Carbon electrodes for the electrocatalytic synthesis of hydrogen peroxide: A review
-
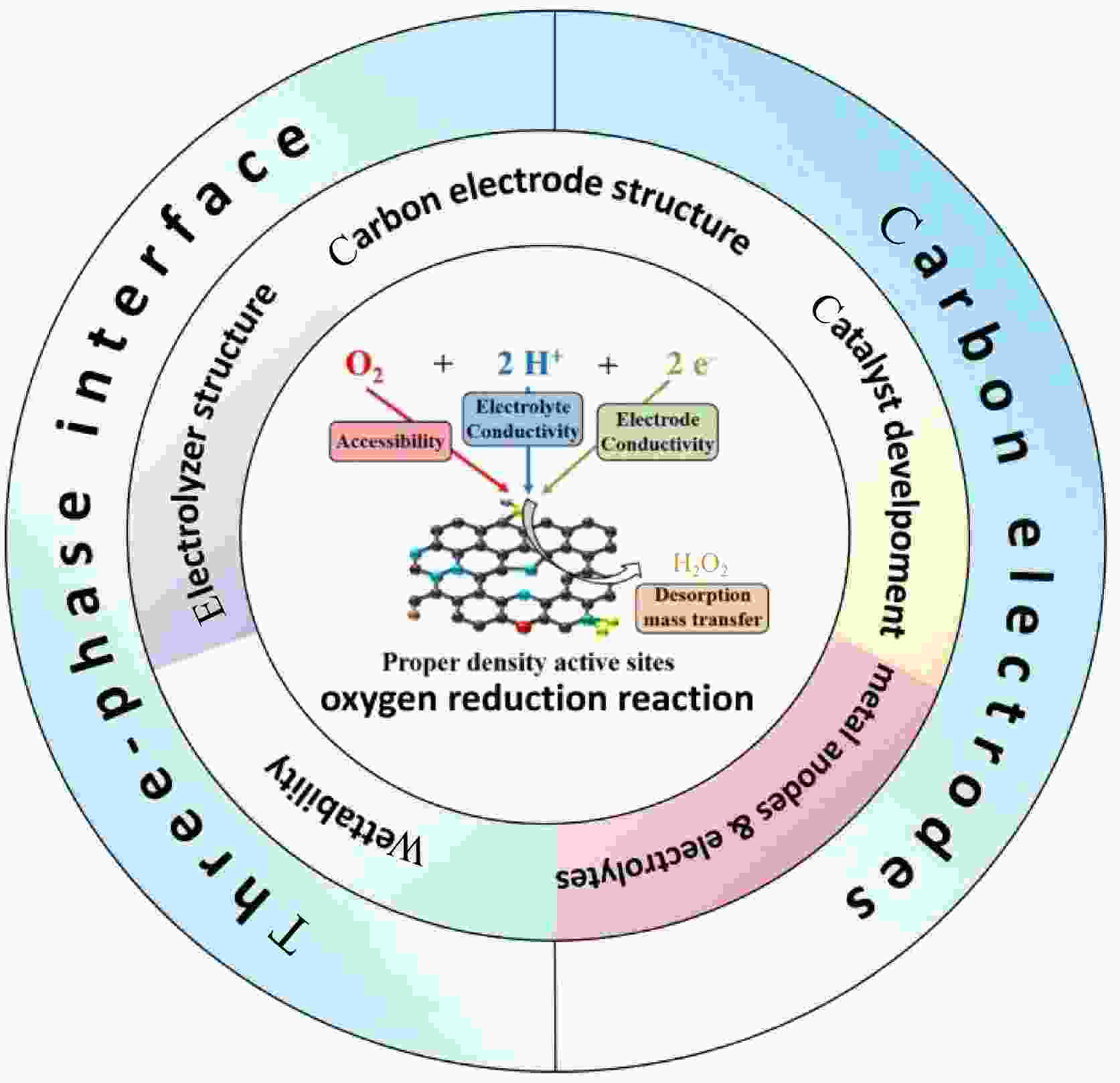
摘要: 通过双电子(2e−)途径的电催化氧还原方式能够即时合成过氧化氢(H2O2),远超传统的蒽醌工艺。近年来,碳电极因具有良好的催化效果和优越的稳定性在电催化合成H2O2方面受到越来越多的关注。本综述结合材料改性与润湿性调整,从三相界面的角度考虑与H2O2合成速率及使用寿命的关系。介绍了碳电极的结构与电催化合成H2O2的原理,包括单质炭材料、无金属催化剂、贵金属催化剂与非贵金属催化剂4种主流催化剂;金属阳极与电解液对于三相界面的影响;碳电极润湿性与三相界面的关系,指出侧重于提高2e−途径选择性的改性方式也会对电极润湿性造成影响。此外,合理地设计电器原件与提升碳电极合成H2O2功效的关系。最后,讨论了当前碳电极电催化合成H2O2所面临的问题与未来的研究方向。Abstract: Electrocatalytic oxygen reduction by a 2e− pathway enables the instantaneous synthesis of H2O2, a process that is far superior to the conventional anthraquinone process. In recent years, the electrocatalytic synthesis of H2O2 using carbon electrodes has attracted more and more attention because of its excellent catalytic performance and superior stability. The relationship between material modification, wettability and the rate of H2O2 synthesis and service life is considered together with the three-phase interface. The structure of the carbon electrodes and the principles of electrocatalytic H2O2 synthesis are first introduced, and four major catalysts are reviewed, namely, monolithic carbon materials, metal-free catalysts, noble metal catalysts and non-precious metal catalysts. The effects of the metal anode and the electrolyte on the three-phase interface are described. The relationship between carbon electrode wettability and the three-phase interface is described, pointing out that modification focusing on improving the selectivity of the 2e− pathway can also impact electrode wettability. In addition, the relationship between the design of the components in the electrochemical system and their effect on the efficiency of H2O2 synthesis is discussed for carbon electrodes. Finally, we present our analysis of the current problems in the electrocatalytic synthesis of H2O2 for carbon electrodes and future research directions.
-
Key words:
- In situ synthesis /
- H2O2 /
- Carbon electrodes /
- Solid-liquid-gas three-phase interface /
- Wettability
-
Figure 1. Schematic structure of the carbon electrode in the electrochemical synthesis of hydrogen peroxide[15]. Reprinted with permission from Elsevier
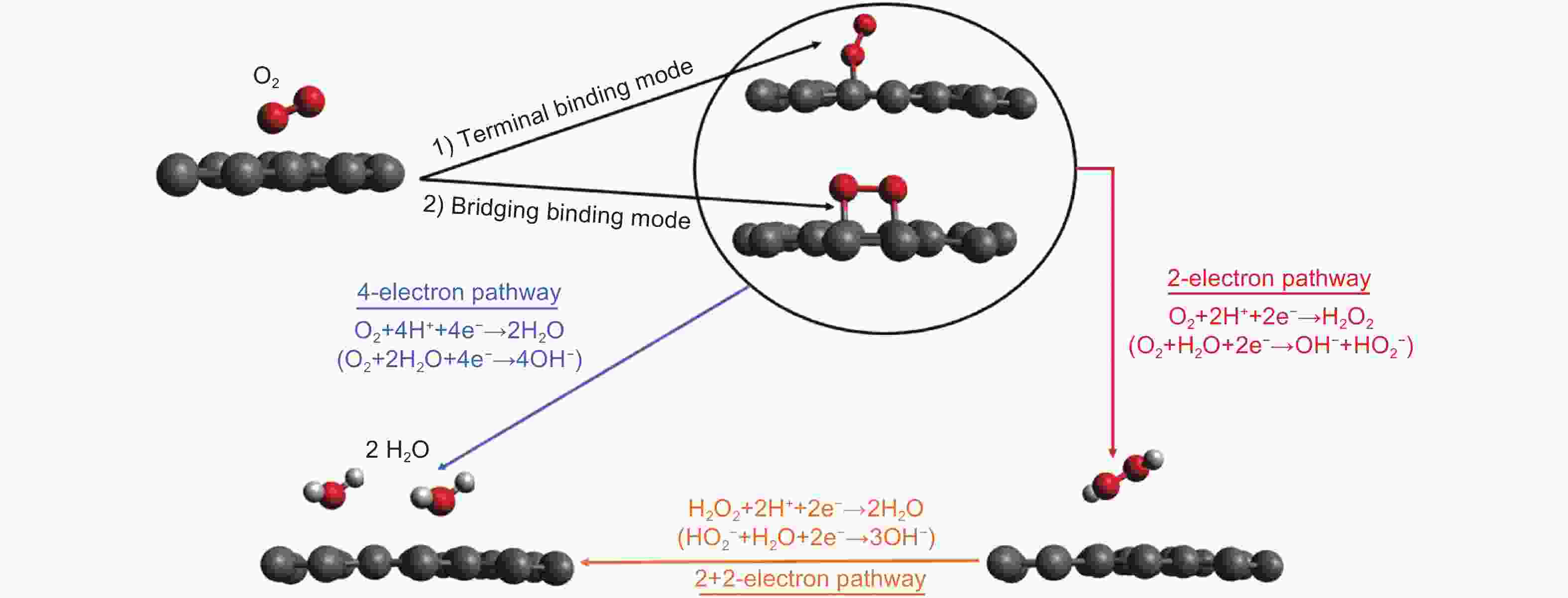
Figure 2. ORR mechanism of oxygen at the air cathode surface in an acidic medium (ORR mechanism at the air cathode surface under alkaline conditions is shown in parentheses)[16]. Reprinted with permission from Elsevier
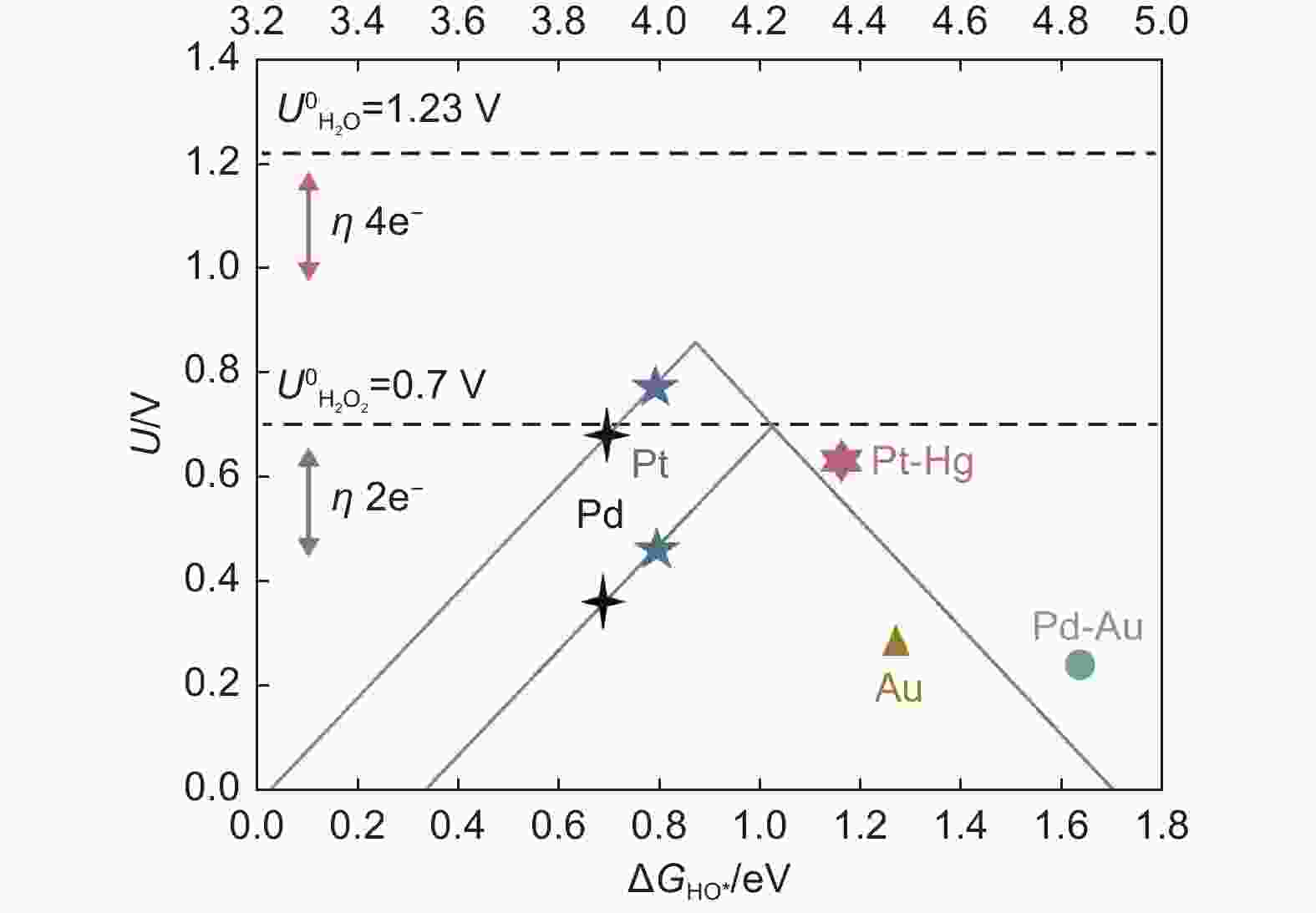
Figure 3. Volcano plot of oxygen reduction activity of different metals as a function of oxygen binding energy(ΔEO)[43]. Reprinted with permission from Elsevier
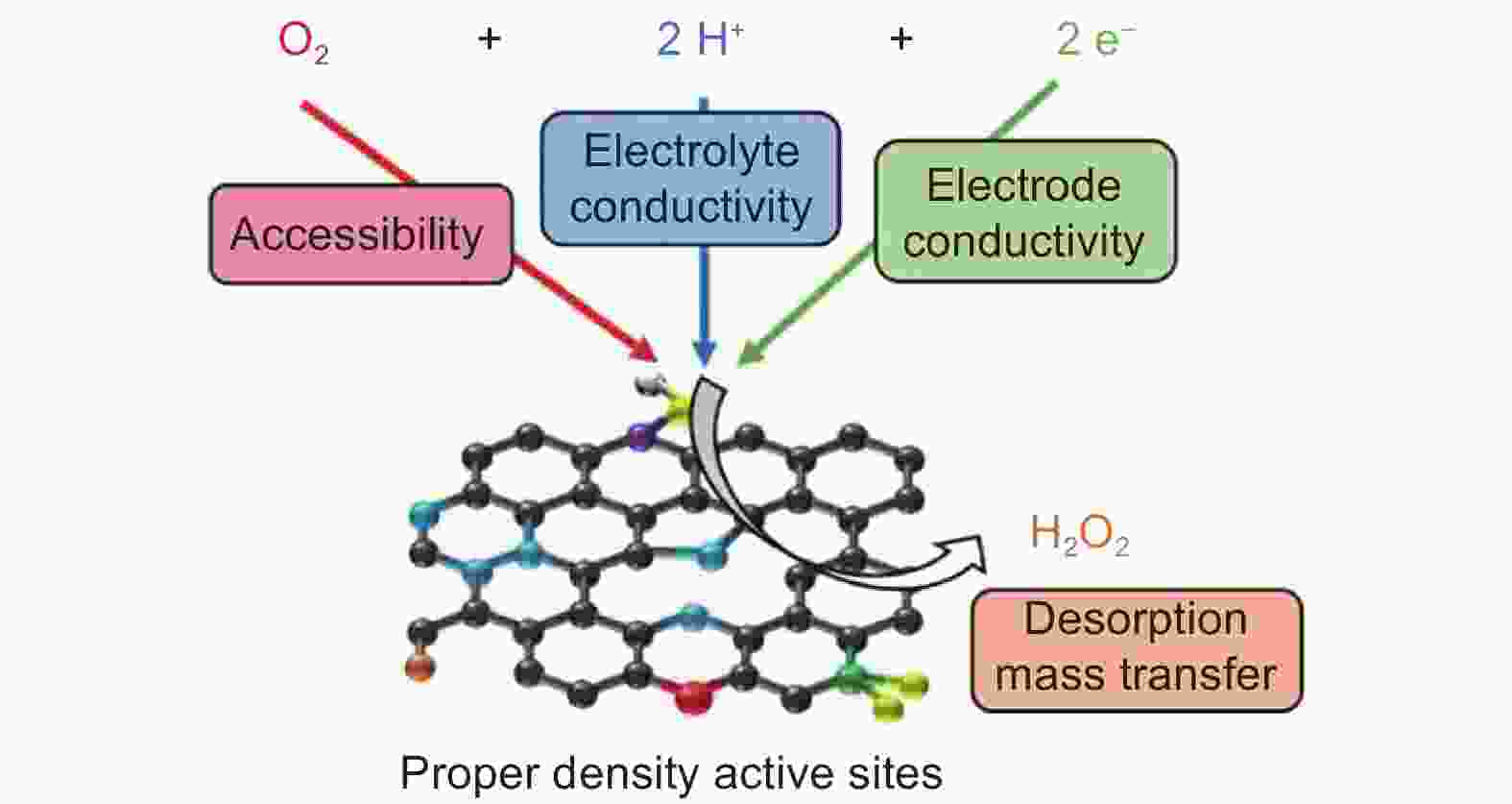
Figure 4. Concept for developing electrocatalysts with appropriate density of active sites: schematic representation of the different parameters controlling the catalytic performance of two-electron ORR catalysts[88]. Reprinted with permission from Elsevier
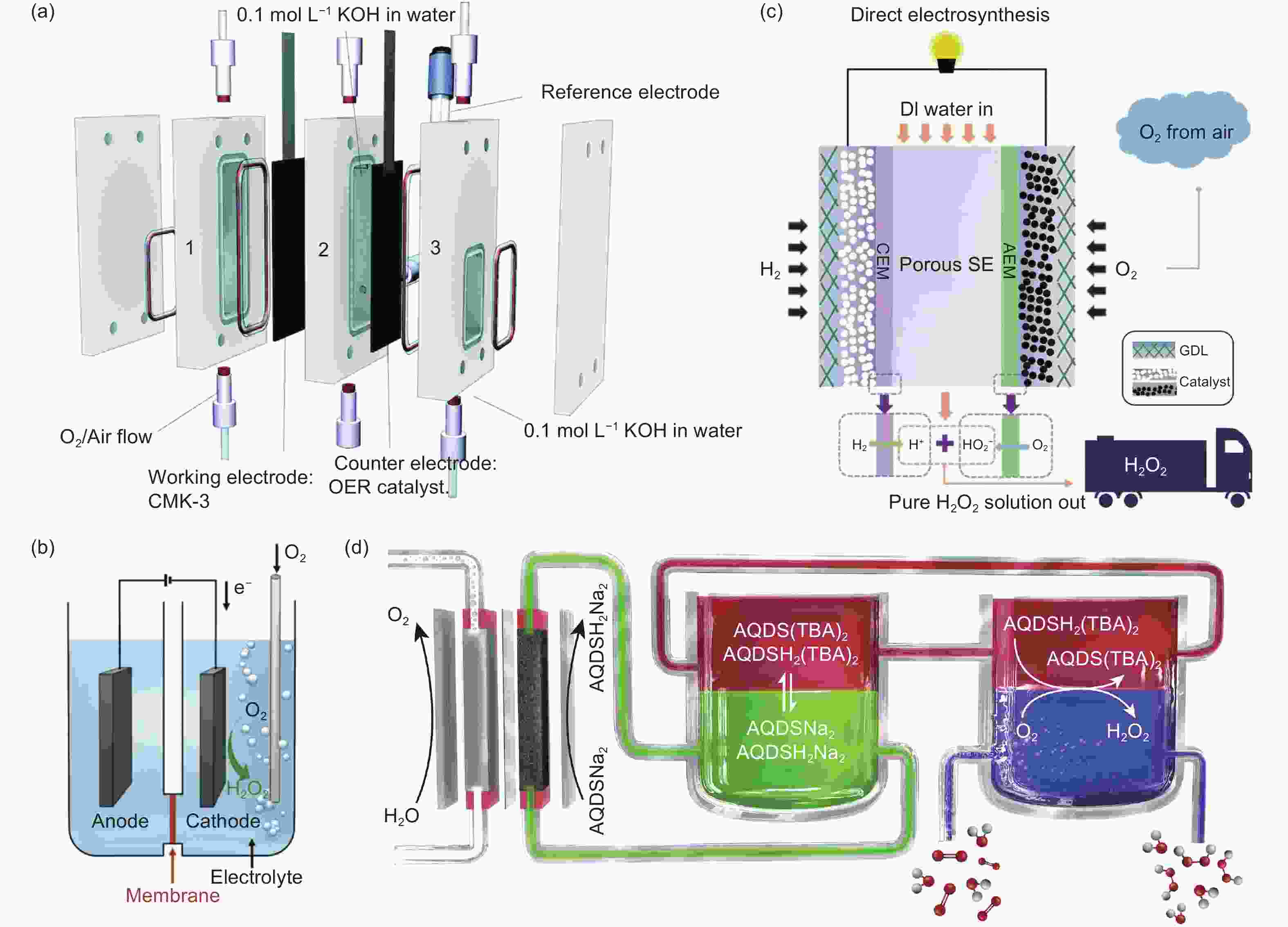
Figure 5. (a) Membrane-free electrolyser[115]. Reprinted with permission from The Royal Society of Chemistry. (b) Conventional polymer electrolyser[116]. Reprinted with permission from American Chemical Society. (c) Double-layer polymer electrolyser[117]. Reprinted with permission from The American Association for the Advancement of Science. (d) Phase transfer device[119]. Reprinted with permission from Elsevier
Table 1. Effect of catalyst engineering on the synthesis of H2O2 from carbon electrodes
Technique Subtrate Electrolytes/pH Wettability H2O2 yield Selectiveness Durability Ref. Monolithic carbon
material1000 °C
1% O2Porous carbon 0.5 mol·L−1 Na2SO4 89.9°→0° 7.9→24.9 mg·L−1 / 20 h [61] 600 °C calcination in air Carbon black 0.1 mol·L−1 Na2SO4 100°→79° 65.3→517.7 mg·L−1 47.1%→56.1% / [62] Coated by electrochemically exfoliated graphene Carbon cloth 0.05 mol·L−1 K2SO4 138.4°→73.2° 251.4→450.8 mg·L−1 / / [63] Metal-free
carbon-based catalystsHeteroatom doping P-CNTs 0.1 mol·L−1 Na2SO4 / 419.5–1291.3 mg·L−1·h−1 88.5% / [64] N-Graphite
(N 60.7%)0.1 mol·L−1 KOH / 1286.9 mmol·g−1·h−1 ~75% / [52] N-CMK3-IL 0.1 mol·L−1 KOH / / 86% / [65] F-mrGO 0.1 mol·L−1 KOH / 430.8 mmol·g−1·h−1 ~100% / [66] Introducing oxygen-containing functional groups Carbon black 0.1 mol·L−1 Na2SO4 57°→45° 83→120 mg·L−1 50% increase [67] O-CNTs 0.1 mol·L−1 KOH ~3950 mg·L−1·h−1 ~90% [68] rGO-KOH 0.1 mol·L−1 KOH / ~100% [69] Precious metal
catalystsPt-Hg 0.1 mol·L−1 HClO4 / / ~90% [70] Au-Pd 0.1 mol·L−1 HClO4 / / ~95% [71] Au-Pd-Ni 0.1 mol·L−1 KOH / 0.0591 mmol·g−1·h−1 / [72] h-Pt1-CuSx 0.1 mol·L−1 HClO4 / ~546 mmo·g−1·h−1 92%–96% / [73] Non-precious
metal catalystsCo-N-C 0.1 mol·L−1 HClO4 / 80–275 mmol·g−1·h−1 ~90% / [74] Ni-N2O2/C 0.1 mol·L−1 KOH / 5.9 mmol·g−1·h−1 96% / [75] Ni-O-C 0.1 mol·L−1 KOH / 59.3 mg·L−1 82% 200 h [76] Co1-NG(O) 0.1 mol·L−1 KOH / ~418 mmol·g−1·h−1 90% / [58] Table 2. Improvement of H2O2 production by wettability modification of carbon electrode
Objective Technique Substrate Detail Wetting angle H2O2 yield Ref. Hydrophilic improvement Functionalization Porous carbon Fabricate honeycomb carbon
nanofibers with abundent OFGs37.7°→ 0° 6 mmol L−1 [106] Physical
methodGraphite sheet Alternating current glow discharge / 35 → 119 µmol L−1 [63] Chemical modification Graphite felt H2SO4, NaNO3, KMnO4 solution treatment followed by 900 °C NH3 activation 120.3° → 61.5° 277 → 479 mg·L−1 [107] Graphite felt 0.5 mol L−1 NaOH treatment followed by NaOH activated at 400 °C 127.3° → 0° 50 → 100 mg·L−1 [108] Electrochemical techniques Graphite felt 0–2 V (vs SCE) cyclical polarization / 85 → 235 mg·L−1 [109] Reticulated vitreous carbon 0–2 V (vs. Ag/AgCl) cyclical / 18 → 64 mg·L−1 [110] Hydrophobic improvement Using hydrophobic auxiliary materials or treatments Graphite felt Loaded with NPC carbonized by ZIF-8 nanocrystals as precursor 112.0° → 125.0° 11 → 113 mg·L−1 [111] CB + graphite + PTFE Decrease PTFE content in CL 107.1° → 141.1° 2131 → 3005 mg·L−1 [112] Graphite felt Modified by CB + PTFE followed by 360 °C calcination 68.4° → 141.0° 25 →
31 mg·cm−2 h−1[93] Constructing amphiphilic interface Constrcting hydrophobic GSL and hydrophilic CL Graphite felt CB + PVDF facilitate hydrophilic; PTFE + 350 °C calcination facilitate hydrophobic CL: 81.2° → 74.4°
GSL: 81.2° → 137.7°3 → 62 mg·L−1 [113] Fabricating Janus electrode Graphite felt PTFE immersion to realize Hydrophobic; galvanostatic anodization to realize hydrophilic CA increased after hydrophobic treatment and decreased after hydrophilic treatment 7 → 50 mg·L−1 [114] -
[1] Gao G, Tian Y, Gong X, et al. Advances in the production technology of hydrogen peroxide[J]. Chinese Journal of Catalysis,2020,41(7):1039-1047. doi: 10.1016/S1872-2067(20)63562-8 [2] Samanta C. Direct synthesis of hydrogen peroxide from hydrogen and oxygen: An overview of recent developments in the process[J]. Applied Catalysis A: General,2008,350(2):133-149. doi: 10.1016/j.apcata.2008.07.043 [3] Kosydar R, Drelinkiewicz A, Ganhy J P. Degradation reactions in anthraquinone process of hydrogen peroxide synthesis[J]. Catalysis Letters,2010,139(3-4):105-113. doi: 10.1007/s10562-010-0413-1 [4] Campos-Martin J M, Blanco-Brieva G, Fierro J L. Hydrogen peroxide synthesis: An outlook beyond the anthraquinone process[J]. Angew Chem Int Ed Engl,2006,45(42):6962-6984. doi: 10.1002/anie.200503779 [5] Robert S, Disselkamp. Convenient storage of concentrated hydrogen peroxide as a CaO2·2H2O2(s)/H2O2(aq) slurry for energy storage applications[J]. Applied Energy,2011,88(11):4214-4217. doi: 10.1016/j.apenergy.2011.02.043 [6] Zhang X, Xia Y, Xia C, et al. Insights into practical-scale electrochemical H2O2 synthesis[J]. Trends in Chemistry,2020,2(10):942-953. doi: 10.1016/j.trechm.2020.07.007 [7] Puértolas B, Hill A K, García T, et al. In-situ synthesis of hydrogen peroxide in tandem with selective oxidation reactions: A mini-review[J]. Catalysis Today,2015,248:115-127. doi: 10.1016/j.cattod.2014.03.054 [8] Yanzheng H, Sisi L, Mengfan W, et al. Deciphering engineering principle of three-phase interface for advanced gas-involved electrochemical reactions[J]. Journal of Energy Chemistry,2023,80(05):302-323. [9] Yan X, Shi W, Wang X. Carbon based electrocatalysts for selective hydrogen peroxide conversion[J]. New Carbon Materials,2022,37(1):223. doi: 10.1016/S1872-5805(22)60582-1 [10] Cai W, Geng J, Zhao S, et al. Simulation of OH– and oxygen transport in the air–cathode catalyst layer of microbial fuel cells[J]. Electrochemistry Communications,2023,151:107494. doi: 10.1016/j.elecom.2023.107494 [11] Cheng S, Liu H, Logan B E. Increased performance of single-chamber microbial fuel cells using an improved cathode structure[J]. Electrochemistry Communications,2006,8(3):489-494. doi: 10.1016/j.elecom.2006.01.010 [12] Ge L, Rabiee H, Li M, et al. Electrochemical CO2 reduction in membrane-electrode assemblies[J]. Chem,2022,8(3):663-692. doi: 10.1016/j.chempr.2021.12.002 [13] Burdyny T, Smith W A. CO2 reduction on gas-diffusion electrodes and why catalytic performance must be assessed at commercially-relevant conditions[J]. Energy & Environmental Science,2019,12(5):1442-1453. [14] Weng L, A T Bell, A Z Weber. Modeling gas-diffusion electrodes for CO2 reduction[J]. Physical Chemistry Chemical Physics: PCCP,2018,20(25):16973-16984. doi: 10.1039/C8CP01319E [15] Li M, Zhu Z, Yuan S, et al. Nitrogen and oxygen co-doped graphite felt gas diffusion electrodes for efficient hydrogen peroxide electrosynthesis[J]. Molecular Catalysis,2023,541:113076. doi: 10.1016/j.mcat.2023.113076 [16] Dessalle A, Quílez-Bermejo J, Fierro V, et al. Recent progress in the development of efficient biomass-based ORR electrocatalysts[J]. Carbon,2023,203:237-260. doi: 10.1016/j.carbon.2022.11.073 [17] Peng W, Tan H, Liu X, et al. Perspectives on carbon-based catalysts for the two-electron oxygen reduction reaction for electrochemical synthesis of hydrogen peroxide: A minireview[J]. Energy & Fuels,2023,37(23):17863-17874. [18] Zhang W, Li J, Wei Z. Carbon-based catalysts of the oxygen reduction reaction: Mechanistic understanding and porous structures[J]. Chinese Journal of Catalysis,2023,48:15-31. doi: 10.1016/S1872-2067(23)64427-4 [19] Shi L, Li Ya , Yin H, et al. Carbon-based metal-free nanomaterials for the electrosynthesis of small-molecule chemicals: A review[J]. New Carbon Materials,2024,39(1):42-63. [20] Arici E, Kaplan B Y, Mert A M, et al. An effective electrocatalyst based on platinum nanoparticles supported with graphene nanoplatelets and carbon black hybrid for PEM fuel cells[J]. International Journal of Hydrogen Energy,2019,44(27):14175-14183. doi: 10.1016/j.ijhydene.2018.11.210 [21] Ren Y, Li B, Lv C, et al. Stabilizing platinum-based electrocatalysts for oxygen reduction reaction in acid media: A mini review[J]. International Journal of Hydrogen Energy,2024,51:1-15. [22] Wikander K, Ekström H, Palmqvist A E C, et al. Alternative catalysts and carbon support material for PEMFC[J]. Fuel Cells,2006,6(1):21-25. doi: 10.1002/fuce.200500092 [23] Işıkel Şanlı L, Bayram V, Ghobadi S, et al. Engineered catalyst layer design with graphene-carbon black hybrid supports for enhanced platinum utilization in PEM fuel cell[J]. International Journal of Hydrogen Energy,2017,42(2):1085-1092. doi: 10.1016/j.ijhydene.2016.08.210 [24] Li S, Feng C, Xie Y, et al. Synthesis of nitrogen-rich porous carbon nanotubes coated Co nanomaterials as efficient ORR electrocatalysts via MOFs as precursor[J]. Journal of Alloys and Compounds,2022,911:165060. doi: 10.1016/j.jallcom.2022.165060 [25] Lim H, Park K, Song H, et al. Enhanced power and rechargeability of a Li−O2 battery based on a hierarchical-fibril CNT electrode[J]. Advanced Materials,2013,25(9):1348-1352. doi: 10.1002/adma.201204018 [26] Wu K, Wang D, Lu X, et al. Highly selective hydrogen peroxide electrosynthesis on carbon: In situ interface engineering with surfactants[J]. Chem,2020,6(6):1443-1458. doi: 10.1016/j.chempr.2020.04.002 [27] Sang Z, Hou F, Wang S, et al. Research progress on carbon-based non-metallic nanomaterials as catalysts for the two-electron oxygen reduction for hydrogen peroxide production[J]. New Carbon Materials,2022,37(1):136-151. doi: 10.1016/S1872-5805(22)60583-3 [28] Zhang D, Mitchell E, Lu X, et al. Metal-free carbon-based catalysts design for oxygen reduction reaction towards hydrogen peroxide: From 3D to 0D[J]. Materials Today,2023,63:339-359. doi: 10.1016/j.mattod.2023.02.004 [29] Peng W, Liu J, Liu X, et al. Facilitating two-electron oxygen reduction with pyrrolic nitrogen sites for electrochemical hydrogen peroxide production[J]. Nature Communications,2023,14(1):4430. doi: 10.1038/s41467-023-40118-y [30] Liu K, Huang X, Wang H, et al. Co3O4-CeO2/C as a highly active electrocatalyst for oxygen reduction reaction in Al-air batteries[J]. ACS Appl Mater Interfaces,2016,8(50):34422-34430. doi: 10.1021/acsami.6b12294 [31] Kabir S, Artyushkova K, Serov A, et al. Role of nitrogen moieties in N-doped 3D-graphene nanosheets for oxygen electroreduction in acidic and alkaline media[J]. ACS Applied Materials & Interfaces,2018,10(14):11623-11632. [32] Liu Z, Gao D, Hu L, et al. Metal-free boron-rich borocarbonitride catalysts for high-efficient oxygen reduction to produce hydrogen peroxide[J]. Chemistry Select,2022,7(5):1-8. [33] Yuan W, Liu J, Yi W, et al. Boron and nitrogen co-doped double-layered mesopore-rich hollow carbon microspheres as high-performance electrodes for supercapacitors[J]. Journal of Colloid and Interface Science,2020,573:232-240. doi: 10.1016/j.jcis.2020.03.126 [34] San Roman D, Krishnamurthy D, Garg R, et al. Engineering three-dimensional (3D) out-of-plane graphene edge sites for highly selective two-electron oxygen reduction electrocatalysis[J]. ACS Catalysis,2020,10(3):1993-2008. doi: 10.1021/acscatal.9b03919 [35] Yu L, Tang L, Guo W, et al. Disclosing the natures of carbon edges with gradient nanocarbons for electrochemical hydrogen peroxide production[J]. Matter,2022,5(6):1909-1923. doi: 10.1016/j.matt.2022.04.010 [36] Lim J S, Kim J H, Woo J, et al. Designing highly active nanoporous carbon H2O2 production electrocatalysts through active site identification[J]. Chem,2021,7(11):3114-3130. doi: 10.1016/j.chempr.2021.08.007 [37] Xin S, Li Y, Guan J, et al. Electrocatalytic oxygen reduction to hydrogen peroxide through a biomass-derived nitrogen and oxygen self-doped porous carbon metal-free catalyst[J]. Journal of Materials Chemistry. A, Materials for Energy and Sustainability,2021,9(44):25136-25149. doi: 10.1039/D1TA06955A [38] Gutru R, Turtayeva Z, Xu F, et al. Recent progress in heteroatom doped carbon based electrocatalysts for oxygen reduction reaction in anion exchange membrane fuel cells[J]. International Journal of Hydrogen Energy,2023,48(9):3593-3631. doi: 10.1016/j.ijhydene.2022.10.177 [39] Yang Y, Liu W, Wang Y, et al. A PtRu catalyzed rechargeable oxygen electrode for Li-O2 batteries: performance improvement through Li2O2 morphology control[J]. Physical Chemistry Chemical Physics: PCCP,2014,16(38):2618-2623. [40] Cao Y, Zhao C, Fang Q, et al. Hydrogen peroxide electrochemical synthesis on hybrid double-atom (Pd–Cu) doped N vacancy g-C3N4: A novel design strategy for electrocatalyst screening[J]. Journal of Materials Chemistry. A, Materials for Energy and Sustainability,2020,8(5):2672-2683. doi: 10.1039/C9TA12468C [41] Yang L, Fan J, Xiao B, et al. Unveiling “Sabatier principle” for electrocatalytic nitric oxide reduction on single cluster catalysts: A DFT and machine learning guideline[J]. Chemical Engineering Journal,2023,468:143823. doi: 10.1016/j.cej.2023.143823 [42] Siahrostami S, Verdaguer-Casadevall A, Karamad M, et al. Enabling direct H2O2 production through rational electrocatalyst design[J]. Nature Materials,2013,12(12):1137-1143. doi: 10.1038/nmat3795 [43] An J, Feng Y, Zhao Q, et al. Electrosynthesis of H2O2 through a two-electron oxygen reduction reaction by carbon based catalysts: From mechanism, catalyst design to electrode fabrication[J]. Environmental Science and Ecotechnology,2022,11:100170. doi: 10.1016/j.ese.2022.100170 [44] Tao L, Huang B, Jin F, et al. Atomic PdAu interlayer sandwiched into Pd/Pt core/shell nanowires achieves superstable oxygen reduction catalysis[J]. ACS Nano,2020,14(9):11570-11578. doi: 10.1021/acsnano.0c04061 [45] Xu G, Yang L, Li J, et al. Strategies for improving stability of Pt-based catalysts for oxygen reduction reaction[J]. Advanced Sensor and Energy Materials,2023,2(2):100058. doi: 10.1016/j.asems.2023.100058 [46] Nørskov J K, Rossmeisl J, Logadottir A, et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode[J]. The Journal of Physical Chemistry. B,2004,108(46):17886-17892. doi: 10.1021/jp047349j [47] Mounfield W P, Garg A, Shao-Horn Y, et al. Electrochemical oxygen reduction for the production of hydrogen peroxide[J]. Chem,2018,4(1):18-19. doi: 10.1016/j.chempr.2017.12.015 [48] Ma D D, Zhu Q L. MOF-based atomically dispersed metal catalysts: Recent progress towards novel atomic configurations and electrocatalytic applications[J]. Coordination Chemistry Reviews,2020,422:213483. doi: 10.1016/j.ccr.2020.213483 [49] Ko M, Pham L T M, Sa Y J, et al. Unassisted solar lignin valorisation using a compartmented photo-electro-biochemical cell[J]. Nature Communications,2019,10(1):5110-5123. doi: 10.1038/s41467-019-12959-z [50] Sun Y, Silvioli L, Sahraie N R, et al. Activity–selectivity trends in the electrochemical production of hydrogen peroxide over single-site metal–nitrogen–carbon catalysts[J]. Journal of the American Chemical Society,2019,141(31):12372-12381. doi: 10.1021/jacs.9b05576 [51] Ma F, Wang S, Liang X, et al. Ni3B as a highly efficient and selective catalyst for the electrosynthesis of hydrogen peroxide[J]. Applied catalysis. B, Environmental,2020,279:119371. doi: 10.1016/j.apcatb.2020.119371 [52] Zhang J, Zhang G, Jin S, et al. Graphitic N in nitrogen-doped carbon promotes hydrogen peroxide synthesis from electrocatalytic oxygen reduction[J]. Carbon,2020,163:154-161. doi: 10.1016/j.carbon.2020.02.084 [53] Liu X, Chen R, Peng W, et al. Multiatom activation of single-atom electrocatalysts via remote coordination for ultrahigh-rate two-electron oxygen reduction[J]. Journal of Energy Chemistry,2023,76:622-630. doi: 10.1016/j.jechem.2022.10.015 [54] Xie X, He C, Li B, et al. Performance enhancement and degradation mechanism identification of a single-atom Co–N–C catalyst for proton exchange membrane fuel cells[J]. Nature Catalysis,2020,3(12):1044-1054. doi: 10.1038/s41929-020-00546-1 [55] Fan M, Cui J, Wu J, et al. Improving the catalytic activity of carbon-supported single atom catalysts by polynary metal or heteroatom doping[J]. Small,2020,16(22):1906782-1-1906782-15. doi: 10.1002/smll.201906782 [56] Zhao C, Ren D, Wang J, et al. Regeneration of single-atom catalysts deactivated under acid oxygen reduction reaction conditions[J]. Journal of Energy Chemistry,2022,73:478-484. doi: 10.1016/j.jechem.2022.06.005 [57] Tian Y, Chen R, Liu X, et al. Fluorine-regulated carbon nanotubes decorated with Co single atoms for multi-site electrocatalysis toward two-electron oxygen reduction[J]. EcoMat,2023,5(5):e12336. doi: 10.1002/eom2.12336 [58] Gao J, Yang H B, Huang X, et al. Enabling direct H2O2 production in acidic media through rational design of transition metal single atom catalyst[J]. Chem,2020,6(3):658-674. doi: 10.1016/j.chempr.2019.12.008 [59] Liu T, Zuo M, Ju Y, et al. An efficient self-assembly CuO/Cu2O@Ni–B porous catalysts with three-dimensional skeleton structure[J]. Materials Letters,2023,344:134422. doi: 10.1016/j.matlet.2023.134422 [60] Musarurwa H, Tavengwa N T. Advances in the application of chitosan-based metal organic frameworks as adsorbents for environmental remediation[J]. Carbohydrate Polymers,2022,283:119153. doi: 10.1016/j.carbpol.2022.119153 [61] Le T X H, Charmette C, Bechelany M, et al. Facile preparation of porous carbon cathode to eliminate paracetamol in aqueous medium using electro-fenton system[J]. Electrochimica Acta,2016,188:378-384. doi: 10.1016/j.electacta.2015.12.005 [62] Zhang H C, Li Y J, Zhao Y S, et al. Carbon black oxidized by air calcination for enhanced H2O2 generation and effective organics degradation[J]. ACS applied materials & interfaces,2019,11(31):27846-27853. [63] Garcia-Rodriguez O, Lee Y Y, Olvera-Vargas H, et al. Mineralization of electronic wastewater by electro-Fenton with an enhanced graphene-based gas diffusion cathode[J]. Electrochimica Acta,2018,276:12-20. doi: 10.1016/j.electacta.2018.04.076 [64] Xia Y, Shang H, Zhang Q, et al. Electrogeneration of hydrogen peroxide using phosphorus-doped carbon nanotubes gas diffusion electrodes and its application in electro-Fenton[J]. Journal of Electroanalytical Chemistry,2019,840:400-408. doi: 10.1016/j.jelechem.2019.04.009 [65] Sun Y, Sinev I, Ju W, et al. Efficient electrochemical hydrogen peroxide production from molecular oxygen on nitrogen-doped mesoporous carbon catalysts[J]. ACS Catalysis,2018,8(4):2844-2856. doi: 10.1021/acscatal.7b03464 [66] Kim H W, Ross M B, Kornienko N, et al. Efficient hydrogen peroxide generation using reduced graphene oxide-based oxygen reduction electrocatalysts[J]. Nature Catalysis,2018,1(4):282-290. doi: 10.1038/s41929-018-0044-2 [67] Antonin V S, Parreira L S, Aveiro L R, et al. W@Au nanostructures modifying carbon as materials for hydrogen peroxide electrogeneration[J]. Electrochimica Acta,2017,231:713-720. doi: 10.1016/j.electacta.2017.01.192 [68] Lu Z, Chen G, Siahrostami S, et al. High-efficiency oxygen reduction to hydrogen peroxide catalysed by oxidized carbon materials[J]. Nature Catalysis,2018,1(2):156-162. doi: 10.1038/s41929-017-0017-x [69] Zhu J, Xiao X, Zheng K, et al. KOH-treated reduced graphene oxide: 100% selectivity for H2O2 electroproduction[J]. Carbon,2019,153:6-11. doi: 10.1016/j.carbon.2019.07.009 [70] Siahrostami S, Verdaguer-Casadevall A, Karamad M, et al. Erratum: Enabling direct H2O2 production through rational electrocatalyst design[J]. Nature Materials,2014,13(2):213. doi: 10.1038/nmat3871 [71] Jirkovský J S, Panas I, Ahlberg E, et al. Single Atom hot-spots at Au–Pd nanoalloys for electrocatalytic H2O2 production[J]. Journal of the American Chemical Society,2011,133(48):19432-19441. doi: 10.1021/ja206477z [72] Zheng Z, Ng Y H, Wang D, et al. Epitaxial growth of Au-Pt-Ni nanorods for direct high selectivity H2O2 production[J]. Advanced Materials,2016,28(45):9949-9955. doi: 10.1002/adma.201603662 [73] Shen R, Chen W, Peng Q, et al. High-concentration single atomic Pt sites on hollow CuSx for selective O2 reduction to H2O2 in acid solution[J]. Chem,2019,5(8):2099-2110. doi: 10.1016/j.chempr.2019.04.024 [74] Euiyeon J, Heejong S, Byoung-Hoon L, et al. Atomic-level tuning of Co–N–C catalyst for high-performance electrochemical H2O2 production[J]. Nature Materials,2020,19(4):436-442. doi: 10.1038/s41563-019-0571-5 [75] Wang Y, Shi R, Shang L, et al. High-efficiency oxygen reduction to hydrogen peroxide catalyzed by nickel single-atom catalysts with tetradentate N2O2 coordination in a three-phase flow cell[J]. Angewandte Chemie,2020,59(31):13057-13062. doi: 10.1002/anie.202004841 [76] Xu W, Liang Z, Gong S, et al. Fast and stable electrochemical production of H2O2 by electrode architecture engineering[J]. ACS Sustainable Chemistry & Engineering,2021,9(20):7120-7129. [77] Chernyaev A, Zou Y, Wilson B P, et al. The interference of copper, iron and aluminum with hydrogen peroxide and its effects on reductive leaching of LiNi1/3Mn1/3Co1/3O2[J]. Separation and Purification Technology,2022,281:119903. doi: 10.1016/j.seppur.2021.119903 [78] Meng F, Liu Q, Kim R, et al. Selective recovery of valuable metals from industrial waste lithium-ion batteries using citric acid under reductive conditions: Leaching optimization and kinetic analysis[J]. Hydrometallurgy,2020,191:105160. doi: 10.1016/j.hydromet.2019.105160 [79] Cheng Q, Chirdon W M, Lin M, et al. Characterization, modeling, and optimization of a single-step process for leaching metallic ions from LiNi1/3Co1/3Mn1/3O2 cathodes for the recycling of spent lithium-ion batteries[J]. Hydrometallurgy,2019,185:1-11. doi: 10.1016/j.hydromet.2019.01.003 [80] Yamanaka I, Murayama T. Neutral H2O2 synthesis by electrolysis of water and O2[J]. Angewandte Chemie,2008,47(10):1900-1902. doi: 10.1002/anie.200704431 [81] Exner K S, Lim T, Joo S H. Circumventing the OCl versus OOH scaling relation in the chlorine evolution reaction: Beyond dimensionally stable anodes[J]. Current Opinion in Electrochemistry,2022,34:100979. doi: 10.1016/j.coelec.2022.100979 [82] Zeradjanin A R, Menzel N, Schuhmann W, et al. On the faradaic selectivity and the role of surface inhomogeneity during the chlorine evolution reaction on ternary Ti-Ru-Ir mixed metal oxide electrocatalysts[J]. Physical Chemistry Chemical Physics:PCCP,2014,16(27):13741-13747. doi: 10.1039/C4CP00896K [83] Duan Y, Fang D, Wang J, et al. Determination of persulfate based on the principle of the oxidation of chloride ion[J]. Journal of environmental sciences, 2023. DOI: https://doi.org/10.1016/j.jes.2023.11.028. [84] Rastinfard A, Dalisson B, Barralet J. Aqueous decomposition behavior of solid peroxides: Effect of pH and buffer composition on oxygen and hydrogen peroxide formation[J]. Acta Biomaterialia,2022,145:390-402. doi: 10.1016/j.actbio.2022.04.004 [85] Qiang Z, Chang J, Huang C. Electrochemical generation of hydrogen peroxide from dissolved oxygen in acidic solutions[J]. Water research,2002,36(1):85-94. doi: 10.1016/S0043-1354(01)00235-4 [86] Brillas E, Sirés I, Oturan M A. Electro-fenton process and related electrochemical technologies based on fenton’s reaction chemistry[J]. Chemical reviews,2009,109(12):6570-6631. doi: 10.1021/cr900136g [87] He Z, Huang Y, Manohar A K, et al. Effect of electrolyte pH on the rate of the anodic and cathodic reactions in an air-cathode microbial fuel cell[J]. Bioelectrochemistry,2008,74(1):78-82. doi: 10.1016/j.bioelechem.2008.07.007 [88] Li N, Huang C, Wang X, et al. Electrosynthesis of hydrogen peroxide via two-electron oxygen reduction reaction: A critical review focus on hydrophilicity/hydrophobicity of carbonaceous electrode[J]. Chemical Engineering Journal,2022,450:138246. doi: 10.1016/j.cej.2022.138246 [89] Zhang J, Xia C, Wang H, et al. Recent advances in electrocatalytic oxygen reduction for on-site hydrogen peroxide synthesis in acidic media[J]. Journal of Energy Chemistry,2022,67:432-450. doi: 10.1016/j.jechem.2021.10.013 [90] Zhou Q, Ma J, Dong S, et al. Intermolecular chemistry in solid polymer electrolytes for high-energy-density lithium batteries[J]. Advanced Materials,2019,31(50):e1902029. doi: 10.1002/adma.201902029 [91] Yang Q, Wang A, Luo J, et al. Improving ionic conductivity of polymer-based solid electrolytes for lithium metal batteries[J]. Chinese Journal of Chemical Engineering,2022,43(3):202-215. [92] Pollet B G, Franco A A, Su H, et al. 1-Proton Exchange Membrane Fuel Cells[M]. Barbir F, Basile A, Veziroğlu T N. Compendium of Hydrogen Energy. Oxford: Woodhead Publishing, 2016: 3-56. [93] Zhang Q, Zhou M, Ren G, et al. Highly efficient electrosynthesis of hydrogen peroxide on a superhydrophobic three-phase interface by natural air diffusion[J]. Nature Communications,2020,11(1):1711-1731. doi: 10.1038/s41467-020-15548-7 [94] Wang X Y, Xia X H, Wang H X, et al. Special-wettability-mediating electrode interfaces for new energy devices: Opportunities and challenges[J]. Nano Energy,2024,120:109185. doi: 10.1016/j.nanoen.2023.109185 [95] Maja M, Orecchia C, Strano M, et al. Effect of structure of the electrical performance of gas diffusion electrodes for metal air batteries[J]. Electrochimica Acta,2000,46(2):423-432. [96] Zhou H, Luo J, Chen Y. Nitrogen moieties-dominated Co-N-doped nanoparticle-modified cathodes in heterogeneous-electro-Fenton-like system for catalytic decontamination of EDTA-Ni(II)[J]. Chemosphere,2020,239:124743. doi: 10.1016/j.chemosphere.2019.124743 [97] Bidault F, Brett D J L, Middleton P H, et al. Review of gas diffusion cathodes for alkaline fuel cells[J]. Journal of Power Sources,2009,187(1):39-48. doi: 10.1016/j.jpowsour.2008.10.106 [98] Squadrito G. Chapter 6-Preparation of MEA[M]. Hacker V, Mitsushima S. Fuel Cells and Hydrogen. Elsevier, 2018: 117-138. [99] Dong H, Yu H, Yu H, et al. Enhanced performance of activated carbon–polytetrafluoroethylene air-cathode by avoidance of sintering on catalyst layer in microbial fuel cells[J]. Journal of Power Sources,2013,232:132-138. doi: 10.1016/j.jpowsour.2013.01.036 [100] Carmo M, Linardi M, Poco J G R. Characterization of nitric acid functionalized carbon black and its evaluation as electrocatalyst support for direct methanol fuel cell applications[J]. Applied catalysis. A, General,2009,355(1):132-138. [101] Wang Y, Zhou W, Gao J, et al. Oxidative modification of graphite felts for efficient H2O2 electrogeneration: Enhancement mechanism and long-term stability[J]. Journal of Electroanalytical Chemistry,2019,833:258-268. doi: 10.1016/j.jelechem.2018.11.051 [102] Gurzęda B, Florczak P, Kempiński M, et al. Synthesis of graphite oxide by electrochemical oxidation in aqueous perchloric acid[J]. Carbon,2016,100:540-545. doi: 10.1016/j.carbon.2016.01.044 [103] Wang J, Li C, Rauf M, et al. Gas diffusion electrodes for H2O2 production and their applications for electrochemical degradation of organic pollutants in water: A review[J]. The Science of the Total Environment,2021,759:143459. doi: 10.1016/j.scitotenv.2020.143459 [104] Kim D, Jung H, Chun H, et al. Enhanced membrane electrode assembly performance by adding PTFE/carbon black for high temperature polymer electrolyte membrane fuel cell[J]. International Journal of Hydrogen Energy,2021,46(57):29424-29431. doi: 10.1016/j.ijhydene.2021.02.120 [105] Li W, Feng Y, An J, et al. Thermal reduced graphene oxide enhanced in-situ H2O2 generation and electrochemical advanced oxidation performance of air-breathing cathode[J]. Environmental Research,2022,204:112327. doi: 10.1016/j.envres.2021.112327 [106] Dong K, Liang J, Wang Y, et al. Honeycomb carbon nanofibers: A superhydrophilic O2-entrapping electrocatalyst enables ultrahigh mass activity for the two-electron oxygen reduction reaction[J]. Angewandte Chemie (International ed. ),2021,60(19):10583-10587. doi: 10.1002/anie.202101880 [107] Ou B, Wang J, Wu Y, et al. A highly efficient cathode based on modified graphite felt for aniline degradation by electro-Fenton[J]. Chemosphere,2019,235:49-57. doi: 10.1016/j.chemosphere.2019.06.144 [108] Lai W, Xie G, Dai R, et al. Kinetics and mechanisms of oxytetracycline degradation in an electro-Fenton system with a modified graphite felt cathode[J]. Journal of Environmental Management,2020,257:109968. doi: 10.1016/j.jenvman.2019.109968 [109] Zhou L, Zhou M, Zhang C, et al. Electro-Fenton degradation of p-nitrophenol using the anodized graphite felts[J]. Chemical Engineering Journal,2013,233:185-192. doi: 10.1016/j.cej.2013.08.044 [110] Jin Y, Shi Y, Chen R, et al. Electrochemical disinfection using a modified reticulated vitreous carbon cathode for drinking water treatment[J]. Chemosphere,2019,215:380-387. doi: 10.1016/j.chemosphere.2018.10.057 [111] Yu F, Tao L, Cao T. High yield of hydrogen peroxide on modified graphite felt electrode with nitrogen-doped porous carbon carbonized by zeolitic imidazolate framework-8(ZIF-8) nanocrystals[J]. Environmental Pollution,2019,255(2):113-119. [112] Zhao Q, An J, Wang S, et al. Superhydrophobic air-breathing cathode for efficient hydrogen peroxide generation through two-electron pathway oxygen reduction reaction[J]. ACS applied Materials & Interfaces,2019,11(38):35410-35419. [113] Li Y, Zhang H, Han N, et al. Janus electrode with simultaneous management on gas and liquid transport for boosting oxygen reduction reaction[J]. Nano Research,2019,12(1):177-182. doi: 10.1007/s12274-018-2199-1 [114] Zhou W, Meng X, Gao J, et al. Janus graphite felt cathode dramatically enhance the H2O2 yield from O2 electroreduction by the hydrophilicity-hydrophobicity regulation[J]. Chemosphere,2021,278:130382. doi: 10.1016/j.chemosphere.2021.130382 [115] Chen Z, Chen S, Siahrostami S, et al. Development of a reactor with carbon catalysts for modular-scale, low-cost electrochemical generation of H2O2[J]. Reaction Chemistry & Engineering,2017,2(2):239-245. [116] Yang S, Verdaguer-Casadevall A, Arnarson L, et al. Toward the decentralized electrochemical production of H2O2: A focus on the catalysis[J]. ACS Catalysis,2018,8(5):4064-4081. doi: 10.1021/acscatal.8b00217 [117] Xia C, Xia Y, Zhu P, et al. Direct electrosynthesis of pure aqueous H2O2 solutions up to 20% by weight using a solid electrolyte[J]. Science,2019,366(6462):226-231. doi: 10.1126/science.aay1844 [118] Xia C, Back S, Ringe S, et al. Confined local oxygen gas promotes electrochemical water oxidation to hydrogen peroxide[J]. Nature Catalysis,2020,3(2):125-134. doi: 10.1038/s41929-019-0402-8 [119] Murray A T, Voskian S, Schreier M, et al. Electrosynthesis of hydrogen peroxide by phase-transfer catalysis[J]. Joule,2019,3(12):2942-2954. doi: 10.1016/j.joule.2019.09.019 -





 下载:
下载: