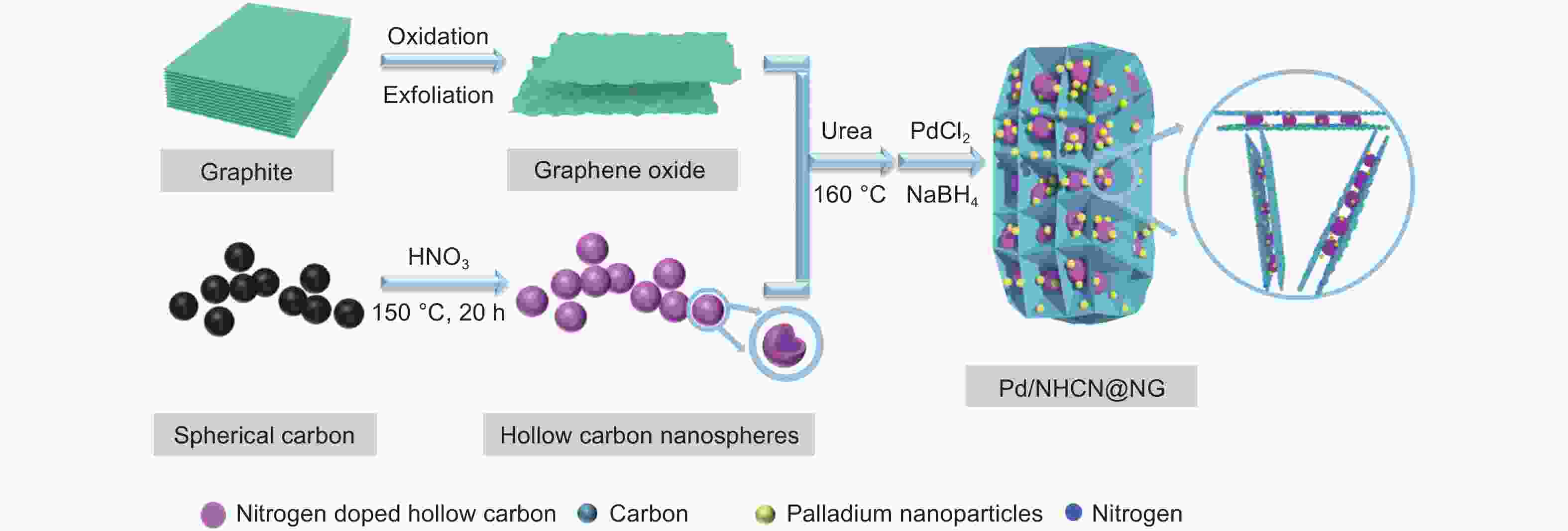
N-doped hollow carbon nanospheres embedded in N-doped graphene loaded with palladium nanoparticles as an efficient electrocatalyst for formic acid oxidation
-
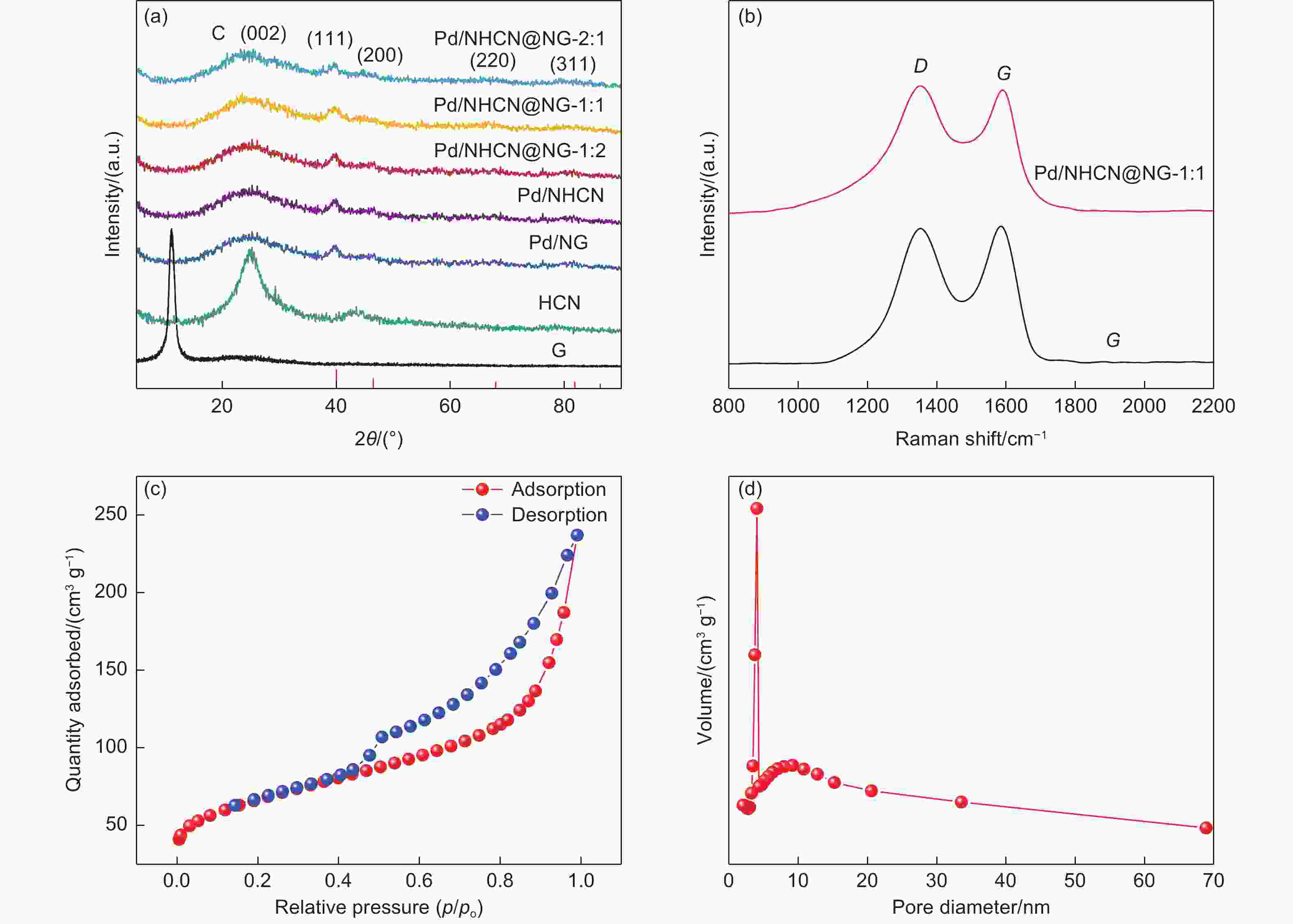
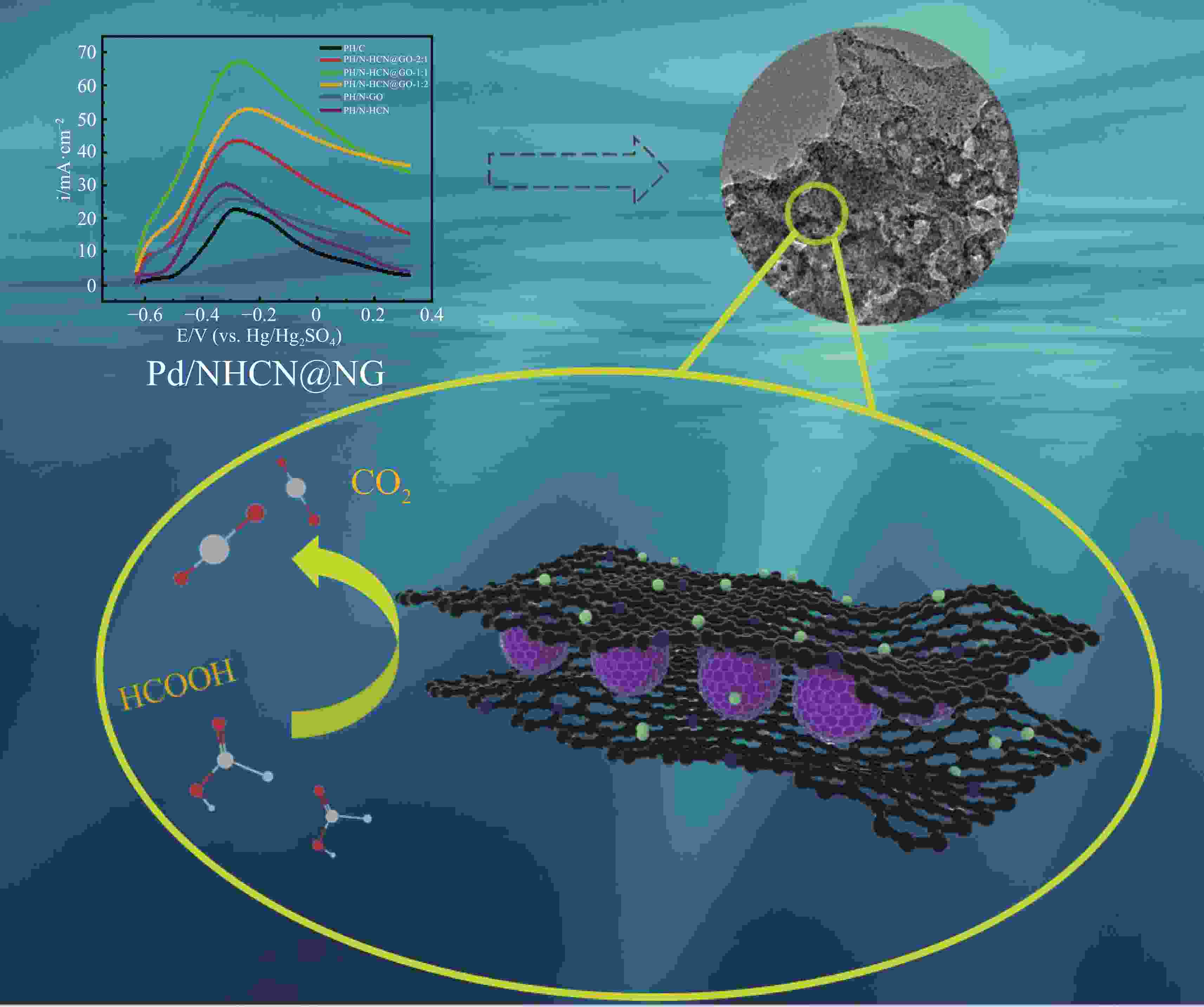
摘要: 低成本、高活性、耐久性好的高效电催化剂对直接甲酸燃料电池的应用起着至关重要的作用。本文采用简单经济的方法,研究了以三维层状多孔结构嵌入氮掺杂石墨烯(NG)的氮掺杂空心碳纳米球(NHCN)负载Pd纳米粒子作为直接甲酸燃料电池催化剂。由于具有独特的氮原子掺杂三维互联层状多孔结构,Pd纳米颗粒尺寸较小的Pd/NHCN@NG催化剂具有较大的催化活性表面积、优越的电催化活性、较高的稳态电流密度和较强的抗CO中毒能力,明显超过传统的Pd/C、Pd/NG和Pd/NHCN催化剂对甲酸电氧化的催化性能。通过优化HCN/GO比,当HCN/GO质量比为1∶1时,Pd/NHCN@NG催化剂对甲酸的催化氧化性能最佳,其活性是Pd/C的4.21倍。本工作开发了一种优越的碳基电催化剂载体材料,为燃料电池的发展带来了广阔的应用前景。
-
关键词:
- 甲酸电氧化 /
- 氮掺杂中空碳纳米球 /
- 氮掺杂石墨烯 /
- 载体材料 /
- 三维互连层状多孔结构
Abstract: Efficient electrocatalysts with a low cost, high activity and good durability play a crucial role in the use of direct formic acid fuel cells. Pd nanoparticles supported on N-doped hollow carbon nanospheres (NHCNs) embedded in an assembly of N-doped graphene (NG) with a three-dimensional (3D) porous structure by a simple and economical method were investigated as direct formic acid fuel cell catalysts. Because of the unique porous configuration of interconnected layers doped with nitrogen atoms, the Pd/NHCN@NG catalyst with Pd nanoparticles has a large catalytic active surface area, superior electrocatalytic activity, a high steady-state current density, and a strong resistance to CO poisoning, far surpassing those of conventional Pd/C, Pd/NG, and Pd/NHCN catalysts for formic acid electrooxidation. When the HCN/GO mass ratio was 1∶1, the Pd/NHCN@NG catalyst had an outstanding performance in the catalytic oxidation of formic acid, with an activity 4.21 times that of Pd/C. This work indicates a way to produce superior carbon-based support materials for electrocatalysts, which will be beneficial for the development of fuel cells. -
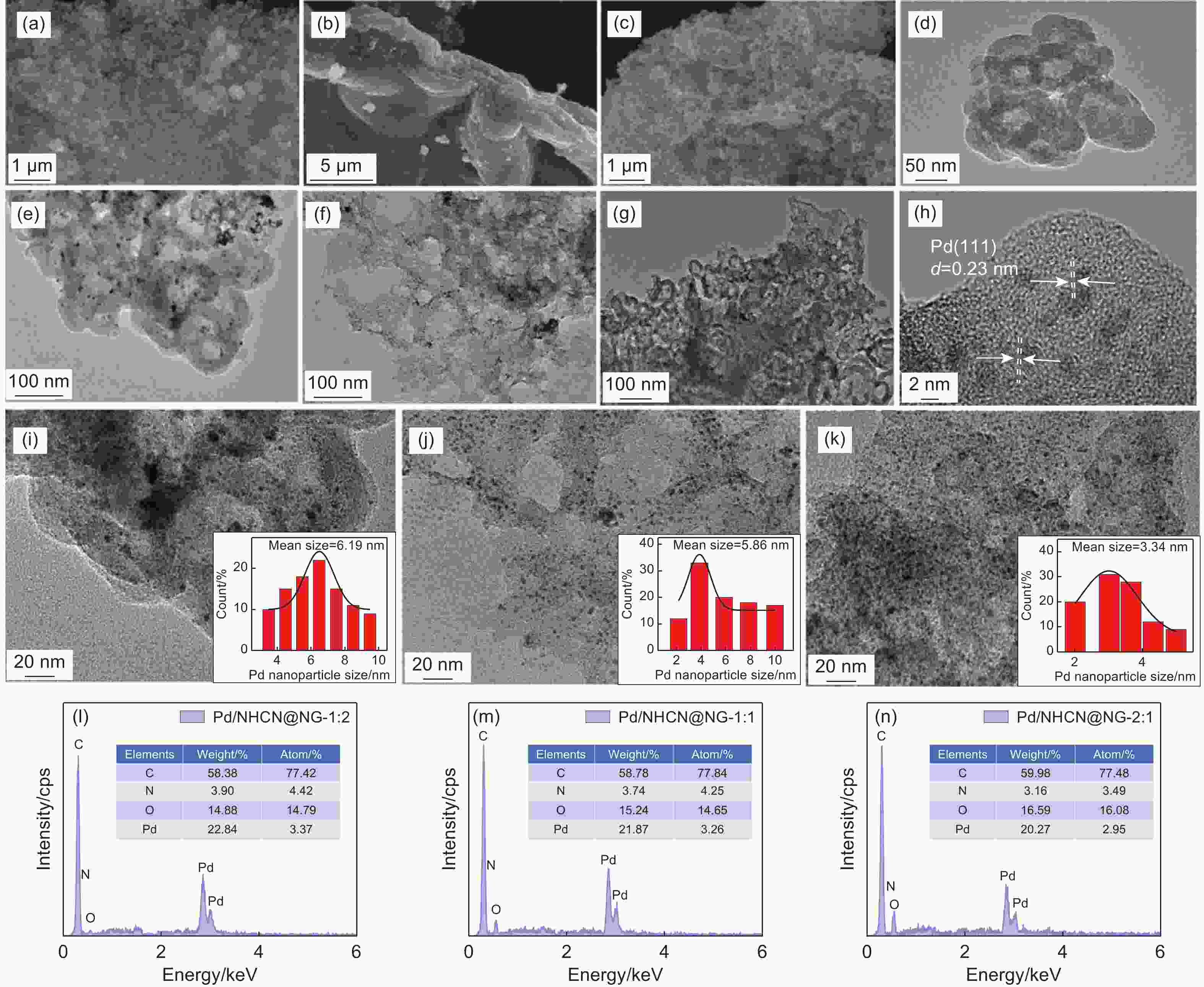
Figure 2. (a-c) SEM and (d-k) TEM images of (d) HCNs, (a, e, i) Pd/NHCN, (b, f, j) Pd/NG, (c, g, k) Pd/NHCN@NG-1:1. (h) HRTEM images Pd/NHCN@NG-1:1. The insets are the particle size distribution of Pd nanoparticles corresponding to the catalysts. EDS spectra of (l) Pd/NHCN@NG-1:2, (m) Pd/NHCN@NG-1:1 and (n) Pd/NHCN@NG-2:1 catalysts
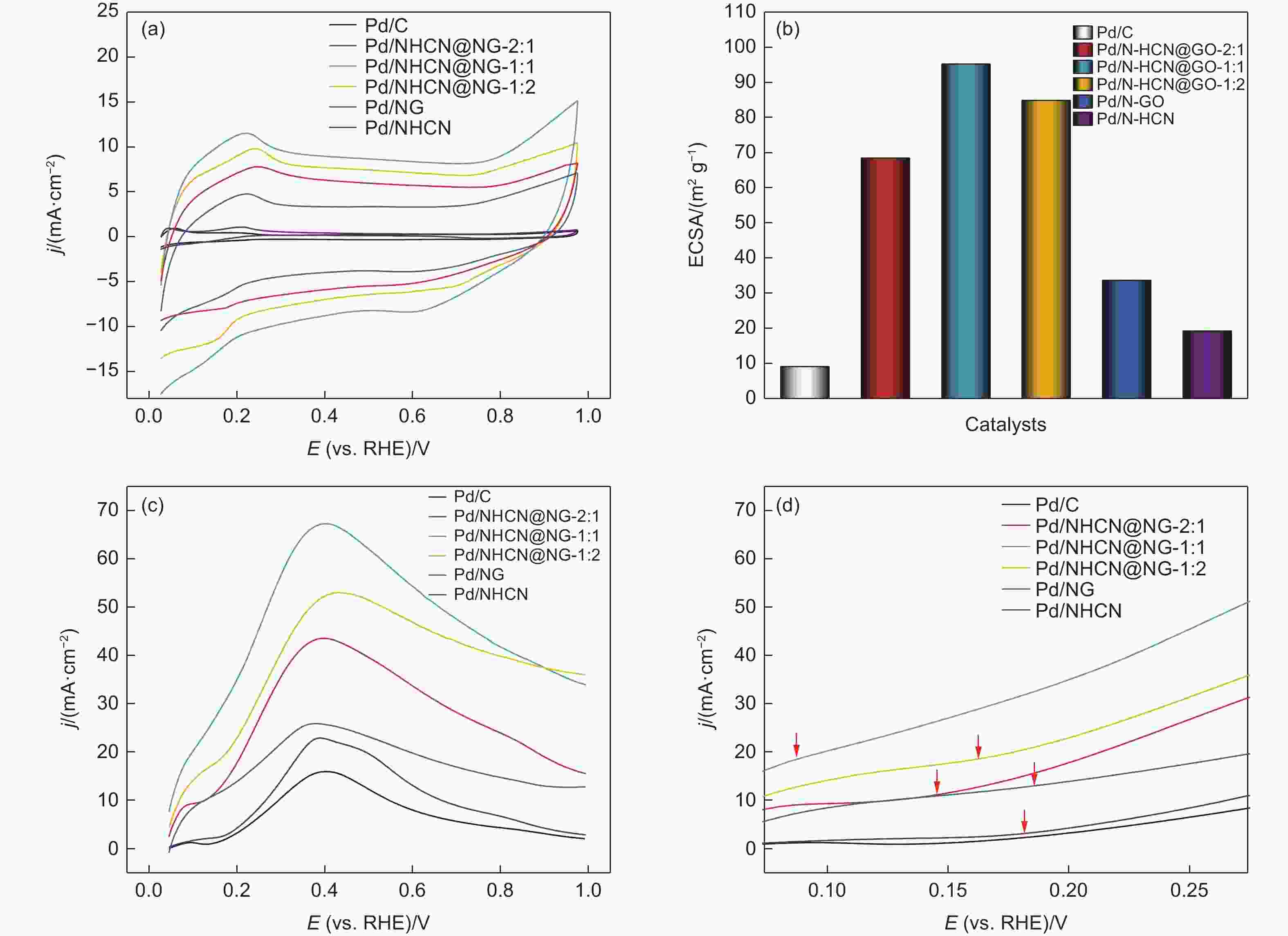
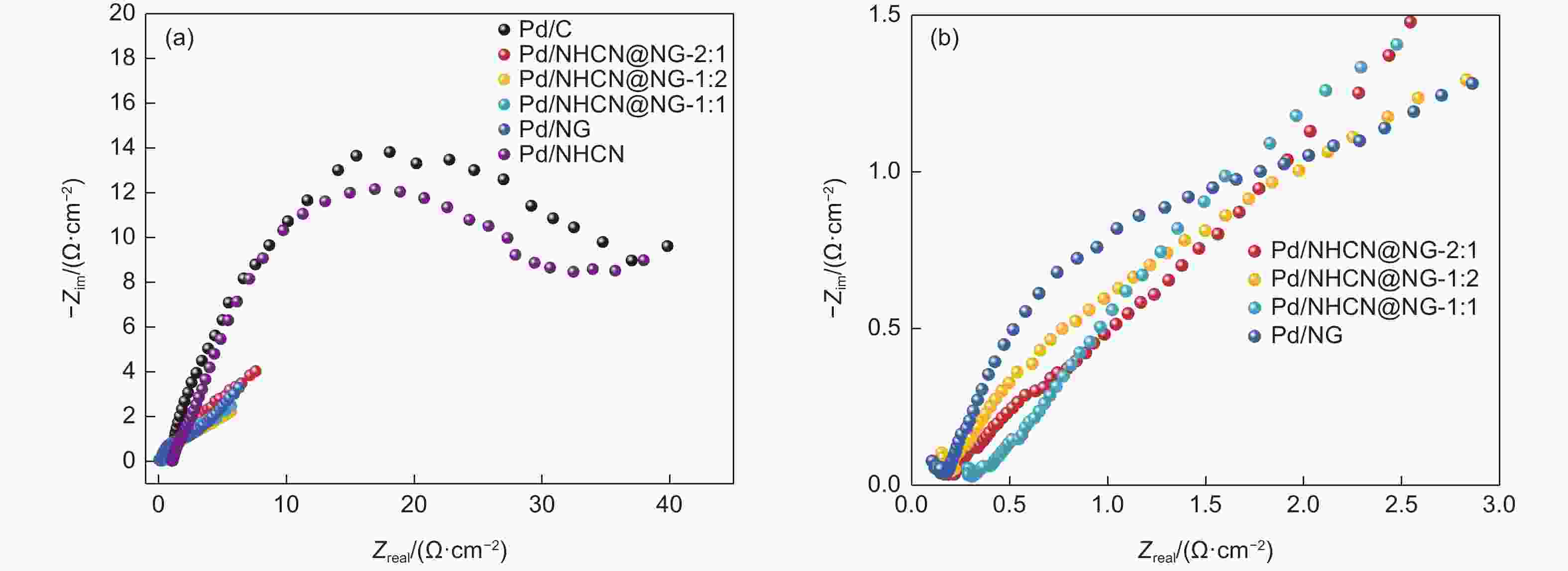
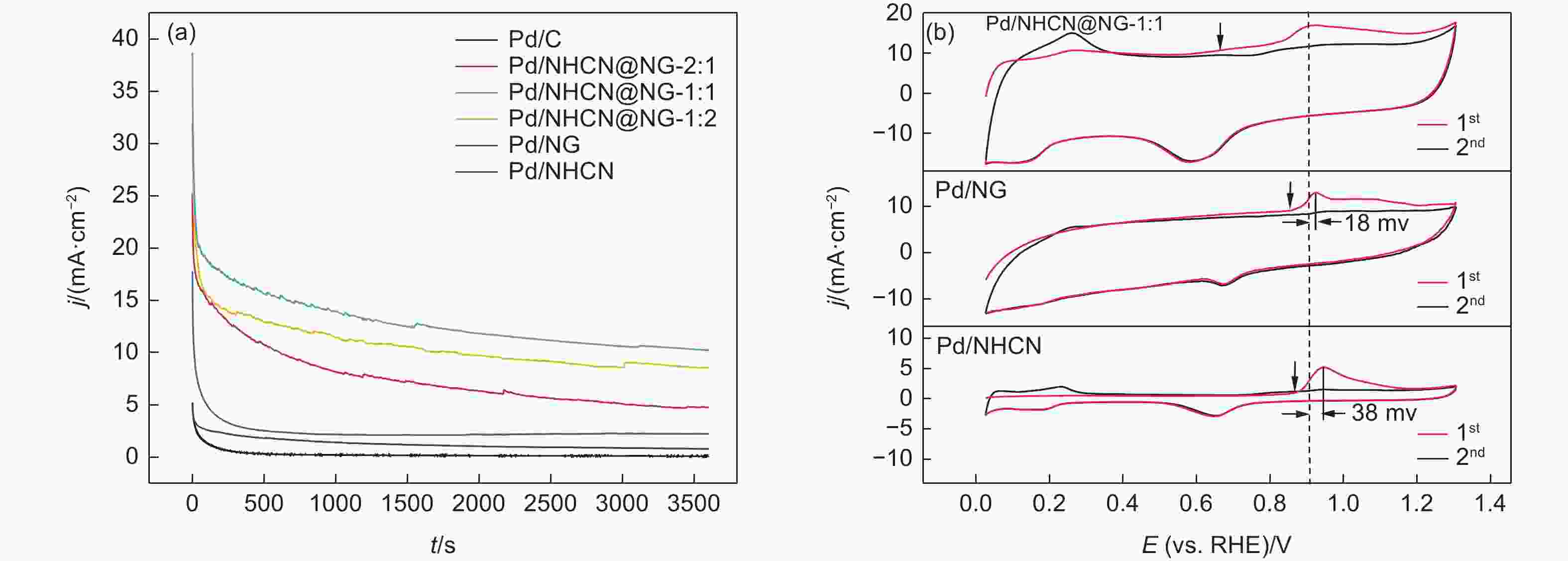
Figure 5. (a) Cyclic voltammetry curves of Pd/NHCN@NG-1:2, Pd/NHCN@NG-1:1, Pd/NHCN@NG-2:1, Pd/NG, Pd/NHCN and Pd/C catalyst in 0.5 mol L−1 H2SO4. (b) Specific ECSA values for different catalysts. (c) Linear sweep voltammetry curves in the 0.5 mol L−1 H2SO4 + 0.5 mol L−1 HCOOH solution with a scan rate of 50 mV s−1. (d) LSV amplified parts before 0.275 V.
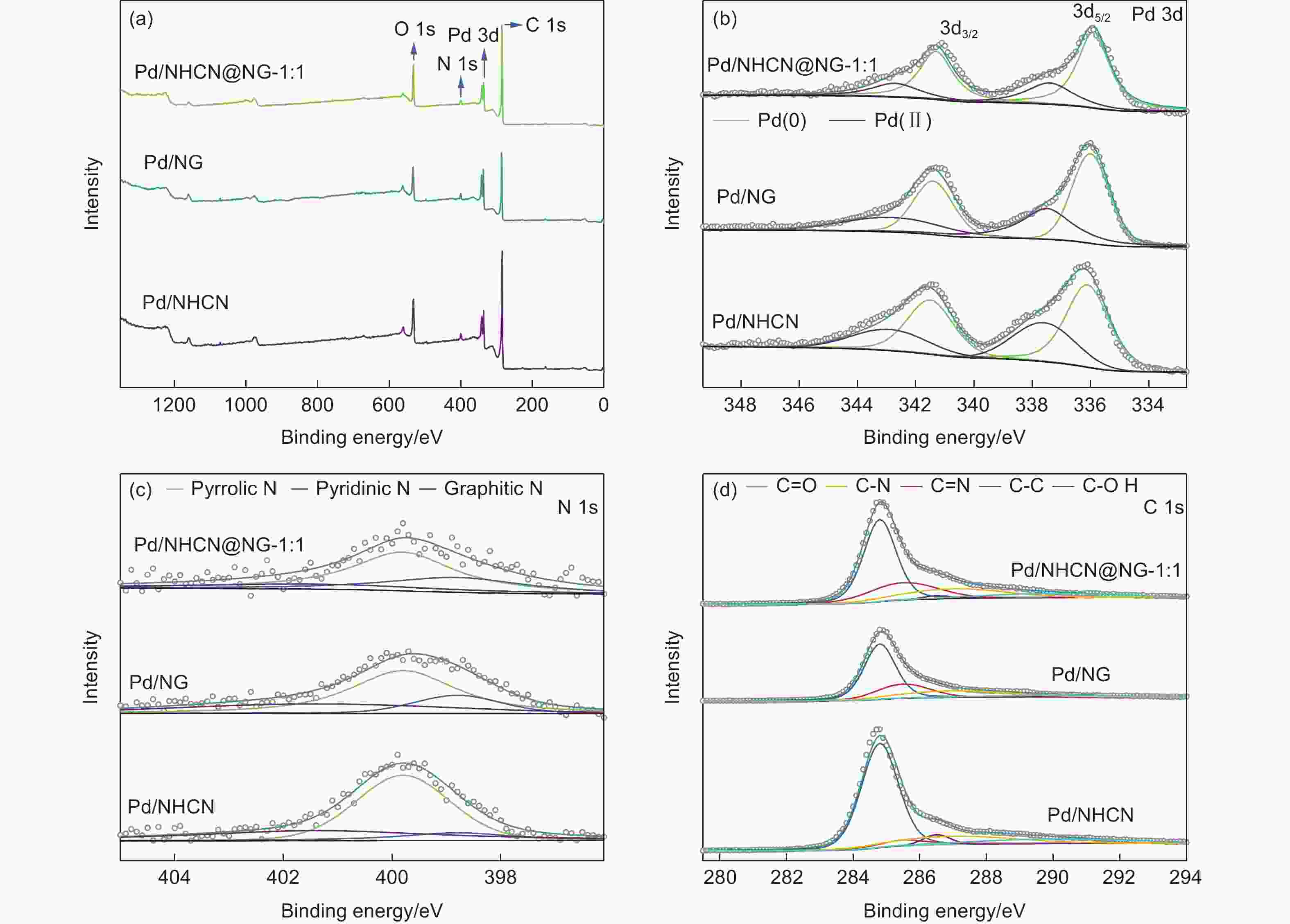
Table 1. Results of the fits of the Pd3d spectra for Pd/NHCN, Pd/NG and Pd/NHCN@NG-1:1 catalysts
Catalyst Binding energy/eV species Relative ratio/% Pd/NHCN 336.1 Pd(0) 38.39 337.6 Pd(Ⅱ) 21.61 341.5 Pd(0) 25.59 342.9 Pd(Ⅱ) 14.41 Pd/NG 336.0 Pd(0) 36.42 337.5 Pd(Ⅱ) 23.58 341.4 Pd(0) 24.28 342.8 Pd(Ⅱ) 15.72 Pd/NHCN@NG-1:1 335.9 Pd(0) 42.55 337.4 Pd(Ⅱ) 17.45 341.3 Pd(0) 28.37 342.7 Pd(Ⅱ) 11.63 Table 2. Results of the fits of the N 1s spectra for Pd/NHCN, Pd/NG and Pd/NHCN@NG-1:1 catalyst
Catalyst Binding
energy/eVSpecies Relative ratio/% Pd/NHCN 398.8 Pyridinic N 11.31 399.8 Pyrrolic N 56.44 401.4 Graphitic N 32.25 Pd/NG 398.8 Pyridinic N 16.51 399.8 Pyrrolic N 58.59 401.4 Graphitic N 24.89 Pd/NHCN@NG-1:1 398.8 Pyridinic N 26.92 399.8 Pyrrolic N 54.73 401.4 Graphitic N 18.35 Table 3. Results of the fits of the C 1s spectra for Pd/NHCN, Pd/NG and Pd/NHCN@NG-1:1 catalysts
Catalyst C―C/%
284.8 eVC=N/%
285.5 eVC―OH/%
286.5 eVC―N/%
287.0 eVC=O/%
289.9 eVPd/NHCN 63.73 6.41 4.12 15.36 10.38 Pd/NG 53.04 20.32 0.34 18.69 7.61 Pd/NHCN@
NG-1:151.68 20.36 14.80 17.15 9.31 -
[1] Safdar H S K, Saleem J, Mudassir A A M, et al. Recent advances in anode electrocatalysts for direct formic acid fuel cells part fundamentals and Pd based catalysts[J]. Chemical Record,2022,22(7):e202200045. doi: 10.1002/tcr.202200045 [2] Caglar A, Ulas B, Cogenli M S, et al. Synthesis and characterization of Co, Zn, Mn, V modified Pd formic acid fuel cell anode catalysts[J]. Journal of Electroanalytical Chemistry,2019,850:113402-113411. doi: 10.1016/j.jelechem.2019.113402 [3] Qu W L, Wang Z B, Gao Y, et al. WO3/C supported Pd catalysts for formic acid electro-oxidation activity[J]. International Journal of Hydrogen Energy,2018,43(1):407-416. doi: 10.1016/j.ijhydene.2017.11.046 [4] Zhang W, Huang H, Li F, et al. Palladium nanoparticles supported on graphitic carbon nitride-modified reduced GO as highly efficient catalysts for formic acid and methanol electrooxidation[J]. Journal of Materials Chemistry A,2014,2(44):19084-19094. doi: 10.1039/C4TA03326D [5] Shu Q Z, Xia Z Z, Wei W, et al. A novel gas diffusion layer and its application to direct methanol fuel cells[J]. New Carbon Materials,2021,36(2):409-419. doi: 10.1016/S1872-5805(21)60017-3 [6] Qin Y H, Li Y, Lam T, et al. Nitrogen-doped carbon-TiO2 composite as support of Pd electrocatalyst for formic acid oxidation[J]. Journal of Power Sources,2015,284:186-193. doi: 10.1016/j.jpowsour.2015.03.040 [7] Jang H D, Kim S K, Chang H, et al. Three-dimensional crumpled graphene-based platinum-gold alloy nanoparticle composites as superior electrocatalysts for direct methanol fuel cells[J]. Carbon,2015,93:869-877. doi: 10.1016/j.carbon.2015.06.009 [8] Mazurkiewicz-Pawlicka M, Malolepszy A, Mikolajczuk -Zychora A, et al. A simple method for enhancing the catalytic activity of Pd deposited on carbon nanotubes used in direct formic acid fuel cells[J]. Applied Surface Science,2019,476:806-814. doi: 10.1016/j.apsusc.2019.01.114 [9] Bieloshapka I, Jiricek P, Vorokhta M, et al. Pd-catalysts for DFAFC prepared by magnetron sputtering[J]. Applied Surface Science,2017,419:838-846. doi: 10.1016/j.apsusc.2017.05.035 [10] Zhang R L, Duan J J, Han Z, et al. One-step aqueous synthesis of hierarchically multi-branched PdRuCu nanoassemblies with highly boosted catalytic activity for ethanol and ethylene glycol oxidation reactions[J]. Applied Surface Science,2020,506:144791. doi: 10.1016/j.apsusc.2019.144791 [11] Yazdan-Abad M Z, Alfi N, Farsadrooh M, et al. Deposition of palladium-copper nanostructure on reduced GO by a simple method toward formic acid oxidation[J]. Journal of Electroanalytical Chemistry,2019,848:113299-113321. doi: 10.1016/j.jelechem.2019.113299 [12] Ren J, Zhang J, Yang C, et al. Pd nanocrystals anchored on 3D hybrid architectures constructed from nitrogen-doped graphene and low-defect carbon nanotube as high-performance multifunctional electrocatalysts for formic acid and methanol oxidation[J]. Materials Today Energy,2020,16:100409-100417. doi: 10.1016/j.mtener.2020.100409 [13] Yan H, Jiao Y, Wu A, et al. Synergism of molybdenum nitride and palladium for high-efficiency formic acid electrooxidation[J]. Journal of Materials Chemistry A,2018,6(17):7623-7630. doi: 10.1039/C8TA02488J [14] Yang N, Zhang Z, Chen B, et al. Synthesis of ultrathin Pd Cu alloy nanosheets used as a highly efficient electrocatalyst for formic acid oxidation[J]. Advanced Materials,2017,29(29):1700769-1700775. doi: 10.1002/adma.201700769 [15] Zhao L, Wang Z B, Li J L, et al. A newly-designed sandwich-structured graphene-Pt-graphene catalyst with improved electrocatalytic performance for fuel cells[J]. Journal of Materials Chemistry A,2015,3(10):5313-5320. doi: 10.1039/C4TA06172A [16] Wang F, Xue H, Tian Z, et al. Fe2P as a novel efficient catalyst promoter in Pd/C system for formic acid electro-oxidation in fuel cells reaction[J]. Journal of Power Sources,2018,375:37-42. doi: 10.1016/j.jpowsour.2017.11.055 [17] Cao J, Song L, Tang J, et al. Enhanced activity of Pd nanoparticles supported on Vulcan XC72R carbon pretreated via a modified Hummers method for formic acid electrooxidation[J]. Applied Surface Science,2013,274:138-143. doi: 10.1016/j.apsusc.2013.02.133 [18] Shi L, Li Ya , Yin H, et al. Carbon-based metal-free nanomaterials for the electrosynthesis of small-molecule chemicals: A review[J]. New Carbon Materials,2024,39(1):42-63. [19] Qian H, Huang H, Wang X. Design and synthesis of palladium/graphitic carbon nitride/carbon black hybrids as high-performance catalysts for formic acid and methanol electrooxidation[J]. Journal of Power Sources,2015,275:734-741. doi: 10.1016/j.jpowsour.2014.10.109 [20] Zhu X R, Zhang X H, Li Y F, et al. Exploring transition metal oxide-based oxygen vacancy supercapacitors: A review[J]. Journal of Energy Storage,2024,80:110350. [21] Chen Y X, Zhao X H, Dong P, er al. Carbon-based electrocatalysts for water splitting at high-current-densities: A review[J]. New Carbon Materials,2024,39(1):1-16. [22] Tang X, Zeng Y, Cao L, et al. Anchoring ultrafine Pt nanoparticles on the 3D hierarchical self-assembly of graphene/functionalized carbon black as a highly efficient oxygen reduction catalyst for PEMFCs[J]. Journal of Materials Chemistry A,2018,6(31):15074-15082. doi: 10.1039/C8TA02453G [23] Zhou Y, Hu X C, Fan Q, et al. Three-dimensional crumpled graphene as an electro-catalyst support for formic acid electro-oxidation[J]. Journal of Materials Chemistry A,2016,4(12):4587-4591. doi: 10.1039/C5TA09956K [24] Yan M, Jiang Q, Zhang T, et al. Three-dimensional low-defect carbon nanotube/nitrogen-doped graphene hybrid aerogel-supported Pt nanoparticles as efficient electrocatalysts toward the methanol oxidation reaction[J]. Journal of Materials Chemistry A,2018,6(37):18165-18172. doi: 10.1039/C8TA05124K [25] Gao Z, Li M, Wang J, et al. Pt nanocrystals grown on three-dimensional architectures made from graphene and MoS2 nanosheets: Highly efficient multifunctional electrocatalysts toward hydrogen evolution and methanol oxidation reactions[J]. Carbon,2018,139:369-377. doi: 10.1016/j.carbon.2018.07.006 [26] Wang Z, Qiang H, Zhu Z, et al. Facile synthesis of nitrogen-doped mesoporous HCNs for high-performance supercapacitors[J]. ChemElectroChem,2018,5(16):2242-2249. doi: 10.1002/celc.201800597 [27] He D, Cheng K, Peng T, et al. Graphene/carbon nanospheres sandwich supported PEM fuel cell metal nanocatalysts with remarkably high activity and stability[J]. J Mater Chem A,2013,1(6):2126-2132. doi: 10.1039/C2TA00606E [28] Zhang J, Song L, Zhao C, et al. Co, N co-doped porous carbons as high-performance oxygen reduction electrocatalysts[J]. New Carbon Materials,2021,36(1):209-218. doi: 10.1016/S1872-5805(21)60016-1 [29] Wu Z L, Wang C W, Zhang X X, et al. Graphene-based CO2 reduction electrocatalysts: A review. A review[J]. New Carbon Materials,2024,39(1):100-130. [30] Shan J, Zeng T, Wu W, et al. Enhancement of the performance of Pd nanoclusters confined within ultrathin silica layers for formic acid oxidation[J]. Nanoscale,2020,12(24):12891-12897. doi: 10.1039/D0NR00307G [31] Esrafili M D, Mousavian P. Catalytic role of graphitic nitrogen atoms in the CO oxidation reaction over N -containing graphene: a first-principles mechanistic evaluation[J]. New Journal of Chemistry,2021,45(31):13822-13832. doi: 10.1039/D1NJ01867A [32] Shu C, Song B, Wei X, et al. Mesoporous 3D nitrogen-doped yolk-shelled carbon spheres for direct methanol fuel cells with polymer fiber membranes[J]. Carbon,2018,129:613-620. doi: 10.1016/j.carbon.2017.12.049 [33] Marcano D C, Kosynkin D V, Berlin J M, et al. Improved synthesis of GO[J]. ACS Nano,2010,4(8):4806-4814. doi: 10.1021/nn1006368 [34] Shi Q, Zhou Y, Cheng J, et al. Turning carbon black into HCNs to encapsulate Fe2O3 as high-performance lithium-ion batteries anode[J]. Microporous and Mesoporous Materials,2022,332:111681-111691. doi: 10.1016/j.micromeso.2022.111681 [35] Li S, Shu J, Ma S, et al. Engineering three-dimensional nitrogen-doped carbon black embedding nitrogen-doped graphene anchoring ultrafine surface-clean Pd nanoparticles as efficient ethanol oxidation electrocatalyst[J]. Applied Catalysis B:Environmental,2021,280:119464-119473. doi: 10.1016/j.apcatb.2020.119464 [36] A. Song, L. Cao, W. Yang. Shao, et al. In situ construction of nitrogen-doped graphene with surface-grown carbon nanotubes as a multifactorial synergistic catalyst for oxygen reduction[J]. Carbon,2019,142:40-50. doi: 10.1016/j.carbon.2018.09.088 [37] Zhang Z, Schniepp H C, Adamson D H. Characterization of GO: Variations in reported approaches[J]. Carbon,2019,154:510-521. doi: 10.1016/j.carbon.2019.07.103 [38] Shen B, Huang H, Liu H, et al. Bottom-up construction of three-dimensional porous MXene/nitrogen-doped graphene architectures as efficient hydrogen evolution electrocatalysts[J]. International Journal of Hydrogen Energy,2021,46(58):29984-29993. doi: 10.1016/j.ijhydene.2021.06.157 [39] Zhao L, Wang Z B, Li J L, et al. Hybrid of carbon-supported Pt nanoparticles and three dimensional graphene aerogel as high stable electrocatalyst for methanol electrooxidation[J]. Electrochimica Acta,2016,189:175-183. doi: 10.1016/j.electacta.2015.12.072 [40] Liu M, Peng C, Yang W, et al. Pd nanoparticles supported on three-dimensional graphene aerogels as highly efficient catalysts for methanol electrooxidation[J]. Electrochimica Acta,2015,178:838-846. doi: 10.1016/j.electacta.2015.08.063 [41] Yang Y, Huang H, Yang C, et al. Ultrafine rh-decorated 3D porous noron and nitrogen dual-doped graphene architecture as an efficient electrocatalyst for methanol oxidation reaction[J]. ACS Applied Energy Materials,2021,4(1):376-383. doi: 10.1021/acsaem.0c02293 [42] Zhang W, Yao Q, Wu X, et al. Intimately coupled hybrid of graphitic carbon nitride nanoflakelets with reduced GO for supporting Pd nanoparticles: A stable nanocatalyst with high catalytic activity towards formic acid and methanol electrooxidation[J]. Electrochimica Acta,2016,200:131-141. doi: 10.1016/j.electacta.2016.03.169 [43] Wei Y, Zhang X, Luo Z, et al. Nitrogen-doped carbon nanotube-supported Pd catalyst for improved electrocatalytic performance toward ethanol electrooxidation[J]. Nano-Micro Letters,2017,9(3):1-9. [44] Chang J, Sun X, Feng L, et al. Effect of nitrogen-doped acetylene carbon black supported Pd nanocatalyst on formic acid electrooxidation[J]. Journal of Power Sources,2013,239:94-102. doi: 10.1016/j.jpowsour.2013.03.066 [45] Dong Q, Wu M, Mei D, et al. Multifunctional Pd-Sn electrocatalysts enabled by in situ formed SnOx and TiC triple junctions[J]. Nano Energy,2018,53:940-948. doi: 10.1016/j.nanoen.2018.08.060 [46] Dong Q, Huang M, Guo C, et al. Functionalized titanium carbide as novel catalyst support for Pd catalyzed electrochemical reaction[J]. International Journal of Hydrogen Energy,2017,42(5):3206-3214. doi: 10.1016/j.ijhydene.2016.09.217 -





 下载:
下载: