Cactus-like NC/CoxP electrode enables efficient and stable hydrogen evolution for saline water splitting
-
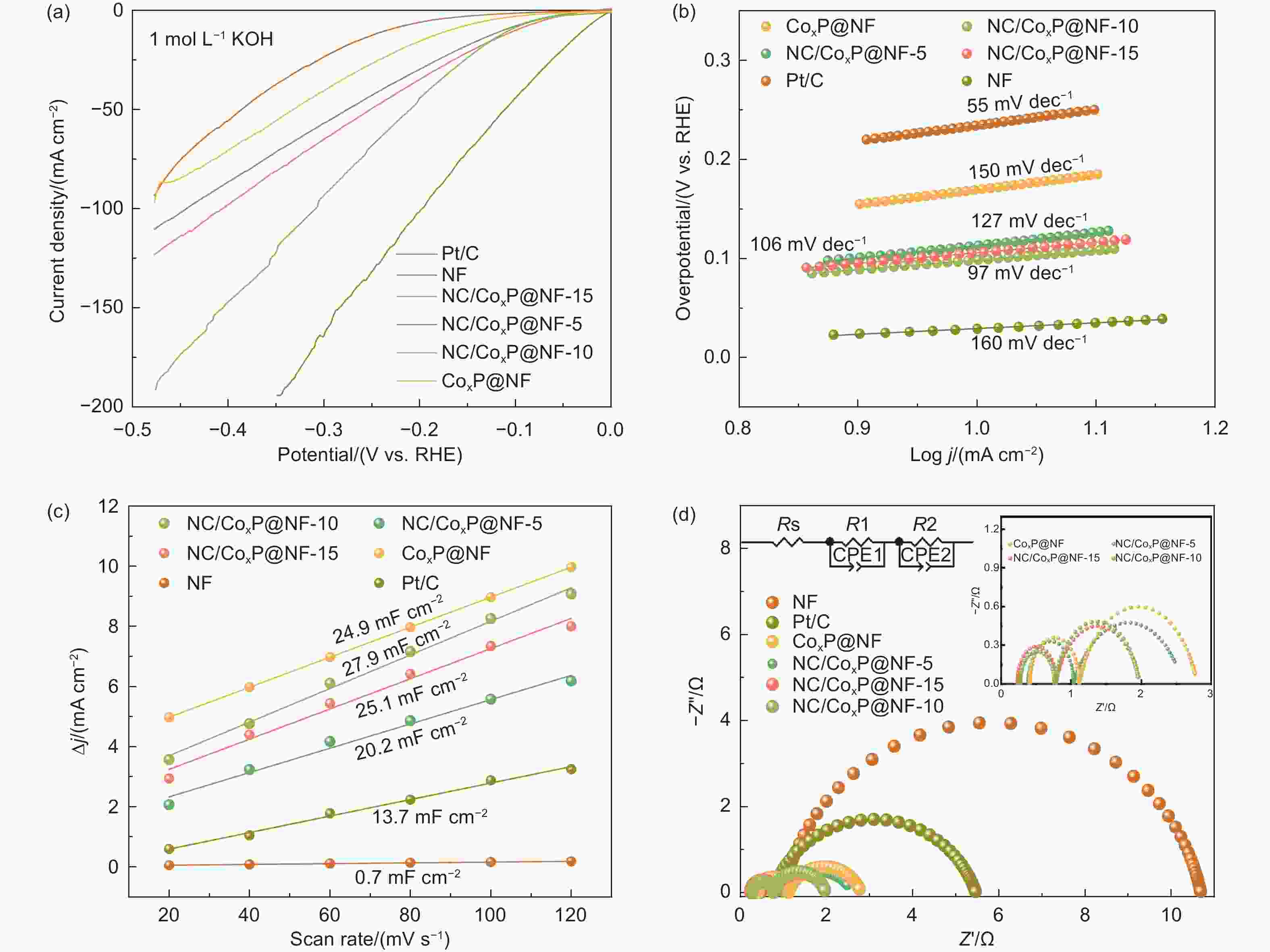
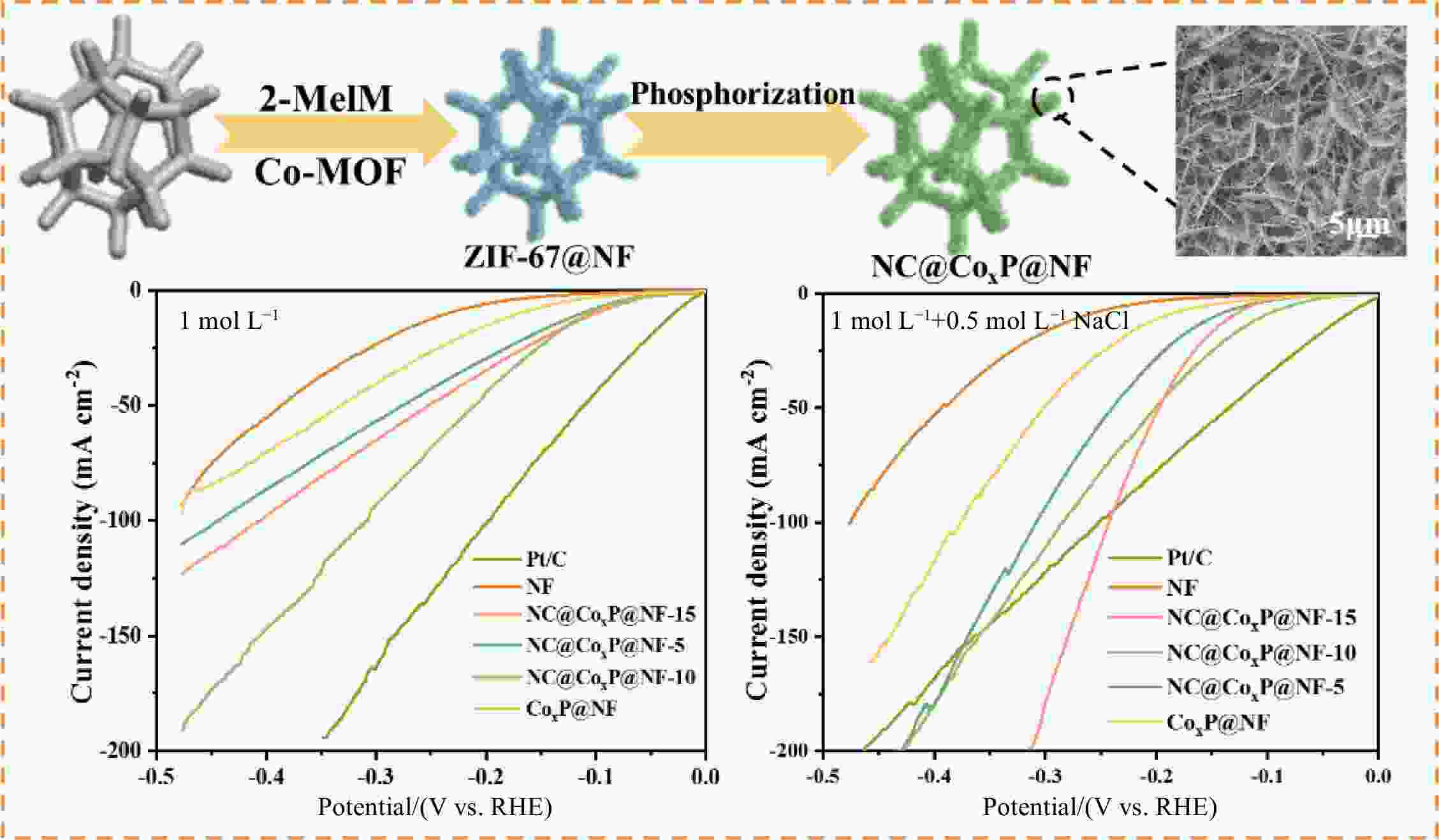
摘要: 设计高效、稳定的析氢催化剂是盐水电解技术发展的必然要求。本文通过原位生长策略在泡沫镍(NF)上生长NC/CoxP@NF催化剂,它由CoxP纳米线阵列与氮掺杂碳纳米片(NC)交替生长组成。在制备过程中,Co(OH)2纳米线通过内源Co2+与2−甲基咪唑的溶解配位作用在NF上原位转化为Co-MOF纳米片。仙人掌状的微观结构使NC/CoxP@NF暴露出丰富的活性位点和离子运输通道,促进了HER催化反应动力学。此外,在分级多孔的NC/CoxP@NF中,纳米线和自支撑纳米片交替生长,进一步增强了材料的结构稳定性。最重要的是,表面聚阴离子(磷酸盐)和NC纳米片保护层的形成提高了催化剂的耐腐性能。最终,NC/CoxP@NF-10表现出优异的析氢性能,在1.0 mol L−1 KOH和1.0 mol L−1 KOH + 0.5 mol L−1 NaCl条件下,分别需要107和133 mV的过电位达到10 mA cm−2的电流密度。Abstract: Designing efficient and robust catalysts for hydrogen evolution reaction (HER) is imperative for saline water electrolysis technology. A catalyst composed of CoxP nanowires array with N-doped carbon nanosheets (NC) was fabricated on Ni foam (NF) by an in-situ growth strategy. The material is designated as NC/CoxP@NF. In the preparation process, Co(OH)2 nanowires were transformed into a metal organic framework of cobalt (ZIF-67) on NF by the dissolution-coordination of endogenous Co2+ and 2-methylimidazole. The resulting cactus-like microstructure gives NC/CoxP@NF abundant exposed active sites and ion transport channels, which improve the HER catalytic reaction kinetics. Furthermore, the interconnected alternating nanowires and free-standing nanosheets in NC/CoxP@NF improve its structural stability, and the formation of surface polyanions (phosphate) and a NC nanosheet protective layer improve the anti-corrosive properties of catalysts. Thus, the NC/CoxP@NF has an excellent performance, requiring overpotentials of 107 and 133 mV for HER to achieve 10 mA cm−2 in 1.0 mol L−1 KOH and 1.0 mol L−1 KOH + 0.5 mol L−1 NaCl, respectively. This in-situ transformation strategy is a new way of constructing highly-efficient HER catalysts for saline water electrolysis.
-
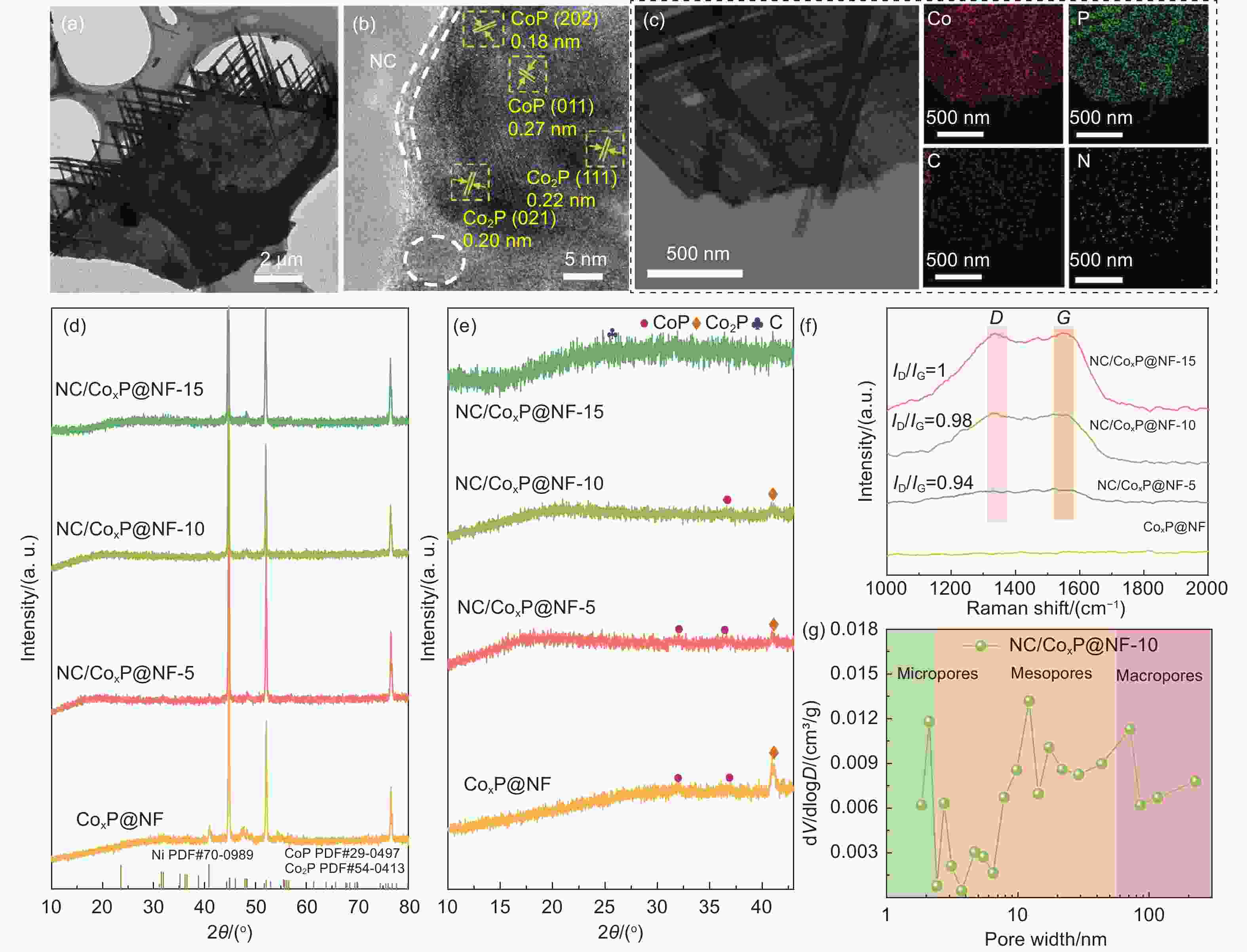
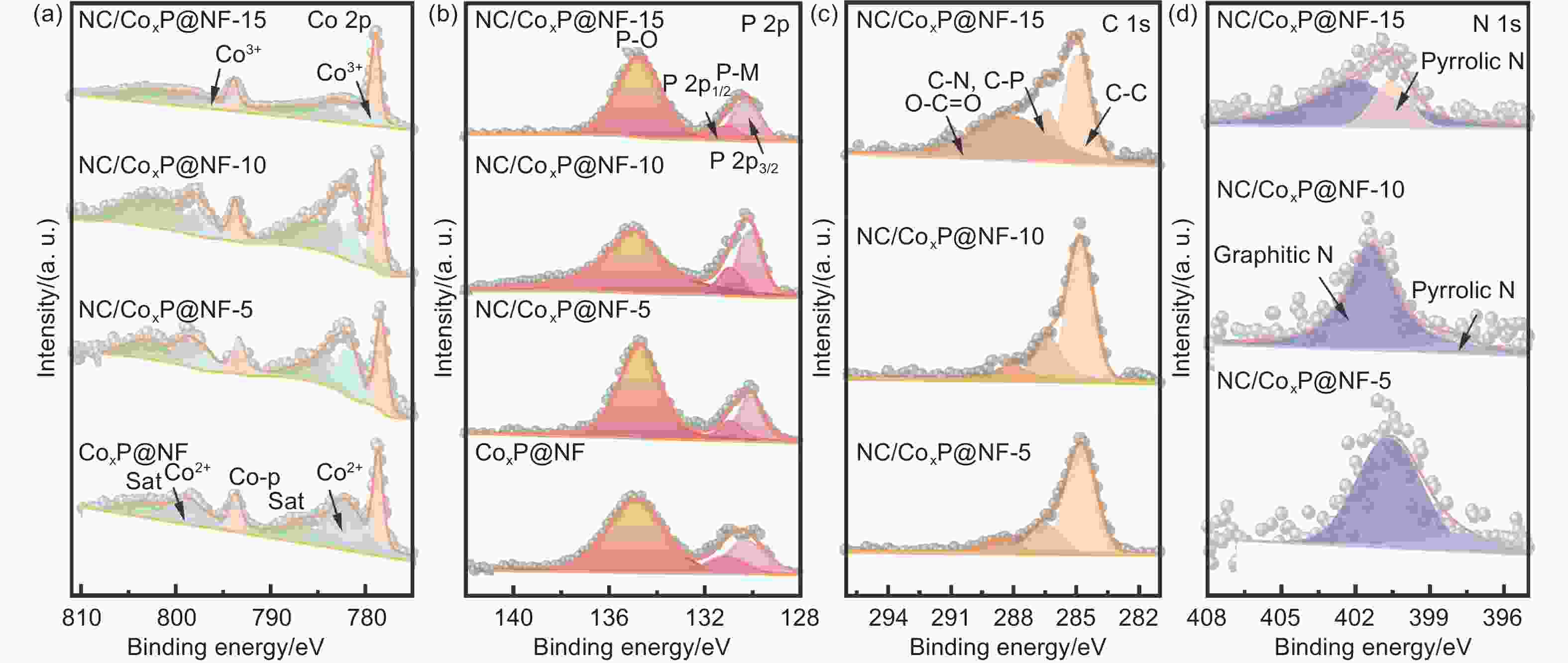
Figure 2. (a) TEM image; (b) HRTEM image and (c) the corresponding elemental mappings of NC/CoxP@NF-10. (d) XRD patterns and (e) the magnified diffraction peaks at the range of 10°-43° of all catalysts. (f) Raman spectra of NC/CoxP@NF-5, NC/CoxP@NF-10 and NC/CoxP@NF-15. (g) Pore distribution curve of NC/CoxP@NF-10
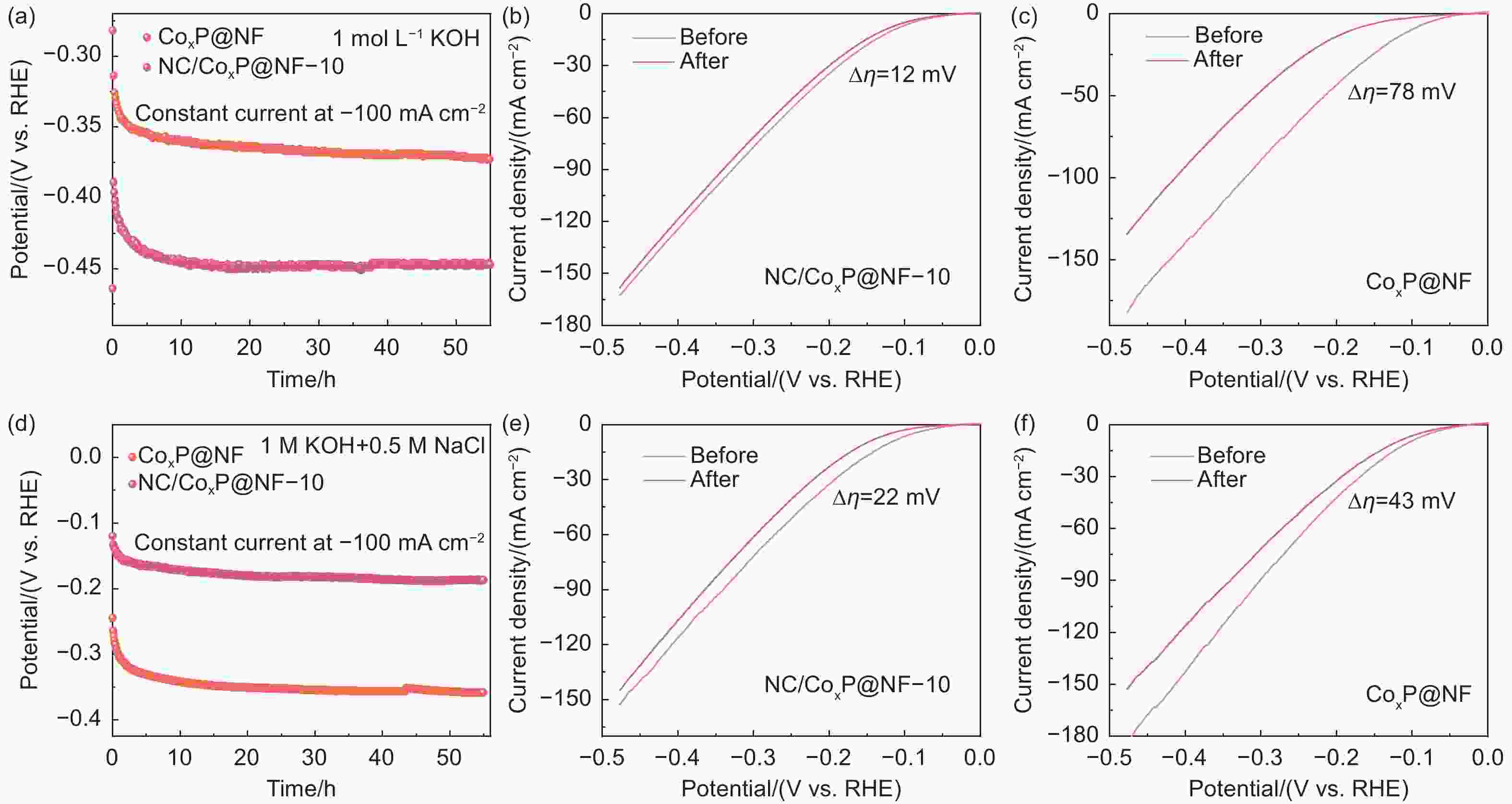
Figure 6. 1 mol L−1 KOH electrolyte: (a) Chronopotentiometric curves conducted at a constant current density of -100 mA cm−2. The LSV curves before and after stability tests for (b) NC/CoxP@NF-10 and (c) CoxP@NF. 1 mol L−1 KOH + 0.5 mol L−1 NaCl solution: (d) Stability tests of NC/CoxP@NF-10 and CoxP@NF. LSV curves before and after stability tests for (e) NC/CoxP@NF-10 and (f) CoxP@NF
-
[1] Yuan H, Zhao L, Chang B, et al. Laser fabrication of Pt anchored Mo2C micropillars as integrated gas diffusion and catalytic electrode for proton exchange membrane water electrolyzer[J]. Applied Catalysis B: Environmental,2022,314:121455. doi: 10.1016/j.apcatb.2022.121455 [2] Gao Y, Qian S, Wang H, et al. Boron-doping on the surface mediated low-valence Co centers in cobalt phosphide for improved electrocatalytic hydrogen evolution[J]. Applied Catalysis B: Environmental,2023,320:122014. doi: 10.1016/j.apcatb.2022.122014 [3] Zheng Y, Qiao S Z. Direct seawater splitting to hydrogen by a membrane electrolyzer[J]. Joule,2023,7(1):20-22. doi: 10.1016/j.joule.2022.12.017 [4] Xie H, Zhao Z, Liu T, et al. A membrane-based seawater electrolyser for hydrogen generation[J]. Nature,2022,612:73-678. [5] Wu L, Yu L, Zhang F, et al. Heterogeneous bimetallic phosphide Ni2P-Fe2P as an efficient bifunctional catalyst for water/seawater Splitting[J]. Advanced Functional Materials,2020,31(1):2006484. [6] Zhu J, Chi J, Cui T, et al. F doping and P vacancy engineered FeCoP nanosheets for efficient and stable seawater electrolysis at large current density[J]. Applied Catalysis B: Environmental,2023,328:122487. doi: 10.1016/j.apcatb.2023.122487 [7] Wang X, Liu X, Wu S, et al. Phosphorus vacancies enriched cobalt phosphide embedded in nitrogen doped carbon matrix enabling seawater splitting at ampere-level current density[J]. Nano Energy,2023,109:108292. doi: 10.1016/j.nanoen.2023.108292 [8] Wu D, Liu B, Li R, et al. Fe-regulated amorphous-crystal NiFeP2 nanosheets coupled with Ru powerfully drive seawater splitting at large current density[J]. Small,2023,19(36):2300030. doi: 10.1002/smll.202300030 [9] Yu W, Liu H, Zhao Y, et al. Amorphous NiOn coupled with trace PtOx toward superior electrocatalytic overall water splitting in alkaline seawater media[J]. Nano Research,2023,16:6517-6530. doi: 10.1007/s12274-022-5369-0 [10] Chen Z, Li Q, Xiang H, et al. Hierarchical porous NiFe-P@NC as an efficient electrocatalyst for alkaline hydrogen production and seawater electrolysis at high current density[J]. Inorganic Chemistry Frontiers,2023,10(5):1493-1500. doi: 10.1039/D2QI02703H [11] Jung Kim S, Choi H, Ho Ryu J, et al. Zn-doped nickel iron (oxy)hydroxide nanocubes passivated by polyanions with high catalytic activity and corrosion resistance for seawater oxidation[J]. Journal of Energy Chemistry,2023,81:82-92. doi: 10.1016/j.jechem.2023.02.033 [12] Li J, Yu T, Wang K, et al. Multiscale engineering of nonprecious metal electrocatalyst for realizing ultrastable seawater splitting in weakly alkaline solution[J]. Advanced Science,2022,9(25):2202387. doi: 10.1002/advs.202202387 [13] Ma T, Xu W, Li B, et al. The critical role of additive sulfate for stable alkaline seawater oxidation on nickel-based electrodes[J]. Angewandte Chemie-International Edition,2021,60(42):22740-22744. doi: 10.1002/anie.202110355 [14] Zhou S, Wang J, Li J, et al. Surface-growing organophosphorus layer on layered double hydroxides enables boosted and durable electrochemical freshwater/seawater oxidation[J]. Applied Catalysis B: Environmental,2023,332:122749. doi: 10.1016/j.apcatb.2023.122749 [15] Chen D, Bai H, Zhu J, et al. Multiscale hierarchical structured NiCoP enabling ampere-level water splitting for multi-scenarios green energy-to-hydrogen systems[J]. Advanced Energy Materials,2023,13(22):2300499. doi: 10.1002/aenm.202300499 [16] Li J, Song M, Hu Y, et al. Hybrid heterostructure Ni3N|NiFeP/FF self-supporting electrode for high-current-density alkaline water Electrolysis[J]. Small Methods,2023,7(4):2201616. doi: 10.1002/smtd.202201616 [17] Loomba S, Khan M W, Haris M, et al. Nitrogen-doped porous nickel molybdenum phosphide sheets for efficient seawater splitting[J]. Small,2023,19(18):2207310. doi: 10.1002/smll.202207310 [18] Chen N, Che S, Yuan Y, et al. Self-supporting electrocatalyst constructed from in-situ transformation of Co(OH)2 to metal-organic framework to Co/CoP/NC nanosheets for high-current-density water splitting[J]. Journal of Colloid and Interface Science,2023,645:513-524. doi: 10.1016/j.jcis.2023.04.089 [19] Popczun E J, Read C G, Roske C W, et al. Highly active electrocatalysis of the hydrogen evolution reaction by cobalt phosphide nanoparticles[J]. Angewandte Chemie-International Edition,2014,53(21):5427-5430. doi: 10.1002/anie.201402646 [20] Wang K, Zhao R, Wang Z, et al. Controlled tuning the morphology of CoNiP catalysts with ultra-high activity for water splitting at large current densities in alkaline medium[J]. Applied Surface Science,2023,626:157218. doi: 10.1016/j.apsusc.2023.157218 [21] Yu M, Li J, Liu F, et al. Anionic formulation of electrolyte additive towards stable electrocatalytic oxygen evolution in seawater splitting[J]. Journal of Energy Chemistry,2022,72:361-369. doi: 10.1016/j.jechem.2022.04.004 [22] Obata K, Takanabe K. A permselective CeOx coating to improve the stability of oxygen evolution electrocatalysts[J]. Angewandte Chemie-International Edition,2018,57(6):1616-1620. doi: 10.1002/anie.201712121 [23] Chang J, Wang G, Yang Z, et al. Dual-doping and synergism toward high-performance seawater electrolysis[J]. Advanced Materials,2021,33(33):2101425. doi: 10.1002/adma.202101425 [24] Sun Z, Chu B, Wang S, et al. Hydrogen-bond induced and hetero coupling dual effects in N-doped carbon coated CrN/Ni nanosheets for efficient alkaline freshwater/seawater hydrogen evolution[J]. Journal of Colloid and Interface Science,2023,646:361-369. doi: 10.1016/j.jcis.2023.05.006 [25] Li J, Hu Y, Huang X, et al. Bimetallic phosphide heterostructure coupled with ultrathin carbon layer boosting overall alkaline water and seawater splitting[J]. Small,2023,19(20):2206533. doi: 10.1002/smll.202206533 [26] Yu Q, Liu X, Liu G, et al. Constructing three‐phase heterojunction with 1D/3D hierarchical structure as efficient trifunctional electrocatalyst in alkaline eeawater[J]. Advanced Functional Materials,2022,32(46):2205767. doi: 10.1002/adfm.202205767 [27] Tan Y, Feng J, Dong H, et al. The edge effects boosting hydrogen evolution performance of platinum/transition bimetallic phosphide hybrid electrocatalysts[J]. Advanced Functional Materials,2022,33(4):2209967. [28] Li T, Zhao X, Getaye Sendeku M, et al. Phosphate-decorated Ni3Fe-LDHs@CoPx nanoarray for near-neutral seawater splitting[J]. Chemical Engineering Journal,2023,460:141413. doi: 10.1016/j.cej.2023.141413 [29] Song Y, Sun M, Zhang S, et al. Alleviating the work function of Vein-Like CoXP by Cr doping for enhanced seawater electrolysis[J]. Advanced Functional Materials,2023,33(30):2214081. doi: 10.1002/adfm.202214081 [30] Liu S S, Ma L J, Li J S. Dual-metal-organic-framework derived CoP/MoP hybrid as an efficient electrocatalyst for acidic and alkaline hydrogen evolution reaction[J]. Journal of Colloid and Interface Science,2022,631:147-153. [31] Li L, Wen Y, Han G, et al. Tailoring the stability of Fe-N-C via pyridinic nitrogen for acid oxygen reduction reaction[J]. Chemical Engineering Journal,2022,437:135320. doi: 10.1016/j.cej.2022.135320 [32] Ye G, Liu S, Huang K, et al. Domain-confined etching strategy to regulate defective sites in carbon for high-efficiency electrocatalytic oxygen reduction[J]. Advanced Functional Materials,2022,32(18):2111396. doi: 10.1002/adfm.202111396 [33] Han N, Feng S, Liang Y, et al. Achieving efficient electrocatalytic oxygen evolution in acidic media on yttrium ruthenate pyrochlore through cobalt incorporation[J]. Advanced Functional Materials,2023,33(20):2208399. doi: 10.1002/adfm.202208399 [34] Zhu J, Li P, Wang G, et al. Design strategy for high-performance bifunctional electrode materials with heterogeneous structures formed by hydrothermal sulfur etching[J]. Journal of Colloid Interface Science,2022,633:608-618. [35] Liu Y, Zhang H, Song W, et al. In-situ growth of ReS2/NiS heterostructure on Ni foam as an ultra-stable electrocatalyst for alkaline hydrogen generation[J]. Chemical Engineering Journal,2023,451:138905. doi: 10.1016/j.cej.2022.138905 [36] Lv X, Wan S, Mou T, et al. Atomic-level surface engineering of nickel phosphide nanoarrays for efficient electrocatalytic water splitting at large current density[J]. Advanced Functional Materials,2022,33(4):2205161. [37] Jin X, Jang H, Jarulertwathana N, et al. Atomically thin holey two-dimensional Ru2P nanosheets for enhanced hydrogen evolution electrocatalysis[J]. ACS Nano,2022,16(10):16452-16461. doi: 10.1021/acsnano.2c05691 [38] Hong C-B, Li X, Wei W-B, et al. Nano-engineering of Ru-based hierarchical porous nanoreactors for highly efficient pH-universal overall water splitting[J]. Applied Catalysis B: Environmental,2021,294:120230. doi: 10.1016/j.apcatb.2021.120230 [39] Zhang K, Wang H, Qiu J, et al. Multi-dimensional Pt/Ni(OH)2/nitrogen-doped graphene nanocomposites with low platinum content for methanol oxidation reaction with highly catalytic performance[J]. Chemical Engineering Journal,2021,421:127786. doi: 10.1016/j.cej.2020.127786 [40] Han Y, Duan H, Liu W, et al. Engineering the electronic structure of platinum single-atom sites via tailored porous carbon nanofibers for large-scale hydrogen production[J]. Applied Catalysis B: Environmental,2023,335:122898. doi: 10.1016/j.apcatb.2023.122898 [41] Wang R, Liu J, Xie J, et al. Hollow nanocage with skeleton Ni-Fe sulfides modified by N-doped carbon quantum dots for enhancing mass transfer for oxygen electrocatalysis in zinc-air battery[J]. Applied Catalysis B: Environmental,2023,324:122230. doi: 10.1016/j.apcatb.2022.122230 [42] Nie N, Zhang D, Wang Z, et al. Stable PtNb-Nb2O5 heterostructure clusters @CC for high-current-density neutral seawater hydrogen evolution[J]. Applied Catalysis B: Environmental,2022,318:121808. doi: 10.1016/j.apcatb.2022.121808 [43] Liu H, Li J, Zhang Y, et al. Boosted water electrolysis capability of NixCoyP via charge redistribution and surface activation[J]. Chemical Engineering Journal,2023,473:145397. doi: 10.1016/j.cej.2023.145397 [44] Yan H, Jiang Z, Deng B, et al. Ultrathin carbon coating and defect engineering promote RuO2 as an efficient catalyst for acidic oxygen evolution reaction with super‐high durability[J]. Advanced Energy Materials,2023,13(23):2300152. doi: 10.1002/aenm.202300152 [45] Wang H-Y, Ren J-T, Wang L, et al. Synergistically enhanced activity and stability of bifunctional nickel phosphide/sulfide heterointerface electrodes for direct alkaline seawater electrolysis[J]. Journal of Energy Chemistry,2022,75:66-73. doi: 10.1016/j.jechem.2022.08.019 [46] Ren J T, Chen L, Tian W W, et al. Rational synthesis of core-shell-structured nickel sulfide-based nanostructures for efficient seawater electrolysis[J]. Small,2023,19(27):2300194. doi: 10.1002/smll.202300194 [47] Liu X, Zhao X, Cao S, et al. Local hydroxyl enhancement design of NiFe sulfide electrocatalyst toward efficient oxygen evolution reaction[J]. Applied Catalysis B: Environmental,2023,331:122715. doi: 10.1016/j.apcatb.2023.122715 -
 ncm2023-0179_Revised+supporting+information_新型炭材料(中英文).pdf
ncm2023-0179_Revised+supporting+information_新型炭材料(中英文).pdf

-





 下载:
下载: