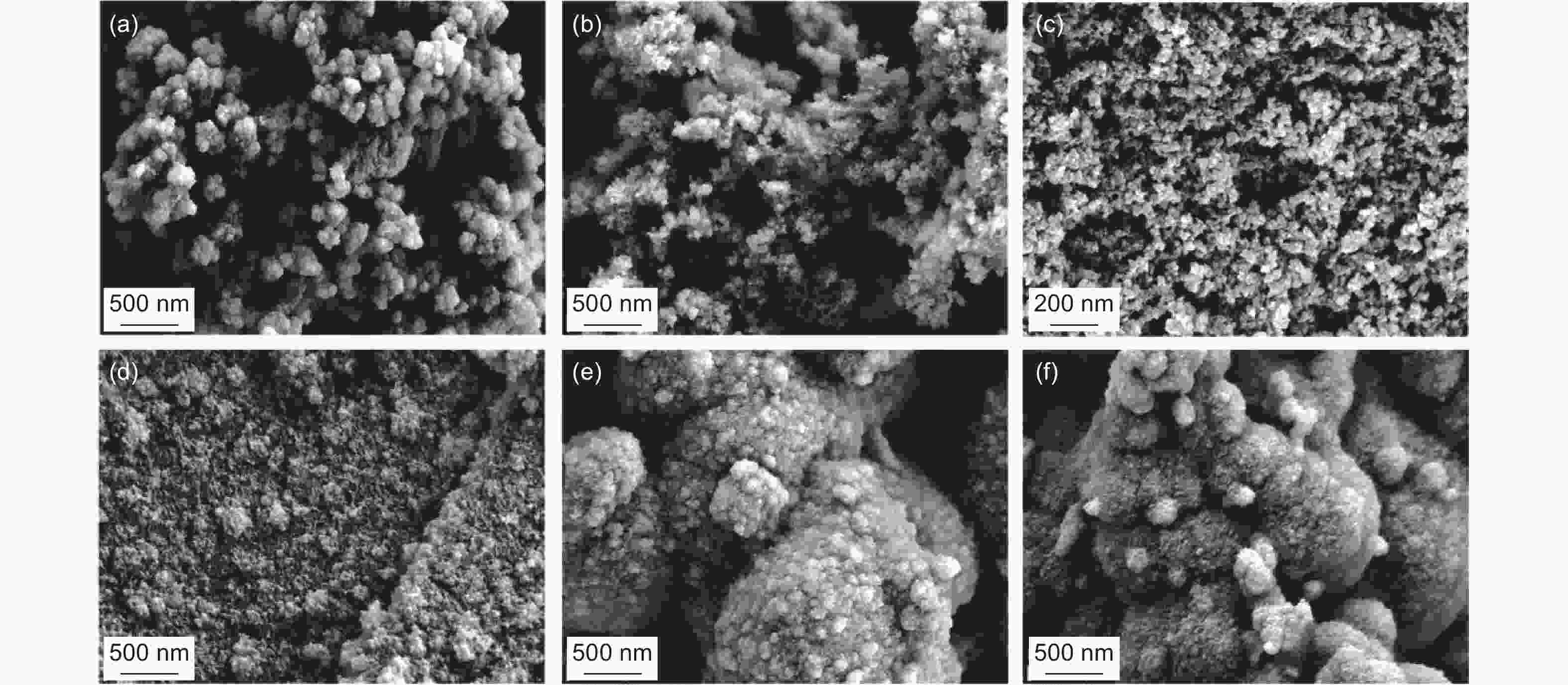
Figure 11. SEM images of GDY produced using various catalysts: (a) CuCl; (b) CuI; (c) Cu(OAc)2; (d) CuSO4; (e) Pd(OAc)2; (f) [(C6H5)3P]2·PdCl2. Reprinted with permission from[82]
Figure 12. (a) GDY-sponge extracting an organic solvent from a combination of oil and water. (b) GDY- melamine sponge adsorption capacity. (c) Recyclability of GDY-melamine sponge. Reprinted with permission from[80]. Copyright © 2019, American Chemical Society
Figure 13. GDY-melamine sponge as a filtering system for separating oil from water mixtures. Reprinted with permission from[80]. Copyright © 2019, American Chemical Society
Table 1. Various characterization techniques to study GDY
Table 2. XPS data for GDY
Table 3. Raman spectroscopy data for GDY
Raman peaks calculated Raman spectra peak (6 main peaks)/cm−1 Significance Ref. 956 Oscillation of alkynes and aromatic ring [110] 1364 The scissoring oscillation of atom in benzene 1478 Oscillation of C―C
triple bond1521 Oscillation of C=C bonds 2142 Synchronous contracting/ stretching of C≡C 2221 C≡C stretching mode with out of phase vibration Experimental Raman spectra
peaks (4 main peaks)1384 D band: vibration of sp2 carbon [72] 1569 G band: sp2 carbon (stretching oscillation) 1940 The diyne linkage 2181 Carbon-carbon triple bond -
[1] Rashid H, Manzoor M M, Mukhtar S. Urbanization and its effects on water resources: An exploratory analysis[J]. Asian Journal of Water, Environment and Pollution,2018,15(1):67-74. doi: 10.3233/AJW-180007 [2] Sarker B, Keya K N, Mahir F I, et al. Surface and ground water pollution: causes and effects of urbanization and industrialization in South Asia[J]. Scientific Review,2021,7(3):32-41. [3] Zamora-Ledezma C, Negrete-Bolagay D, Figueroa F, et al. Heavy metal water pollution: A fresh look about hazards, novel and conventional remediation methods[J]. Environmental Technology & Innovation,2021,22:101504. [4] Obinna I B, Ebere E C. A review: Water pollution by heavy metal and organic pollutants: Brief review of sources, effects and progress on remediation with aquatic plants[J]. Analytical Methods in Environmental Chemistry Journal,2019,2(3):5-38. [5] Ismail M, Akhtar K, Khan M, et al. Pollution, toxicity and carcinogenicity of organic dyes and their catalytic bio-remediation[J]. Current Pharmaceutical Design,2019,25(34):3645-3663. doi: 10.2174/1381612825666191021142026 [6] Hube S, Eskafi M, Hrafnkelsdóttir K F, et al. Direct membrane filtration for wastewater treatment and resource recovery: A review[J]. Science of the Total Environment,2020,710:136375. doi: 10.1016/j.scitotenv.2019.136375 [7] Martínez-Huitle C A, Panizza M. Electrochemical oxidation of organic pollutants for wastewater treatment[J]. Current Opinion in Electrochemistry,2018,11:62-71. doi: 10.1016/j.coelec.2018.07.010 [8] Sharma J, Dhiman P, Kumar A, et al. 2D-2D g-C3N5/Bi24O31Br10 S-scheme nanostructures with increased photocatalytic efficiency for crystal violet removal[J]. Chemical Engineering Research and Design,2023,195:432-446. doi: 10.1016/j.cherd.2023.05.059 [9] Sharma P, Kumar A, Zheng G, et al. Current scenario in ternary metal indium sulfides-based heterojunctions for photocatalytic energy and environmental applications: A Review [J]. Materials Today Communications, 2023: 106741. [10] Sharma G, García-Peñas A, Verma Y, et al. Tailoring homogeneous hydrogel nanospheres by facile ultra-sonication assisted cross-linked copolymerization for rhodamine B dye adsorption[J]. Gels,2023,9(10):770. doi: 10.3390/gels9100770 [11] Rajasulochana P, Preethy V. Comparison on efficiency of various techniques in treatment of waste and sewage water–A comprehensive review[J]. Resource-Efficient Technologies,2016,2(4):175-184. doi: 10.1016/j.reffit.2016.09.004 [12] Safarpour M, Khataee A. Graphene-based materials for water purification[J]. Nanoscale materials in water purification,2019:383-430. [13] He K, Chen G, Zeng G, et al. Three-dimensional graphene supported catalysts for organic dyes degradation[J]. Applied Catalysis B:Environmental,2018,228:19-28. doi: 10.1016/j.apcatb.2018.01.061 [14] Rao N R, Sood A K, Subrahmanyam K S, et al. Graphene: the new two-dimensional nanomaterial[J]. Angewandte Chemie International Edition,2009,48(42):7752-7777. doi: 10.1002/anie.200901678 [15] Zhu X R, Zhang X H, Li Y F, et al. Exploring transition metal oxide-based oxygen vacancy supercapacitors: A review[J]. Journal of Energy Storage,2024,80:110350. [16] Zuo Z, Li Y. Emerging electrochemical energy applications of graphdiyne[J]. Joule,2019,3(4):899-903. doi: 10.1016/j.joule.2019.01.016 [17] Liu R, Liu H, Li Y, et al. Nitrogen-doped graphdiyne as a metal-free catalyst for high-performance oxygen reduction reactions[J]. Nanoscale,2014,6(19):11336-11343. doi: 10.1039/C4NR03185G [18] Xue Z, Zhu M, Dong Y, et al. An integrated targeting drug delivery system based on the hybridization of graphdiyne and MOFs for visualized cancer therapy[J]. Nanoscale,2019,11(24):11709-11718. doi: 10.1039/C9NR02017A [19] Yu H, Xue Y, Li Y. Graphdiyne and its assembly architectures: synthesis, functionalization, and applications[J]. Advanced Materials,2019,31(42):1803101. doi: 10.1002/adma.201803101 [20] Khan K, Tareen A K, Iqbal M, et al. Novel emerging graphdiyne based two dimensional materials: Synthesis, properties and renewable energy applications[J]. Nano Today,2021,39:101207. doi: 10.1016/j.nantod.2021.101207 [21] Ghosh A, Orasugh J T, Ray S S, et al. Prospects of 2D graphdiynes and their applications in desalination and wastewater remediation [J]. RSC advances, 2023, 13: (27): 18568-18604. [22] Gao X, Liu H, Wang D, et al. Graphdiyne: synthesis, properties, and applications[J]. Chemical Society Reviews,2019,48(3):908-936. doi: 10.1039/C8CS00773J [23] Haley M M, Brand S C, Pak J J. Carbon networks based on dehydrobenzoannulenes: Synthesis of graphdiyne substructures[J]. Angewandte Chemie International Edition in English,1997,36(8):836-838. doi: 10.1002/anie.199708361 [24] Li G, Li Y, Liu H, et al. Architecture of graphdiyne nanoscale films[J]. Chemical Communications,2010,46(19):3256-3258. doi: 10.1039/b922733d [25] Rana S, Kumar A, Dhiman P, et al. Progress in graphdiyne and phosphorene based composites and heterostructures as new age materials for photocatalytic hydrogen evolution[J]. Fuel,2024,356:129630. doi: 10.1016/j.fuel.2023.129630 [26] Long M, Tang L, Wang D, et al. Electronic structure and carrier mobility in graphdiyne sheet and nanoribbons: Theoretical predictions[J]. ACS nano,2011,5(4):2593-2600. doi: 10.1021/nn102472s [27] Zheng Y, Chen Y, Lin L, et al. Intrinsic magnetism of graphdiyne[J]. Applied Physics Letters,2017,111(3):033101. doi: 10.1063/1.4993916 [28] Zhang M, Wang X, Sun H, et al. Enhanced paramagnetism of mesoscopic graphdiyne by doping with nitrogen[J]. Scientific Report,2017,7:11535. doi: 10.1038/s41598-017-11698-9 [29] Meng Z, Zhang X, Zhang Y, et al. Graphdiyne as a high-efficiency membrane for separating oxygen from harmful gases: a first-principles study[J]. ACS Applied Materials & Interfaces,2016,8(41):28166-28170. [30] Qiu H, Xue M, Shen C, et al. Graphynes for water desalination and gas separation[J]. Advanced Materials,2019,31(42):1803772. doi: 10.1002/adma.201803772 [31] Fang L, Cao Z. Isoelectronic doping and external electric field regulate the gas-separation performance of graphdiyne[J]. The Journal of Physical Chemistry C,2020,124(4):2712-2720. doi: 10.1021/acs.jpcc.9b11062 [32] Srinivasu K, Ghosh S K. Graphyne and graphdiyne: Promising materials for nanoelectronics and energy storage applications[J]. The Journal of Physical Chemistry C,2012,116(9):5951-5956. doi: 10.1021/jp212181h [33] Wu T, Sun M, Huang B, et al. Graphdiyne based catalysts for energy applications[J]. Materials Chemistry Frontiers,2021,5:7369-7383. doi: 10.1039/D1QM00796C [34] Li J, Wan C, Wang C, et al. 2D material chemistry: Graphdiyne-based biochemical sensing[J]. Chemical Research in Chinese Universities,2020,36(4):622-630. doi: 10.1007/s40242-020-0181-4 [35] Kaur H, Thakur V K, Siwal S S. Recent advancements in graphdiyne-based nano-materials for biomedical applications[J]. Materials Today:Proceedings,2022,56:112-120. doi: 10.1016/j.matpr.2021.12.355 [36] Zhang Z, Muhammad Y, Chen Y, et al. Construction of ultra-stable and Z-scheme Fe-Graphdiyne/MIL-100 (Fe) photo-Fenton catalyst with C=C―Fe|O interface for the highly enhanced catalytic degradation of dinotefuran[J]. Chemical Engineering Journal,2021,426:131621. doi: 10.1016/j.cej.2021.131621 [37] Haley M M. Synthesis and properties of annulenic subunits of graphyne and graphdiyne nanoarchitectures[J]. Pure and Applied Chemistry,2008,80(3):519-532. doi: 10.1351/pac200880030519 [38] Kong Y, Li J, Zeng S, et al. Bridging the gap between reality and ideality of graphdiyne: The advances of synthetic methodology[J]. Chem,2020,6(8):1933-1951. doi: 10.1016/j.chempr.2020.06.011 [39] Yang Y, Xu X. Mechanical properties of graphyne and its family–A molecular dynamics investigation[J]. Computational Materials Science,2012,61:83-88. doi: 10.1016/j.commatsci.2012.03.052 [40] Wan Y, Xiong S, Ouyang B, et al. Thermal transport engineering in graphdiyne and graphdiyne nanoribbons[J]. ACS omega,2019,4(2):4147-4152. doi: 10.1021/acsomega.9b00074 [41] Fairlamb I J, Bäuerlein P S, Marrison L R, et al. Pd-catalysed cross coupling of terminal alkynes to diynes in the absence of a stoichiometric additive[J]. Chemical Communications,2003(5):632-633. doi: 10.1039/b212430k [42] Hay A S. Oxidative coupling of acetylenes. II1[J]. The Journal of Organic Chemistry,1962,27(9):3320-3321. doi: 10.1021/jo01056a511 [43] Yan H C, Hui B L, Yu L L. Progress and prospect of two dimensional carbon graphdiyne[J]. Chinese Science Bulletin,2016,61(26):2901-2912. doi: 10.1360/N972016-00483 [44] Huang C, Li Y, Wang N, et al. Progress in research into 2D graphdiyne-based materials [J]. Chemical Reviews, 2018, 118: (16) 7744-7803. [45] Li Y, Xu L, Liu H, et al. Graphdiyne and graphyne: From theoretical predictions to practical construction[J]. Chemical Society Reviews,2014,43(8):2572-2586. doi: 10.1039/c3cs60388a [46] Liu R, Gao X, Zhou J, et al. Chemical vapor deposition growth of linked carbon monolayers with acetylenic scaffoldings on silver foil[J]. Advanced Materials,2017,29(18):1604665. doi: 10.1002/adma.201604665 [47] Qian X, Liu H, Huang C, et al. Self-catalyzed growth of large-area nanofilms of two-dimensional carbon[J]. Scientific reports,2015,5(1):1-7. doi: 10.9734/JSRR/2015/14076 [48] Wang F, Zuo Z, Shang H, et al. Ultrafastly interweaving graphdiyne nanochain on arbitrary substrates and its performance as a supercapacitor electrode[J]. ACS Applied Materials & Interfaces,2018,11(3):2599-2607. [49] Zhang Y, Pei Q, Wang C. Mechanical properties of graphynes under tension: A molecular dynamics study[J]. Applied Physics Letters,2012,101(8):081909. doi: 10.1063/1.4747719 [50] Clair S, de Oteyza D G. Controlling a chemical coupling reaction on a surface: Tools and strategies for on-surface synthesis[J]. Chemical Reviews,2019,119(7):4717-4776. doi: 10.1021/acs.chemrev.8b00601 [51] Ebrahimiasl S, Behmagham F, Abdolmohammadi S, et al. Recent advances in the application of nanometal catalysts for Glaser coupling[J]. Current Organic Chemistry,2019,23(22):2489-2503. [52] Albrecht F, Rey D, Fatayer S, et al. Intramolecular coupling of terminal alkynes by atom manipulation[J]. Angewandte Chemie,2020,132(51):23189-23193. doi: 10.1002/ange.202009200 [53] Mirjafary Z, Sadighian H, Piri S, et al. Efficient synthesis of novel 1, 3-diyne-based sulfonamides using CuCl2/Et3N as a robust catalytic system[J]. Journal of Sulfur Chemistry,2017,38(2):188-194. doi: 10.1080/17415993.2016.1263634 [54] Akhtar R, Zahoor A F. Transition metal catalyzed Glaser and Glaser-Hay coupling reactions: Scope, classical/green methodologies and synthetic applications[J]. Synthetic Communications,2020,50(22):3337-3368. doi: 10.1080/00397911.2020.1802757 [55] Hendrich C M, Sekine K, Koshikawa T, et al. Homogeneous and heterogeneous gold catalysis for materials science[J]. Chemical Reviews,2020,121(14):9113-9163. [56] Lin Y, Kang H, Liang M, et al. Hybrid nanostructured MnO2 nanowire/graphdiyne with enhanced lithium-ion performance promoting by interfacial storage[J]. Applied Surface Science,2020,526:146457. doi: 10.1016/j.apsusc.2020.146457 [57] Zhang H, Muhammad Y, Cui X, et al. Engineering bidirectional CMC-foam-supported HKUST-1@ graphdiyne with enhanced heat/mass transfer for the highly efficient adsorption and regeneration of acetaldehyde[J]. Journal of Materials Chemistry A,2021,9(7):4066-4074. doi: 10.1039/D0TA09780B [58] Li M, Li Y, Zhang Y, et al. Strain tunable nanoporous rN-GDY membrane for efficient seawater desalination[J]. Journal of Materials Chemistry A,2022,10(31):16533-16540. doi: 10.1039/D2TA04108A [59] Zhai X, Zuo Z, Xiong Z, et al. Large-scale CuS nanotube arrays@ graphdiyne for high-performance sodium ion battery[J]. 2D Materials,2022,9(2):025024. doi: 10.1088/2053-1583/ac5d84 [60] Zhao F, Li X, He J, et al. Preparation of hierarchical graphdiyne hollow nanospheres as anode for lithium-ion batteries[J]. Chemical Engineering Journal,2021,413:127486. doi: 10.1016/j.cej.2020.127486 [61] Schmidt R, Thorwirth R, Szuppa T, et al. Fast, ligand-and solvent-free synthesis of 1,4-substituted buta-1,3-diynes by Cu-catalyzed homocoupling of terminal alkynes in a ball mill[J]. Chemistry-A European Journal,2011,17(29):8129-8138. doi: 10.1002/chem.201100604 [62] Klappenberger F, Zhang Y Q, Björk J, et al. On-surface synthesis of carbon-based scaffolds and nanomaterials using terminal alkynes[J]. Accounts of chemical research,2015,48(7):2140-2150. doi: 10.1021/acs.accounts.5b00174 [63] Seo S, Choi J, Cho S M, et al. Few-Layered graphyne growth by an on-surface coupling reaction via alkynyl vapour deposition[J]. arXiv preprint arXiv: 2010,1344,6:2020. [64] Zhou J, Li J, Liu Z, et al. Exploring approaches for the synthesis of few-layered graphdiyne[J]. Advanced Materials,2019,31(42):1803758. doi: 10.1002/adma.201803758 [65] Sun Q, Cai L, Ma H, et al. Dehalogenative homocoupling of terminal alkynyl bromides on Au (111): incorporation of acetylenic scaffolding into surface nanostructures[J]. ACS nano,2016,10(7):7023-7030. doi: 10.1021/acsnano.6b03048 [66] Zhang Y Q, Kepčija N, Kleinschrodt M, et al. Homo-coupling of terminal alkynes on a noble metal surface[J]. Nature communications,2012,3(1):1-8. [67] Cirera B, Zhang Y Q, Björk J, et al. Synthesis of extended graphdiyne wires by vicinal surface templating[J]. Nano letters,2014,14(4):1891-1897. doi: 10.1021/nl4046747 [68] Zuo Z, Shang H, Chen Y, et al. A facile approach for graphdiyne preparation under atmosphere for an advanced battery anode[J]. Chemical Communications,2017,53(57):8074-8077. doi: 10.1039/C7CC03200E [69] Ciesielski A, Samorì P. Graphene via sonication assisted liquid-phase exfoliation[J]. Chemical Society Reviews,2014,43(1):381-398. doi: 10.1039/C3CS60217F [70] Niu L, Coleman J N, Zhang H, et al. Production of two-dimensional nanomaterials via liquid-based direct exfoliation[J]. Small,2016,12(3):272-293. doi: 10.1002/smll.201502207 [71] Guan G, Zhang S, Liu S, et al. Protein induces layer-by-layer exfoliation of transition metal dichalcogenides[J]. Journal of the American Chemical Society,2015,137(19):6152-6155. doi: 10.1021/jacs.5b02780 [72] Zhou J, Gao X, Liu R, et al. Synthesis of graphdiyne nanowalls using acetylenic coupling reaction[J]. Journal of the American Chemical Society,2015,137(24):7596-7599. doi: 10.1021/jacs.5b04057 [73] Gao X, Zhou J, Du R, et al. Robust superhydrophobic foam: A graphdiyne-based hierarchical architecture for oil/water separation[J]. Advanced Materials,2016,28(1):168-173. doi: 10.1002/adma.201504407 [74] Wang S S, Liu H B, Kan X N, et al. Superlyophilicity-facilitated synthesis reaction at the microscale: Ordered graphdiyne stripe arrays[J]. Small,2017,13(4):1602265. doi: 10.1002/smll.201602265 [75] Gao X, Zhu Y, Yi D, et al. Ultrathin graphdiyne film on graphene through solution-phase van der Waals epitaxy[J]. Science advances,2018,4(7):eaat6378. doi: 10.1126/sciadv.aat6378 [76] Matsuoka R, Sakamoto R, Hoshiko K, et al. Crystalline graphdiyne nanosheets produced at a gas/liquid or liquid/liquid interface[J]. Journal of the American Chemical Society,2017,139(8):3145-3152. doi: 10.1021/jacs.6b12776 [77] Liu R, Zhou J, Gao X, et al. Graphdiyne filter for decontaminating lead-ion-polluted water[J]. Advanced Electronic Materials,2017,3(11):1700122. doi: 10.1002/aelm.201700122 [78] Mashhadzadeh A H, Vahedi A, Ardjmand M, et al. Investigation of heavy metal atoms adsorption onto graphene and graphdiyne surface: a density functional theory study[J]. Superlattices and Microstructures,2016,100:1094-1102. doi: 10.1016/j.spmi.2016.10.079 [79] Li Y, Huang H, Cui R, et al. Electrochemical sensor based on graphdiyne is effectively used to determine Cd2+ and Pb2+ in water[J]. Sensors and Actuators B:Chemical,2021,332:129519. doi: 10.1016/j.snb.2021.129519 [80] Li J, Chen Y, Gao J, et al. Graphdiyne sponge for direct collection of oils from water[J]. ACS Applied Materials & Interfaces,2018,11(3):2591-2598. [81] Zhu J, Xiang S, Zhang B, et al. Oxygen-defective graphdiyne for ultra-efficient removal of sulfonylurea herbicides from aqueous solution[J]. Journal of Environmental Chemical Engineering,2022,10(3):107724. doi: 10.1016/j.jece.2022.107724 [82] Zhu J, Liu D, Li C, et al. Facile and large-scale synthesis of polymorphic graphdiyne catalyzed by transition metal salts for organic pollutant removal[J]. RSC advances,2021,11(56):35408-35414. doi: 10.1039/D1RA06653F [83] Friedrich B, Hoffmann D, Renn J, et al. One hundred years of chemical warfare: Research, deployment, consequences[J]. Springer Nature, 2017, XI: 408. [84] Chai P R, Hayes B D, Erickson T B, et al. Novichok agents: A historical, current, and toxicological perspective[J]. Toxicology communications,2018,2(1):45-48. doi: 10.1080/24734306.2018.1475151 [85] Sajid H, Khan S, Ayub K, et al. Effective adsorption of A-series chemical warfare agents on graphdiyne nanoflake: A DFT study[J]. Journal of Molecular Modeling,2021,27:1-12. doi: 10.1007/s00894-020-04615-x [86] Chu P K, Li L. Characterization of amorphous and nanocrystalline carbon films[J]. Materials Chemistry and Physics,2006,96(2-3):253-277. doi: 10.1016/j.matchemphys.2005.07.048 [87] Butt H-J, Wolff E, Gould S, et al. Imaging cells with the atomic force microscope[J]. Journal of structural biology,1990,105(1-3):54-61. doi: 10.1016/1047-8477(90)90098-W [88] Albrecht T R, Grütter P, Horne D, et al. Frequency modulation detection using high-Q cantilevers for enhanced force microscope sensitivity[J]. Journal of applied physics,1991,69(2):668-673. doi: 10.1063/1.347347 [89] Cui W, Zhang M, Wang N, et al. High-performance field-effect transistor based on novel conjugated p-o-fluoro-p-alkoxyphenyl-substituted polymers by graphdiyne doping[J]. The Journal of Physical Chemistry C,2017,121(42):23300-23306. doi: 10.1021/acs.jpcc.7b07364 [90] Ren H, Shao H, Zhang L, et al. A new graphdiyne nanosheet/Pt nanoparticle-based counter electrode material with enhanced catalytic activity for dye-sensitized solar cells[J]. Advanced Energy Materials,2015,5(12):1500296. doi: 10.1002/aenm.201500296 [91] Zhang X, Wang Q, Jin Z, et al. Graphdiyne quantum dots for much improved stability and efficiency of perovskite solar cells[J]. Advanced Materials Interfaces,2018,5(2):1701117. doi: 10.1002/admi.201701117 [92] Wang K, Wang N, He J, et al. Preparation of 3D architecture graphdiyne nanosheets for high-performance sodium-ion batteries and capacitors[J]. ACS Applied Materials & Interfaces,2017,9(46):40604-40613. [93] Li C, Lu X, Han Y, et al. Direct imaging and determination of the crystal structure of six-layered graphdiyne[J]. Nano Research,2018,11:1714-1721. doi: 10.1007/s12274-017-1789-7 [94] Pan Q, Chen S, Wu C, et al. Sulfur-rich graphdiyne-containing electrochemical active tetrathiafulvalene for highly efficient lithium storage application[J]. ACS Applied Materials & Interfaces,2019,11(49):46070-46076. [95] Giannuzzi L A, Stevie F A. A review of focused ion beam milling techniques for TEM specimen preparation[J]. Micron,1999,30(3):197-204. doi: 10.1016/S0968-4328(99)00005-0 [96] Chauhan A, Chauhan P. Powder XRD technique and its applications in science and technology[J]. J Anal Bioanal Tech,2014,5(5):1-5. [97] Seah M. The quantitative analysis of surfaces by XPS: A review[J]. Surface and Interface Analysis,1980,2(6):222-239. doi: 10.1002/sia.740020607 [98] Mrđenović D, Cai Z F, Pandey Y, et al. Nanoscale chemical analysis of 2D molecular materials using tip-enhanced Raman spectroscopy[J]. Nanoscale,2023,15:963-974. doi: 10.1039/D2NR05127C [99] Tureček F. UV-vis spectroscopy of gas-phase ions[J]. Mass Spectrometry Reviews,2023,42(1):206-226. doi: 10.1002/mas.21726 [100] Qian X, Ning Z, Li Y, et al. Construction of graphdiyne nanowires with high-conductivity and mobility[J]. Dalton transactions,2012,41(3):730-733. doi: 10.1039/C1DT11641J [101] Suryanarayana C, Grant N. A practical, X-ray diffraction: A practical approach[J]. Microscopy and Microanalysis,1998,4(5):513-515. doi: 10.1017/S143192769800049X [102] Ossiander M, Riemensberger J, Neppl S, et al. Absolute timing of the photoelectric effect[J]. Nature,2018,561(7723):374-377. doi: 10.1038/s41586-018-0503-6 [103] Edgell M, Paynter R, Castle J. High energy XPS using a monochromated Ag Lα source: Resolution, sensitivity and photoelectric cross sections[J]. Journal of electron spectroscopy and related phenomena,1985,37(2):241-256. doi: 10.1016/0368-2048(85)80071-2 [104] Hollander J M, Jolly W L. X-ray photoelectron spectroscopy[J]. Accounts of chemical research,1970,3(6):193-200. doi: 10.1021/ar50030a003 [105] Powell C J, Larson P. Quantitative surface analysis by X-ray photoelectron spectroscopy[J]. Applications of Surface Science,1978,1(2):186-201. doi: 10.1016/0378-5963(78)90014-4 [106] Brown J, Fyfe W, Bancroft G. Semi-quantitative surface analysis of Mt. St. Helens Ash by X-ray photoelectron spectroscopy (XPS)[J]. Applications of Surface Science,1981,7(4):419-424. doi: 10.1016/0378-5963(81)90087-8 [107] Zhang S, Liu H, Huang C, et al. Bulk graphdiyne powder applied for highly efficient lithium storage[J]. Chemical Communications,2015,51(10):1834-1837. doi: 10.1039/C4CC08706B [108] Wokaun A B. Schrader: Infrared and Raman Spectroscopy-Methods and Applications. VCH, Weinheim, 1995, DM 298, ISBN 3-527-26446-9[M]. Wiley Online Library, 1996, 100(7): 1268-1268. [109] Riviere J. Surface Analytical Techniques[M]. Clarendon, in, Oxford, 1990. [110] Zhang S, Wang J, Li Z, et al. Raman spectra and corresponding strain effects in graphyne and graphdiyne[J]. The Journal of Physical Chemistry C,2016,120(19):10605-10613. doi: 10.1021/acs.jpcc.5b12388 [111] Li G, Li Y, Qian X, et al. Construction of tubular molecule aggregations of graphdiyne for highly efficient field emission[J]. The Journal of Physical Chemistry C,2011,115(6):2611-2615. doi: 10.1021/jp107996f [112] Perkampus H H. UV-VIS spectroscopy and its applications[J]. Springer Science & Business Media, 2013. [113] Sun C, Liu Y, Wang Z, et al. Self-assembled g-C3N4 nanotubes/graphdiyne composite with enhanced photocatalytic CO2 reduction[J]. Journal of Alloys and Compounds,2021,868:159045. doi: 10.1016/j.jallcom.2021.159045 [114] Liang S, Deng H, Zhou Z, et al. Fabrication of graphdiyne and its analogues for photocatalytic application [J]. EcoMat, 2022: e12297. [115] Zheng Q, Luo G, Liu Q, et al. Structural and electronic properties of bilayer and trilayer graphdiyne[J]. Nanoscale,2012,4(13):3990-3996. doi: 10.1039/c2nr12026g [116] Kinniburgh D G. General purpose adsorption isotherms[J]. Environmental science & technology,1986,20(9):895-904. [117] Ayawei N, Ebelegi A N, Wankasi D. Modelling and interpretation of adsorption isotherms [J]. Journal of chemistry, 2017, 2017. [118] Liu Y. Some consideration on the Langmuir isotherm equation[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects,2006,274(1-3):34-36. [119] Skopp J. Derivation of the Freundlich adsorption isotherm from kinetics[J]. Journal of Chemical Education,2009,86(11):1341. doi: 10.1021/ed086p1341 [120] Qiu H, Lv L, Pan B-c, et al. Critical review in adsorption kinetic models[J]. Journal of Zhejiang University-Science A,2009,10(5):716-724. doi: 10.1631/jzus.A0820524 [121] Brezesinski G, Möhwald H. Langmuir monolayers to study interactions at model membrane surfaces[J]. Advances in colloid and interface science,2003,100:563-584. [122] Wang J, Guo X. Adsorption isotherm models: Classification, physical meaning, application and solving method[J]. Chemosphere,2020,258:127279. doi: 10.1016/j.chemosphere.2020.127279 [123] Das A, Dash C, Celik M A, et al. Tris (alkyne) and Bis (alkyne) complexes of coinage metals: synthesis and characterization of (cyclooctyne) 3M+ (M= Cu, Ag) and (cyclooctyne) 2Au+ and coinage metal (M= Cu, Ag, Au) family group trends[J]. Organometallics,2013,32(11):3135-3144. doi: 10.1021/om400073a [124] Ferrara J D, Tessier-Youngs C, Youngs W J. Synthesis and characterization of a copper (I) triflate complex of 1, 2: 5, 6: 9, 10-tribenzocyclododeca-1,5,9-triene-3,7,11-triyne[J]. Organometallics,1987,6(3):676-678. doi: 10.1021/om00146a041 [125] Yu H, Gu L, Chen L, et al. Activation of grapefruit derived biochar by its peel extracts and its performance for tetracycline removal[J]. Bioresource technology,2020,316:123971. doi: 10.1016/j.biortech.2020.123971 [126] Yamil L d O, Georgin J, Franco D S, et al. High-performance removal of 2, 4-dichlorophenoxyacetic acid herbicide in water using activated carbon derived from Queen palm fruit endocarp (Syagrus romanzoffiana)[J]. Journal of Environmental Chemical Engineering,2021,9(1):104911. doi: 10.1016/j.jece.2020.104911 [127] Akpinar I, Drout R J, Islamoglu T, et al. Exploiting π–π interactions to design an efficient sorbent for atrazine removal from water[J]. ACS applied materials & interfaces,2019,11(6):6097-6103. [128] Ahmed I, Jhung S H. Applications of metal-organic frameworks in adsorption/separation processes via hydrogen bonding interactions[J]. Chemical Engineering Journal,2017,310:197-215. doi: 10.1016/j.cej.2016.10.115 [129] Yang Q, Yu S, Jiang D. A modular method of developing an eco-product family considering the reusability and recyclability of customer products[J]. Journal of cleaner production,2014,64:254-265. doi: 10.1016/j.jclepro.2013.07.030 -





 下载:
下载: