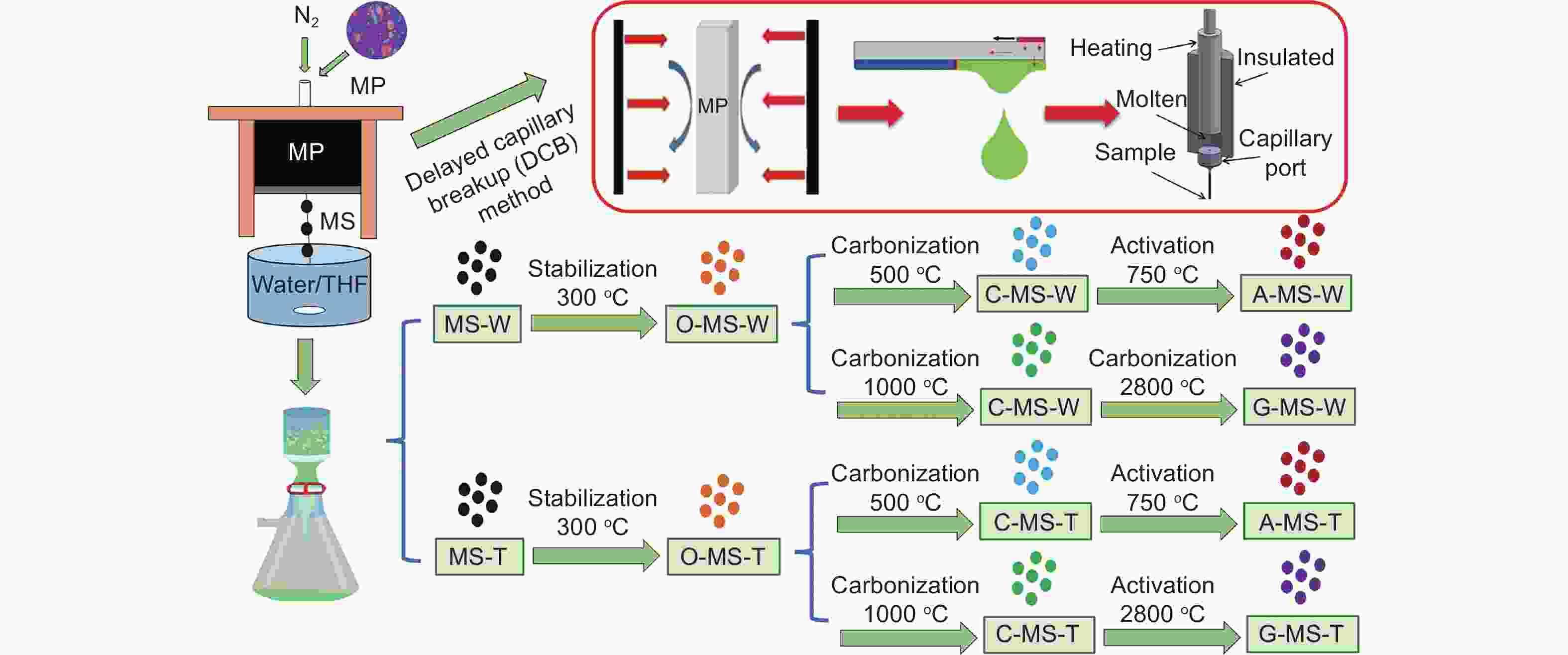
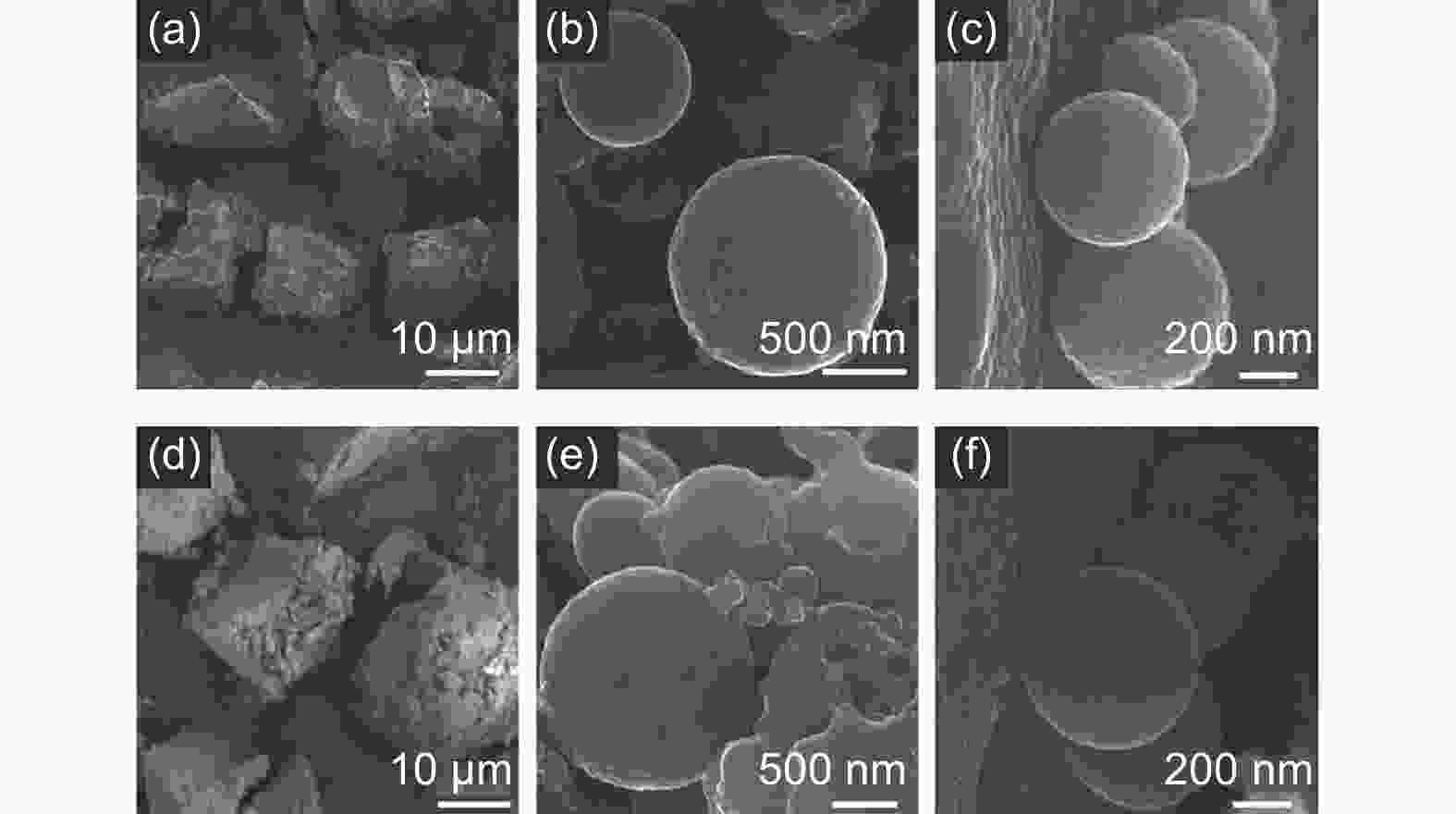
An innovative and efficient method for the preparation of mesocarbon microbeads and their use in the electrodes of lithium ion batteries and electric double layer capacitors
-
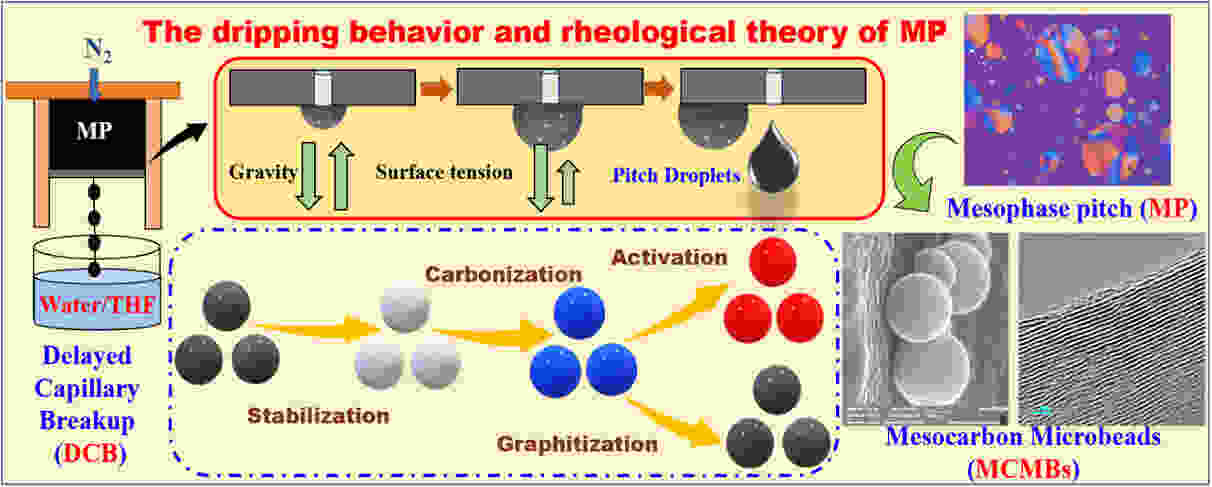
摘要: 基于沥青在熔融纺丝过程中的滴落行为和流变学理论,提出了一种新型高效的中间相炭微球(MCMB)制备方法—毛细管破裂法(DCB法)。本实验以中间相沥青为原料,采用DCB法考察了不同接收相(水或THF)对MCMB制备的影响规律,并系统研究了相应MCMB微观结构的演变规律。在此基础上,所制MCMB经750 °C的KOH活化制备了A-MCMB,以及经2800 °C的石墨化制备了G-MCMB,并分别探究了它们作为超级电容器(EDLC)和锂离子电池(LIB)电极材料的电化学性能。结果表明:采用DCB方法所制备的水接收相的MCMB-W和THF接收相的MCMB-T均呈现尺寸约1~2 μm的球形结构特征。此外,A-MCMB-T具有高比表面积1391 m2 g−1、微孔体积0.55 cm3 g−1和中孔体积0.24 cm3 g−1,作为EDLC的电极材料时,其比电容比MP衍生的炭材料提高了30%,且电容保持率也显著提升。同时,G-MCMB-T具有较高的石墨化度0.895和有序的层状结构,作为LIB的负极材料时,在100 mA g−1下进行100次循环后,具有353.5 mAh g−1的高比容量。因此,本文提出并验证了一种新的MCMB制备方法,有望为储能材料的设计和开发提供了一种思路和途径。Abstract: An innovative and efficient method for preparation of mesocarbon microbeads (MCMBs) was developed based on the dripping behavior and rheological properties of molten pitch during melt-spinning, where a string of beads was formed after the pitch was extruded from spinnerets and dropped into a receiving solvent (tetrohydrofuran or water). The pitch droplets were first carbonized, then activated by KOH or graphitized at 2800 °C to prepare A-MCMBs or G-MCMBs, respectively, and these were respectively used as the electrode materials for electric double layer capacitors (EDLCs) and lithium-ion batteries (LIBs). Results showed that both MCMB-W prepared using water as the receiving solvent and MCMB-T prepared using tetrohydrofuran as the receiving solvent had a spherical shape with sizes of 1-2 μm. A-MCMB-T had a high specific surface area (1 391 m2 g−1), micropore volume (0.55 cm3 g−1) and mesopore volume (0.24 cm3 g−1), with a 30% higher specific capacitance than an activated mesophase carbon prepared under the same conditions, and its capacitance retention was significantly improved when it was used as an electrode material for EDLCs. G-MCMB-T had a high degree of graphitization (0.895) and when it was used as an electrode material for LIBs it had a high specific capacity of 353.5 mAh g−1 after 100 cycles at 100 mA g−1. This work reports a new preparation method for MCMBs, which could be used to prepare energy storage materials.
-
Table 1. Relevant analysis results of samples
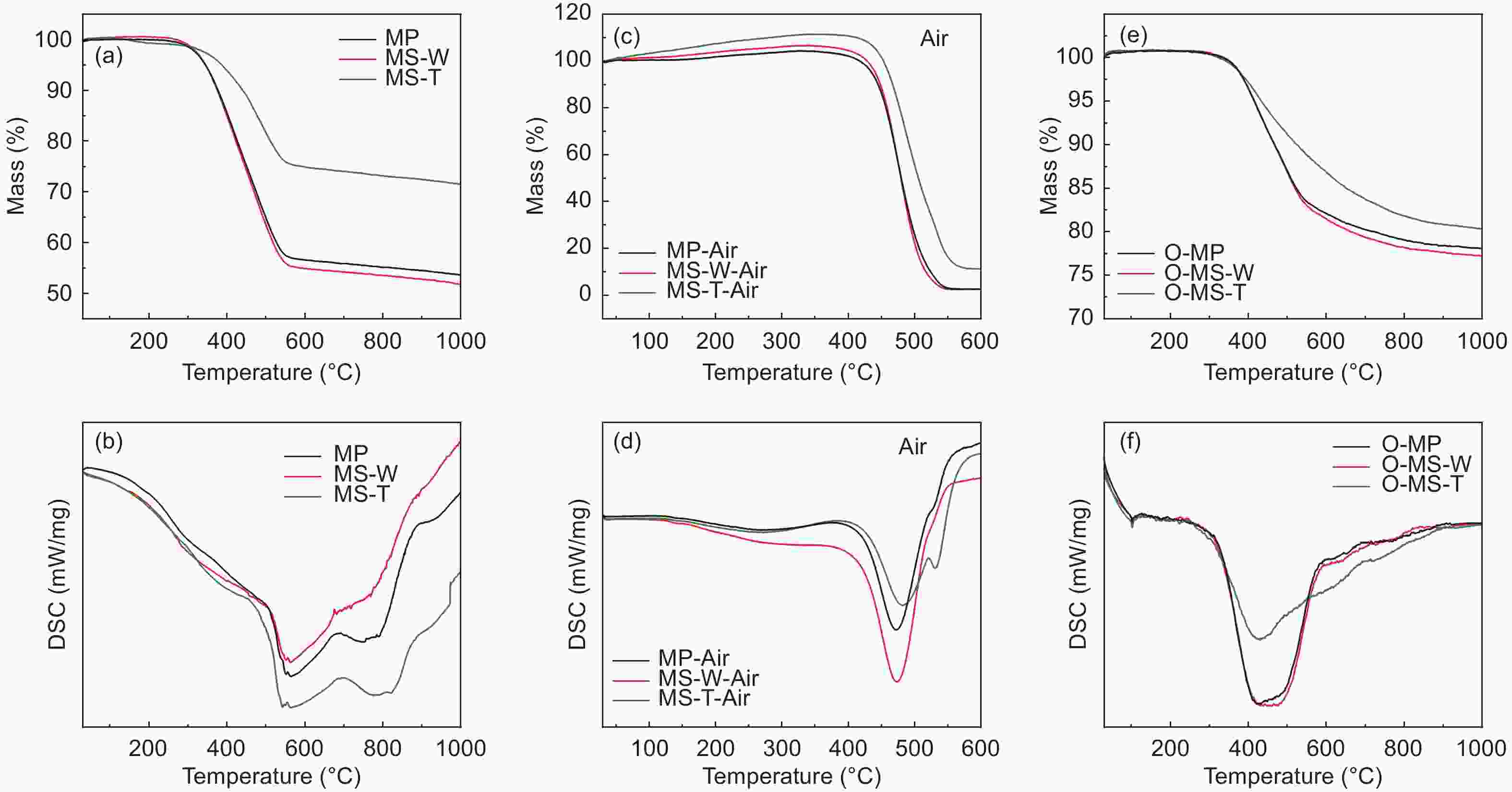
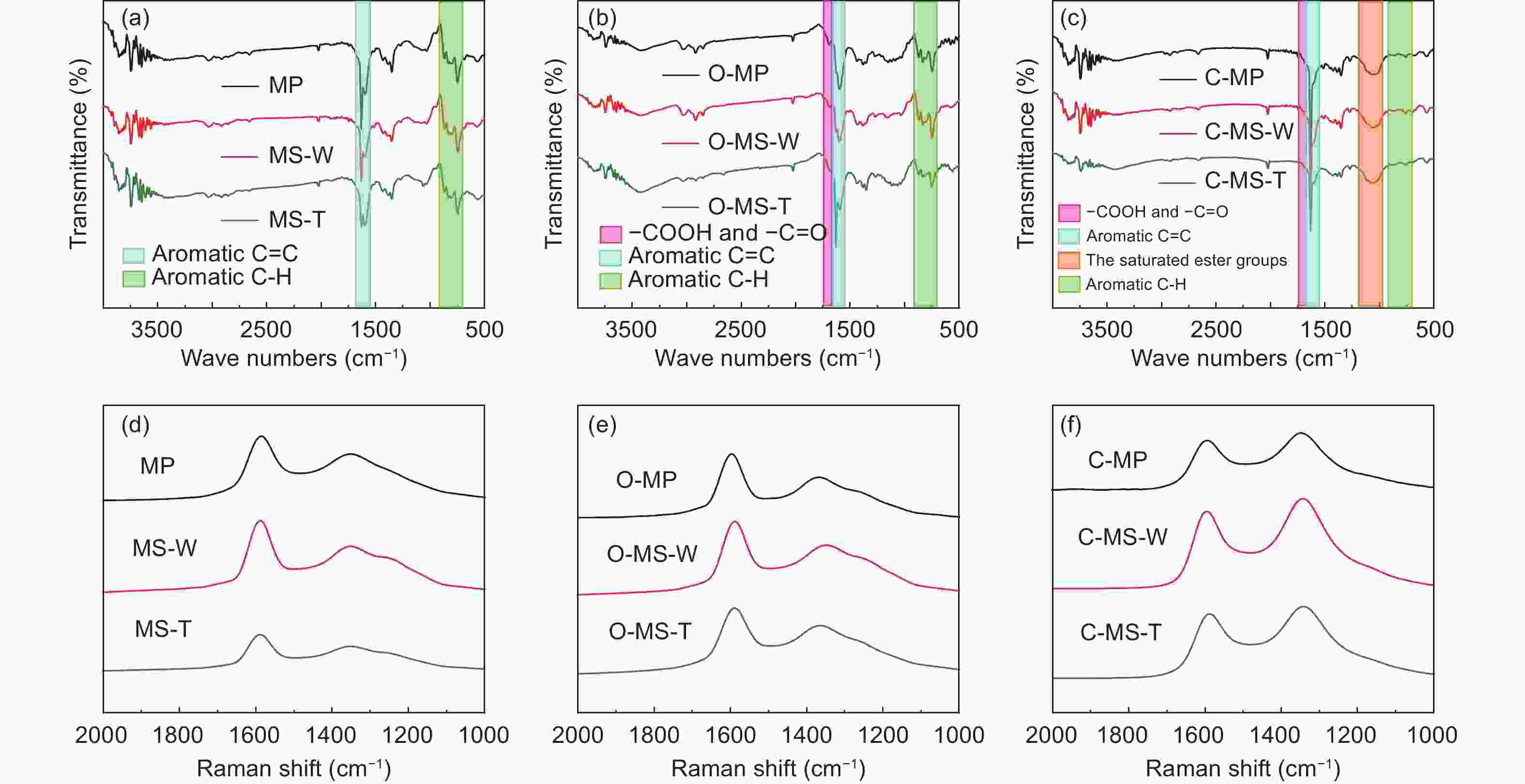
Samples Yield TG (in N2) DSC (in air) FTIR Raman Td
(°C)W △T
(°C)△H
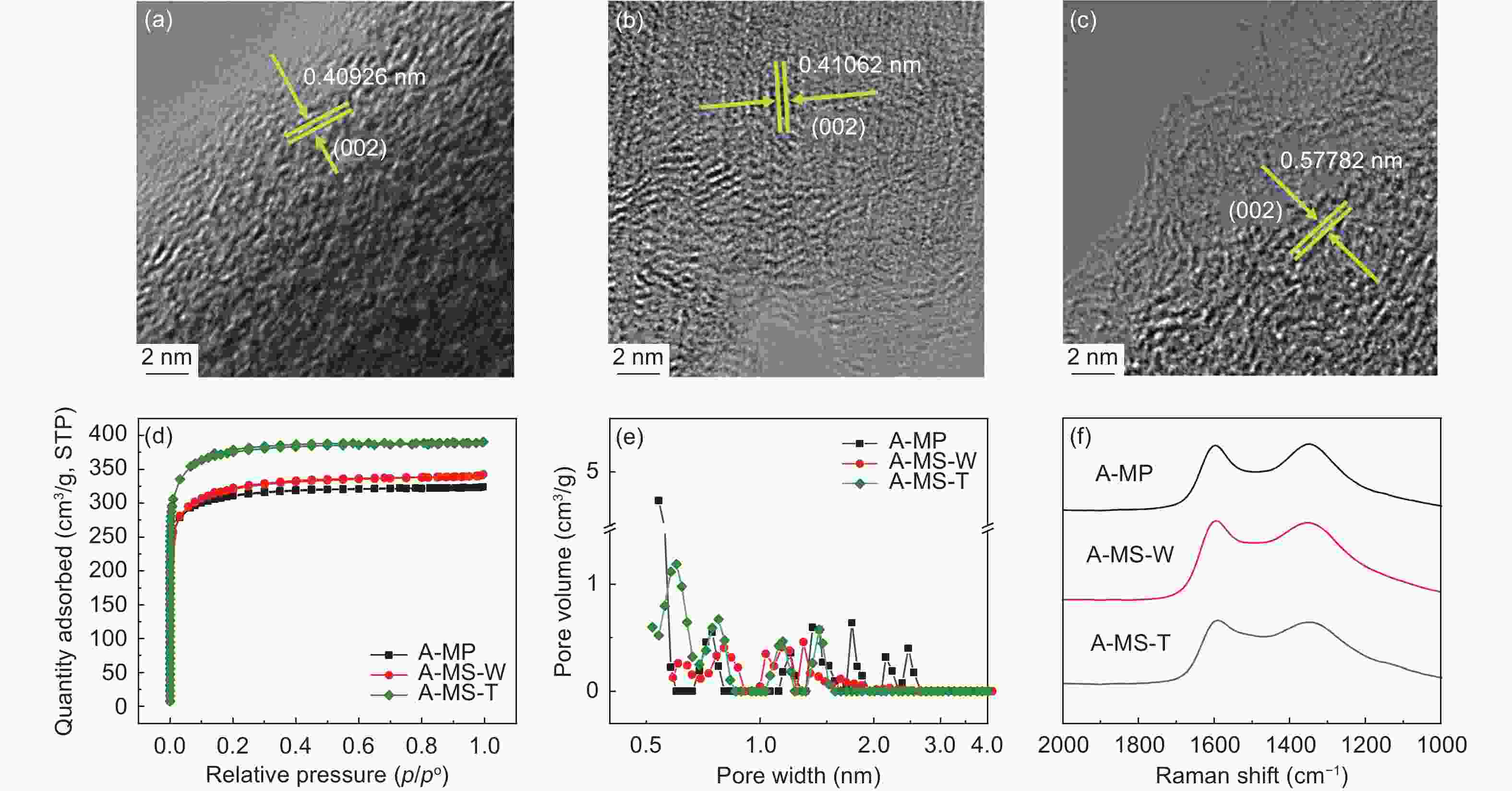
(J/g)△H/△T IOS ICHS IA ID/IG IA/IG MP ─ 235 54% 207 291 1.41 0.225 0.501 0.503 0.69 0.40 MS-W ─ 241 52% 218 405 1.86 0.224 0.500 0.502 0.64 0.35 MS-T ─ 305 72% 221 209 0.95 0.212 0.498 0.501 0.60 0.32 O-MP 103% 291 77% ─ ─ ─ 0.243 0.500 0.500 0.83 0.45 O-MS-W 104% 296 77% ─ ─ ─ 0.241 0.495 0.501 0.69 0.38 O-MS-T 105% 301 80% ─ ─ ─ 0.239 0.494 0.499 0.62 0.33 C-MP 70% ─ ─ ─ ─ ─ 0.250 0.499 0.500 1.14 0.53 C-MS-W 72% ─ ─ ─ ─ ─ 0.249 0.498 0.499 1.12 0.51 C-MS-T 74% ─ ─ ─ ─ ─ 0.247 0.497 0.498 1.08 0.45 Table 2. Microstructure and porosity parameters of A-MCMBs
Samples Raman Parameters of pore structure ID/IG IA/IG SBET
(m2 g−1)Vmic
(cm3 g−1)Vmes
(cm3 g−1)Vtot
(cm3 g−1)D
(nm)A-MP 0.99 0.60 1222 0.16 0.49 0.65 5.04 A-MS-W 0.96 0.71 1190 0.33 0.17 0.50 5.00 A-MS-T 0.94 0.72 1391 0.37 0.24 0.61 4.19 Table 3. FTIR and XPS analysis results of A-MCMBs
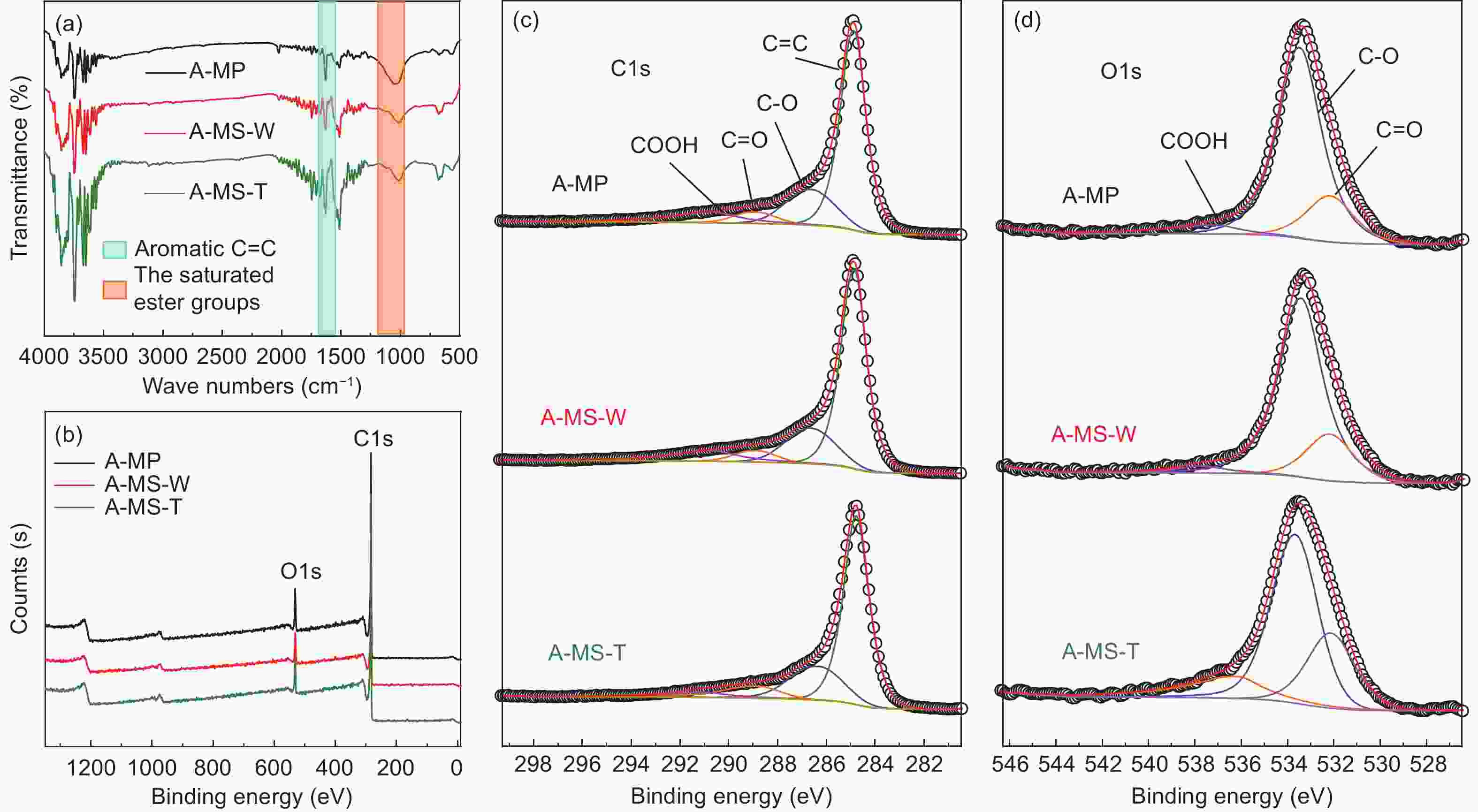
Samples FTIR XPS (at%) percentage of total C1s percentage of total O1s IOS ICHS IA C O C=C C―O C=O COOH C=O C―O COOH A-MP 0.251 0.500 0.502 90.03 8.70 35.29 23.73 19.85 21.13 31.66 35.91 32.43 A-MS-W 0.231 0.480 0.482 90.45 8.39 35.26 23.75 19.87 21.12 32.46 36.04 31.50 A-MS-T 0.220 0.460 0.462 91.28 7.64 34.32 24.28 21.27 20.13 32.96 37.20 29.84 Table 4. Electrochemical properties of different A-MCMBs
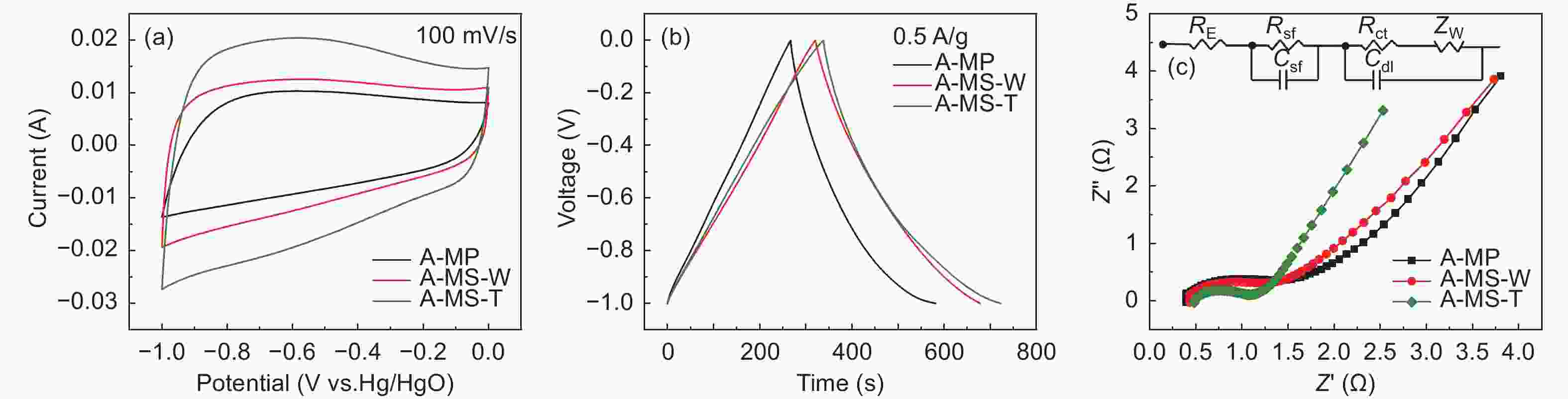
Samples Specific capacitance(F/g) Capacitance retention(%) Rct(Ω) 0.5 A g−1 1 A g−1 2 A g−1 5 A g−1 10 A g−1 A-MP 156.9 131.1 118.8 108.5 100.0 63.7 0.6 A-MS-W 178.6 169.5 153.4 145.0 139.0 77.8 0.5 A-MS-T 193.5 166.2 147.0 135.5 129.0 66.6 0.3 Table 5. Parameters of pore structure and graphite microcrystallite s of G-MCMBs
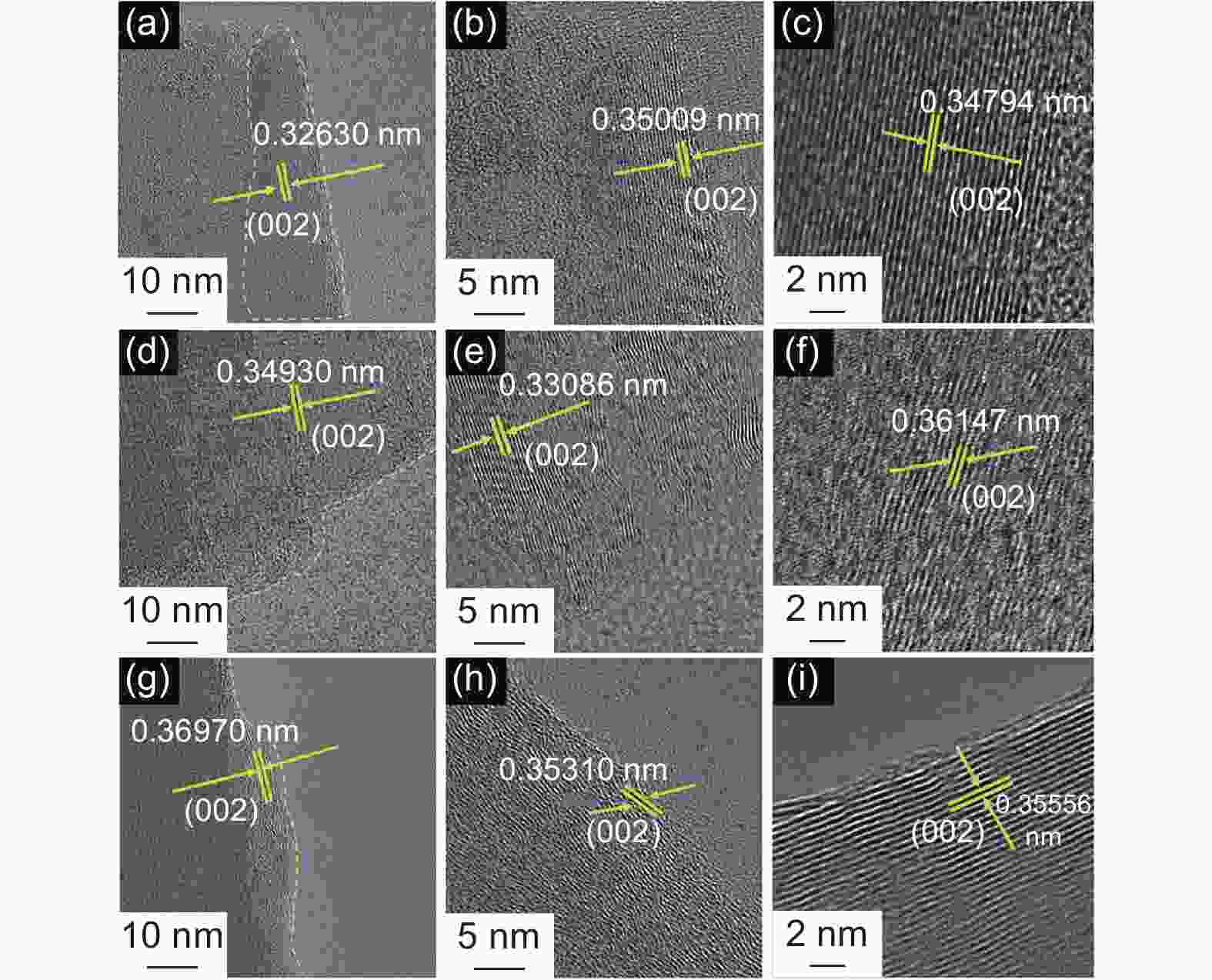
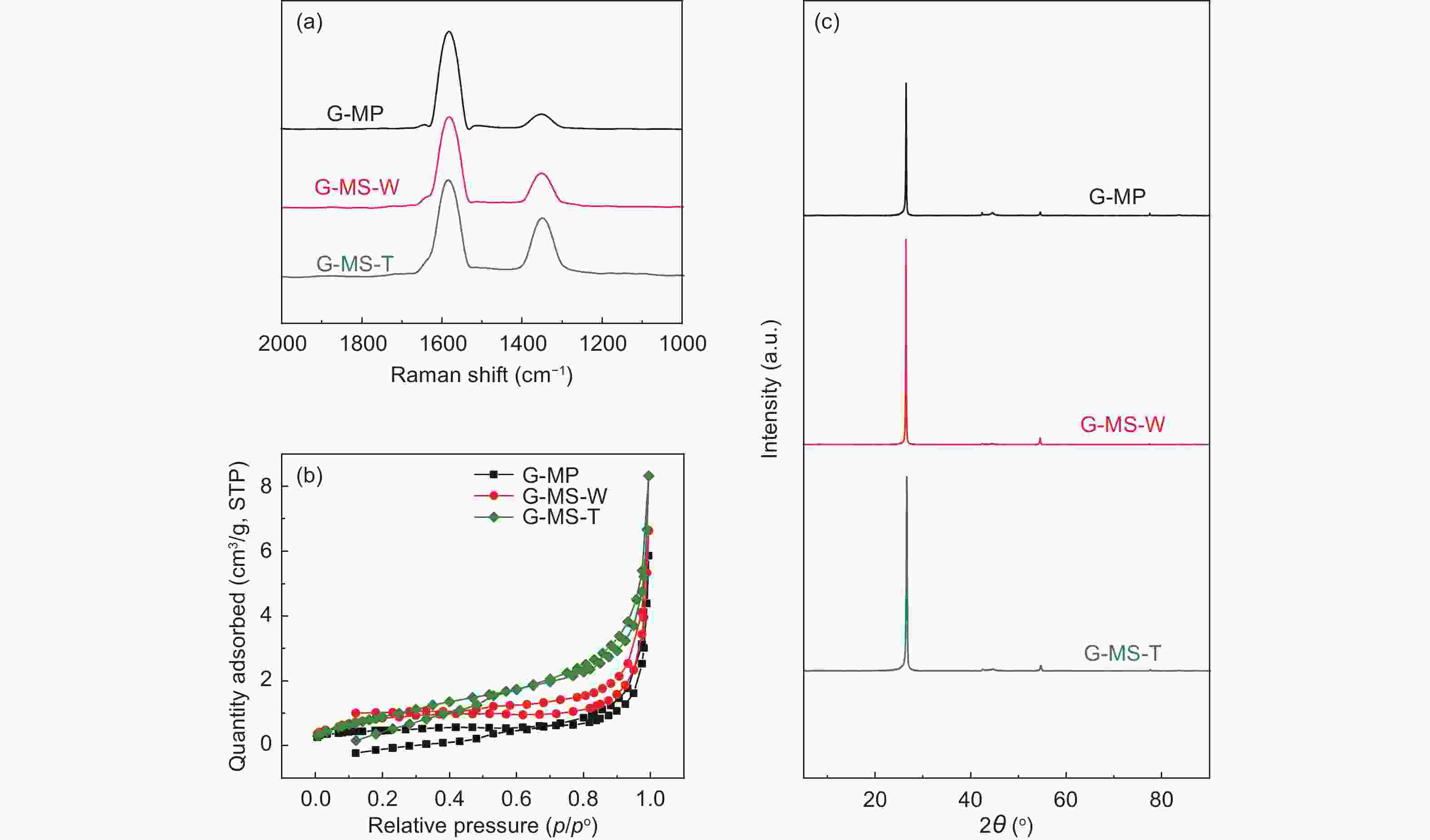
Samples ID/IG ID’/IG SBET
(m2 g−1)d002
(nm)Lc
(nm)La
(nm)N
(n)G G-MP 0.21 0.05 1.60 0.3366 0.6582 1.3976 2.96 0.861 G-MS-W 0.18 0.04 2.25 0.3366 0.6973 1.9862 3.07 0.861 G-MS-T 0.14 0.02 3.53 0.3363 0.5680 2.4556 2.69 0.895 Table 6. Cycle performance of different G-MCMBs
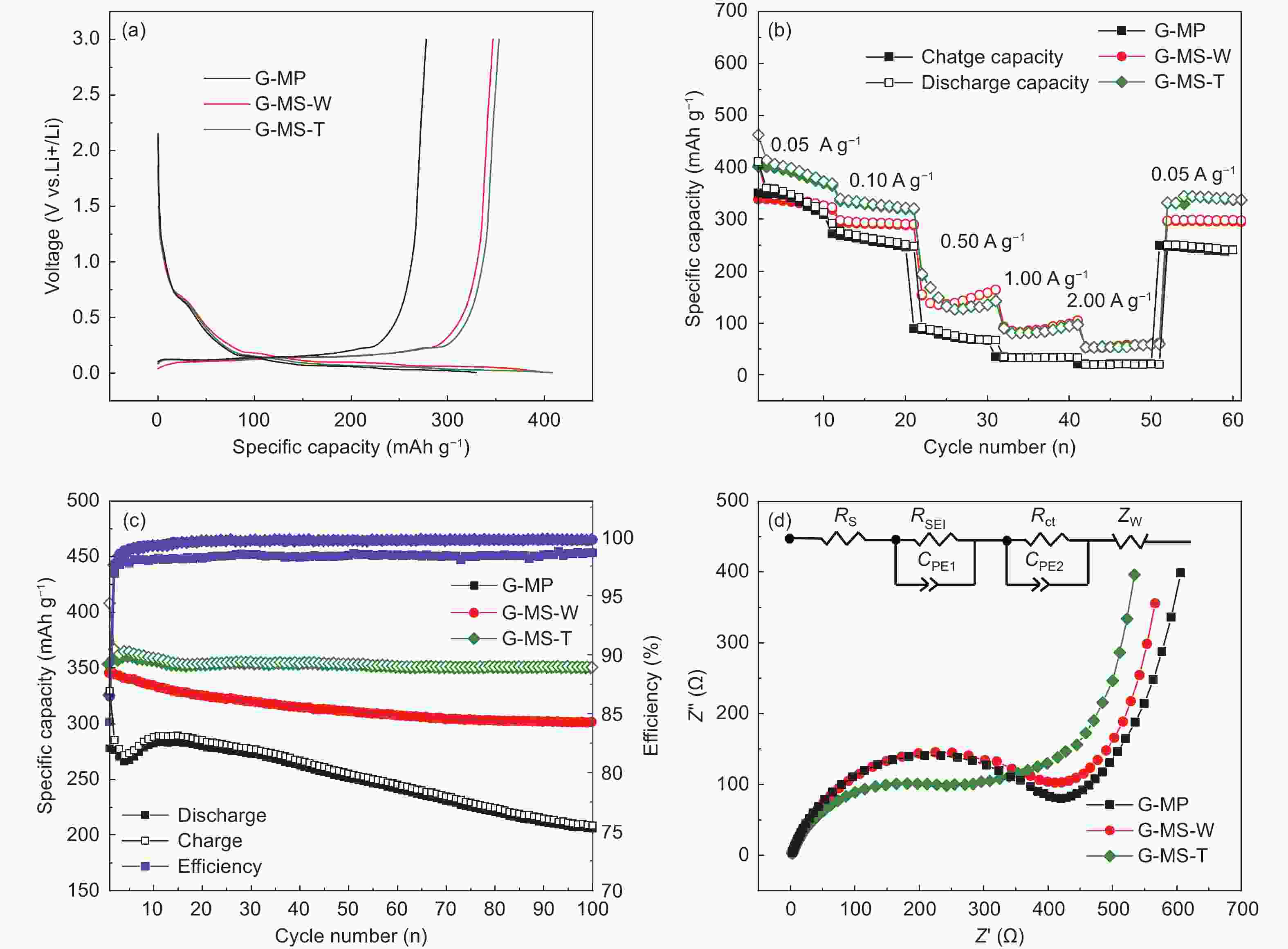
Samples Specific capacity (mAh g−1) ICE
(%)
Capacity retention
(%)Rct
(Ω)D1 C1 D2 D3 D100 G-MP 329.4 277.6 285.1 277.1 255.3 84.27 77.50 295.9 G-MS-W 401.4 346.9 354.0 349.6 313.6 86.42 78.13 301.9 G-MS-T 408.1 353.0 366.6 362.4 353.5 86.50 86.62 95.5 -
[1] Xia H Y, Wang J P, Huang B, et al. The influence of ball-milling on improving the performance of mesocarbon microbeads based carbon blocks[J]. Materials Science & Engineering A,2011,529:282-288. [2] Lin X B, Hui C, Wu J, et al. TiC-modified CNTs as reinforcing fillers for isotropic graphite produced from mesocarbon microbeads[J]. New Carbon Materials,2021,36(5):961-969. doi: 10.1016/S1872-5805(21)60067-7 [3] Zhao G Y, Wei Z H, Zhang N Q, et al. Enhanced low temperature performances of expanded commercial mesocarbon microbeads (MCMB) as lithium ion battery anodes[J]. Materials Letters,2012,89:243-246. doi: 10.1016/j.matlet.2012.07.066 [4] Li F H, Chi W D, Shen Z M, et al. Activation of mesocarbon microbeads with different textures and their application for supercapacitor[J]. Fuel Processing Technology,2010,91(1):17-24. doi: 10.1016/j.fuproc.2009.08.020 [5] Ji Y B, Li T H, Zhu L, et al. Preparation of activated carbons by microwave heating KOH activation[J]. Applied Surface Science,2007,254(2):506-512. doi: 10.1016/j.apsusc.2007.06.034 [6] Liu Y C, Qiu X P, Zhu W T, et al. Impedance studies on mesocarbon microbeads supported Pt-Ru catalytic anode[J]. Journal of Power Sources,2003,114(1):10-14. doi: 10.1016/S0378-7753(02)00527-X [7] Wu B, Gong Q M, Song H H, et al. Prickly structure of multi-walled carbon nanotube reinforced mesocarbon microbead composite with high strength at elevated temperature[J]. Composites Part B-Engineering,2014,56:876-881. doi: 10.1016/j.compositesb.2013.09.033 [8] Cheng Y L, Li T H, Fang C Q, et al. In situ preparation and mechanical properties of CNTs/MCMBs composites[J]. Composites Part B-Engineering,2013,47:290-297. doi: 10.1016/j.compositesb.2012.11.009 [9] Chaudhary A, Teotia S, Kumar R, et al. Multi-component framework derived SiC composite paper to support efficient thermal transport and high EMI shielding performance[J]. Composites Part B-Engineering,2019,176:11. [10] Yong-Gang, Wang, And, et al. Microstructure of mesocarbon microbeads prepared from synthetic isotropic naphthalene pitch in the presence of carbon black[J]. Carbon,1999,37(2):307-314. doi: 10.1016/S0008-6223(98)00179-1 [11] Li F H, Shen Z M, Xue R S, et al. Study on mesocarbon microbeads prepared by the emulsion method[J]. New Carbon Materials,2004,19(1):21-27. [12] Korai Y, Ishida S, Yoon S H, et al. Preparation of mesocarbon microbeads by dispersing mesophase pitch in isotropic pitches[J]. Carbon,1997,35(10):1503-1515. [13] Brooks J D, Taylor G H. Formation of graphitizing carbons from the liquid phase[J]. Nature,1965,3(2):185, IN189, 187-186, IN118, 193. [14] Wang Y G, Chang Y C, Ishida S, et al. Preparation of mesocarbon microbeads from a synthetic isotropic pitch through two stage heat treatment in the presence of carbon black[J]. Carbon,1998,36(36):1231-1233. [15] Ma Z C, Lin Q L, Cai Q H, et al. Effect of rosin addition on preparation of mesocarbon microbeads[J]. Gongneng Cailiao/Journal of Functional Materials,2012,43(8):1052-1055. [16] Yang Y S, Wang C Y, Chen M M. Preparation and structure analysis of nano-iron/mesocarbon microbead composites made from a coal tar pitch with addition of ferrocene[J]. Journal of Physics and Chemistry of Solids,2009,70(10):1344-1347. doi: 10.1016/j.jpcs.2009.07.023 [17] Li T Q, Wang C Y, Liu X J, et al. Characteristics of mesocarbon microbeads generated from a coal tar pitch with addition of micro-alumina powder[J]. Fuel Processing Technology,2005,87(1):77-83. doi: 10.1016/j.fuproc.2005.07.003 [18] Yang Y S, Wang C Y, Chen M M, et al. The role of primary quinoline insoluble on the formation of mesocarbon microbeads[J]. Fuel Processing Technology,2011,92(1):154-157. doi: 10.1016/j.fuproc.2010.08.024 [19] Cheng Y L, Li T H, Hou X L, et al. Effects of AlCl3-NaCl content on the formation of mesocarbon microbeads[J]. International Journal of Chemical Reactor Engineering,2010,8:13. [20] Cheng Y L, Li T H, Li F J, et al. Preparation of mesocarbon microbeads with silicone oil/pitch emulsion[J]. Journal of Materials Engineering,2010,24(3):56-59. [21] Qiu H S, Li Y F, Wang Y P, et al. A novel form of carbon micro-balls from coal[J]. Carbon,2003,41(4):767-772. doi: 10.1016/S0008-6223(02)00392-5 [22] Zhang D K, Zhang L Z, Fang X L, et al. Enhancement of mesocarbon microbead (MCMB) preparation through supercritical fluid extraction and fractionation[J]. Fuel,2019,237:753-762. doi: 10.1016/j.fuel.2018.10.054 [23] Kim Y, Hossain A, Nakamura Y. Numerical modeling of melting and dripping process of polymeric material subjected to moving heat flux: Prediction of drop time[J]. Proceedings of the Combustion Institute,2015,35:2555-2562. doi: 10.1016/j.proci.2014.05.068 [24] Kim Y, Hossain A, Nakamura Y. Numerical study of effect of thermocapillary convection on melting process of phase change material subjected to local heating[J]. Journal of Thermal Science and Technology,2013,8(1):136-151. doi: 10.1299/jtst.8.136 [25] Kim Y, Hossain A, Nakamura Y. Numerical study of melting of a phase change material (PCM) enhanced by deformation of a liquid-gas interface[J]. International Journal of Heat and Mass Transfer,2013,63:101-112. doi: 10.1016/j.ijheatmasstransfer.2013.03.052 [26] Lu Y, Hussein A, Li D Z, et al. Properties of bio-pitch and its wettability on coke[J]. Acs Sustainable Chemistry & Engineering,2020,8(40):15366-15374. [27] Li S Z, Tian Y M, Zhong Y J, et al. Formation mechanism of carbon foams derived from mesophase pitch[J]. Carbon,2011,49(2):618-624. doi: 10.1016/j.carbon.2010.10.007 [28] Watanabe F, Korai Y, Mochida I, et al. Structure of melt-blown mesophase pitch-based carbon fiber[J]. Carbon,2000,38(5):741-747. doi: 10.1016/S0008-6223(99)00148-7 [29] Yu R, Liu D, Lou B, et al. The effect of solvent extraction on petroleum pitch compositions and their pyrolysis behaviors[J]. Fuel,2019,247:97-107. doi: 10.1016/j.fuel.2019.03.041 [30] Li M, Liu D, Lv R Q, et al. Preparation of the mesophase pitch by hydrocracking tail oil from a naphthenic vacuum residue[J]. Energy & Fuels,2015,29(7):4193-4200. [31] Yoon S H, Park Y D, Mochida I. Preparation of carbonaceous spheres from suspensions of pitch materials[J]. Carbon,1992,30(5):781-786. doi: 10.1016/0008-6223(92)90162-P [32] Shi K, Zhang X X, Wu W, et al. Effect of the oxygen content and the functionality of spinnable pitches derived from ethylene tar by distillation on the mechanical properties of carbon fibers[J]. New Carbon Materials,2019,34(1):84-92. doi: 10.1016/S1872-5805(19)60003-X [33] Dungen P, Schlogl R, Heumann S. Non-linear thermogravimetric mass spectrometry of carbon materials providing direct speciation separation of oxygen functional groups[J]. Carbon,2018,130:614-622. doi: 10.1016/j.carbon.2018.01.047 [34] Yuan M, Cao B, Meng C Y, et al. Preparation of pitch-based carbon microbeads by a simultaneous spheroidization and stabilization process for lithium-ion batteries[J]. Chemical Engineering Journal,2020,400:9. [35] Dauché F M, Bolaños G, Blasig A, et al. Control of mesophase pitch properties by supercritical fluid extraction[J]. Carbon,1998,36(7-8):953-961. doi: 10.1016/S0008-6223(97)00212-1 [36] Gong X, Lou B, Yu R, et al. Carbonization of mesocarbon microbeads prepared from mesophase pitch with different anisotropic contents and their application in lithium-ion batteries[J]. Fuel Processing Technology,2021,217:13. [37] ALillo-Ródenas M, Cazorla-Amorós D, Linares-Solano A. Understanding chemical reactions between carbons and NaOH and KOH: An insight into the chemical activation mechanism[J]. Carbone,2003,41(2):267-275. [38] Chmiola J, Yushin G, Gogotsi Y, et al. Anomalous increase in carbon capacitance at pore sizes less than 1 nanometer[J]. Science,2006,313(5794):1760-1763. doi: 10.1126/science.1132195 [39] Shen Z, Xue R. Preparation of activated mesocarbon microbeads with high mesopore content[J]. Fuel Processing Technology,2003,84(1-3):95-103. doi: 10.1016/S0378-3820(03)00050-X [40] Xie L J, Sun G H, Su F Y, et al. Hierarchical porous carbon microtubes derived from willow catkins for supercapacitor applications[J]. Journal of Materials Chemistry A,2016,4(5):1637-1646. doi: 10.1039/C5TA09043A [41] Liu F Y, Wang Z X, Zhang H T, et al. Nitrogen, oxygen and sulfur co-doped hierarchical porous carbons toward high-performance supercapacitors by direct pyrolysis of kraft lignin[J]. Carbon,2019,149:105-116. doi: 10.1016/j.carbon.2019.04.023 [42] Lei Y, Huang Z H, Yang Y, et al. Porous mesocarbon microbeads with graphitic shells: constructing a high-rate, high-capacity cathode for hybrid supercapacitor[J]. Scientific Reports,2013,3:6. [43] Biscoe J, Warren B E. An X‐Ray Study of Carbon Black[J]. Journal of Applied Physics,1942,13(6):364-371. doi: 10.1063/1.1714879 [44] Guo A, Wang F, Jiao S, et al. Preparation of mesocarbon microbeads as anode material for lithium-ion battery by thermal polymerization of a distillate fraction from an FCC slurry oil after hydrofining with suspended catalyst[J]. Fuel,2020,276:11. [45] Zhang Y P, Wang J X, Zhao P Y, et al. Anode performance of NaOH-etched mesocarbon microbeads for sodium-ion batteries[J]. Materials Science and Engineering:B,2021,264:6. [46] Yang S B, Song H H, Chen X H. Expansion of mesocarbon microbeads[J]. Carbon,2006,44(4):730-733. doi: 10.1016/j.carbon.2005.09.019 [47] Wang Y X, Tian W, Wang L H, et al. A tunable molten-salt route for scalable synthesis of ultrathin amorphous carbon nanosheets as high-performance anode materials for lithium-ion batteries[J]. Acs Applied Materials & Interfaces,2018,10(6):5577-5585. [48] Zhong M, Yan J W, Wu H X, et al. Multilayer graphene spheres generated from anthracite and semi-coke as anode materials for lithium-ion batteries[J]. Fuel Processing Technology,2020,198:8. -
 Supporting Information ——20220001.pdf
Supporting Information ——20220001.pdf

-





 下载:
下载: